http://nnr.sagepub.com
Neurorehabilitation and Neural Repair
DOI: 10.1177/1545968309345268
2010; 24; 42 originally published online Sep 3, 2009; Neurorehabil Neural Repair
Keh-chung Lin, Yi-an Chen, Chia-ling Chen, Ching-yi Wu and Ya-fen Chang
A Randomized Controlled Study
The Effects of Bilateral Arm Training on Motor Control and Functional Performance in Chronic Stroke:
http://nnr.sagepub.com/cgi/content/abstract/24/1/42
The online version of this article can be found at:
Published by:
http://www.sagepublications.com On behalf of:
American Society of Neurorehabilitation
can be found at:
Neurorehabilitation and Neural Repair
Additional services and information for
http://nnr.sagepub.com/cgi/alerts Email Alerts: http://nnr.sagepub.com/subscriptions Subscriptions: http://www.sagepub.com/journalsReprints.nav Reprints: http://www.sagepub.com/journalsPermissions.nav Permissions: http://nnr.sagepub.com/cgi/content/refs/24/1/42 Citations
Neurorehabilitation and Neural Repair 24(1) 42 –51 © The Author(s) 2010
Reprints and permission: http://www. sagepub.com/journalsPermissions.nav DOI: 10.1177/1545968309345268 http://nnr.sagepub.com
The Effects of Bilateral Arm Training on
Motor Control and Functional Performance
in Chronic Stroke: A Randomized
Controlled Study
Keh-chung Lin, ScD,
1,2Yi-an Chen, MS,
3Chia-ling Chen, MD, PhD,
4Ching-yi Wu, ScD,
4and Ya-fen Chang, MS
5Abstract
Background. Most studies of bilateral arm training (BAT) did not employ a randomized controlled trial design and involved
very limited functional training tasks. Objective. Compare the effects of BAT with control intervention (CI) on motor control and motor performance of the upper extremity and also functional gains in patients with chronic stroke. Methods. This 2-group randomized controlled trial with pretreatment and posttreatment measures enrolled 33 stroke patients (mean age = 53.85 years) 6 to 67 months after onset of a first stroke. They received either a BAT program concentrating on both upper extremities moving simultaneously in functional tasks by symmetric patterns or CI (control treatment) for 2 hours on weekdays for 3 weeks. Outcome measures included kinematic analyses assessing motor control strategies for unilateral and bimanual reaching and clinical measures involving the Fugl-Meyer Assessment (FMA) of motor-impairment severity and the Functional Independence Measure (FIM) and the Motor Activity Log (MAL) evaluating functional ability. Results. After treatment, the BAT group showed better temporal and spatial efficiency during unilateral and bilateral tasks and less online error correction only during the bilateral task than the control group. The BAT group showed a significantly greater improvement in the FMA than the control group but not in the FIM and MAL. Conclusions. Relative to CI, BAT improved the spatiotemporal control of the affected arm in both bilateral and unilateral tasks, decreased online corrections to perform bilateral tasks, and reduced motor impairment. These findings support the use of BAT to improve motor control and motor function of the affected upper limb in stroke patients.
Keywords
cerebrovascular accident, motor activity, kinematics, rehabilitation, randomized controlled trial, upper extremity
Introduction
Stroke survivors are often left with hemiplegia or hemiparesis of the upper extremity (UE) that makes functioning in their daily living environment extremely challenging.1 The
disrup-tions caused to motor control of the UE by stroke are persistent despite usual treatment, and thus, novel intervention techniques are called for.2 Among the approaches advocated for stroke
motor rehabilitation, bilateral arm training (BAT) has received considerable attention.3-8 Previous studies of BAT, however,
employed very small sample sizes and limited functional train-ing tasks without randomized controlled trials to investigate the effects on motor control. This study investigated the effects of BAT on motor control and performance of the affected UE and daily function in patients with chronic stroke.
BAT involves repetitive practice of symmetrical bilateral movements in different forms, including bilateral isokinema-tic training,3,9-11 which involves spatiotemporally identical
movements with functional activities, and robot-assisted
1National Taiwan University, Taipei, Taiwan 2National Taiwan University Hospital, Taipei, Taiwan 3Yung Cheng Rehabilitation Clinic, Taipei, Taiwan 4Chang Gung Memorial Hospital, Taoyuan, Taiwan 5Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan
Corresponding Author:
Ching-yi Wu, Department of Occupational Therapy, Chang Gung University, 259 Wen-hwa 1st Rd, Kwei-shan, Taoyuan, Taiwan E-mail: cywu@mail.cgu.edu.tw
movement training involving assistive exercise on the arm trainer with5,12-14 or without6,15-17 auditory cueing. A basic
assumption of BAT is that symmetrical bilateral movements activate similar neural networks in both hemispheres when homologous muscle groups are simultaneously activated. Bilateral symmetrical movements, therefore, may allow for the activation of the undamaged hemisphere, thus promoting neural plasticity to increase activation of the damaged hemi-sphere and facilitate movement control of the impaired limb.18
A number of studies have shown that BAT can reduce motor impairment of the affected arm as measured by the Fugl-Meyer Assessment (FMA)5,6,12,14,15,17 and enhance
motor function as evaluated by the Wolf Motor Function Test.12,14 Only a few studies5,12,13 have investigated the
impact of BAT on functional performance during daily activities, and the results were inconclusive. To clarify whether and how the BAT involving functional activities may affect motor control during the performance of func-tional tasks, Mudie and Matyas9,10 visually observed
kinematic aspects of movements (eg, spatiotemporal char-acteristics of movement), and subsequent studies7,19
objectively and directly measured the movement kinemat-ics to underscore the spatial and temporal efficiency and control strategy (preplanning control vs online error correc-tion control) of movements. However, Mudie et al employed a single-subject design, and the test tasks were the same as the training tasks. The positive effects of BAT might be owing to practice effects. Another 2 studies7,19 involved
short treatment duration of 1 week and did not show benefi-cial effects of bilateral training on movement kinematics. One study14 that used robot-assisted BAT with auditory
cues showed beneficial effects of BAT on kinematic mea-sures such as movement efficiency and smoothness.
In addition to the 2 kinematic studies,7,19 some other
research8,11,13,16 also failed to demonstrate the effects of
BAT, though several studies5,6,9,10,12,15,17 showed the benefits
of BAT for improving movement performance after stroke. The small sample size, degree of initial impairment, time since stroke onset, or intensity of treatment might have con-tributed to the lack of significant effects in these negative studies. For example, Lewis and Byblow11 recruited only 6
stroke patients across the 3 phases of recovery (acute, sub-acute, and chronic) and used a low-intensity treatment protocol involving 11 repetitions of each task, 3 tasks at each training session, for a total of about 10 sessions of bilateral training. Lum et al16 recruited only 5 stroke patients
in the bilateral training group, and the study groups were not matched on level of baseline performance. To overcome the limitations in prior research,5-7,9,10,12,15,17,19 we used a
larger sample and implemented a more intense and longer (eg, 3 weeks) treatment protocol in the BAT program.
Because current approaches to stroke rehabilitation emphasize the importance of task-oriented training,20 our
BAT program used repetitive practice on a variety of func-tional tasks with spatiotemporally identical movements. Although some previous studies of BAT have adopted func-tional activities for training, most7-11,19 included only 1 to 4
types of functional tasks (eg, simulated drinking, picking up a cup). In addition, we used kinematic analysis to investigate the effect of BAT on motor control of reaching and reach-to-grasp movements in experimental tasks that were not practiced during the treatment period. Study of motor control strategy is important for understanding the mechanisms
underlying therapeutic gains. Combining kinematic analysis and clinical evaluation might enable comprehensive assess-ment of the change in control strategies and motor performance after BAT.14
We predicted that BAT might confer therapeutic benefits for enhancing motor control strategy during upper limb activity and reduce motor and functional deficits in stroke patients. Specifically, we hypothesized that stroke patients receiving 3 weeks of BAT, compared with patients receiv-ing control intervention (CI), would exhibit better motor control performance in the affected UE during unilateral and bilateral testing tasks (ie, increased movement effi-ciency and better control strategy) and achieve greater motor (higher FMA scores) and functional gains (greater functional independence).
Methods
Design
This study was a randomized pretest and posttest control group design. Participants were individually randomized to the BAT group or a control group (Figure 1). All patients were blind to the study hypotheses. The outcome measures were administered before and after a 3-week intervention. Training took place during regularly scheduled occupa-tional therapy sessions, and all other routine stroke rehabilitations (eg, physical therapy or speech therapy) that did not involve UE training proceeded as usual.
Participants
We recruited 33 stroke patients (19 men, 14 women; mean age = 53.85 years) after onset of an ischemic or hemor-rhagic stroke (onset mean = 13.52 months; range = 6-57 months) from the rehabilitation departments of 3 participat-ing hospitals. All patients signed informed consent forms approved by the institutional review board. The patients were all right-hand dominant before the stroke by self-report.
Inclusion criteria were as follows: (a) clinical diagnosis of a first or recurrent unilateral stroke; (b) the ability to reach Brunnstrom stage III or above in the proximal and distal part of arm21; (c) no serious cognitive deficits
(Mini-Mental State Examination score ≥24); (d) no excessive spasticity in the affected arm, including shoulder, elbow, wrist, and fingers (Modified Ashworth Scale22 score ≤2 in
any joint) that might preclude the functional movements; (e) no other neurologic, neuromuscular, or orthopedic dis-ease; and (f) lack of participation in any experimental rehabilitation or drug studies. Patients would have been excluded if they had a stroke relapse or seizure attack during the intervention but none did. All potential participants received independent examinations by 4 occupational ther-apists to determine their eligibility for inclusion.
44 Neurorehabilitation and Neural Repair 24(1)
Intervention
The intervention was provided at 3 participating hospitals under the supervision of 3 occupational therapists. The treating therapists were trained in the administration of the BAT protocol by the investigators and completed a written competency test before subject treatment.
Bilateral arm training group. The BAT group concentrated
on both the affected and unaffected UEs moving simultane-ously in functional tasks with symmetric patterns for 2 hours per day, 5 days a week, for 3 weeks. The subjects had one-on-one supervision as they practiced on a variety of functional tasks typically found difficult by stroke patients. The partici-pants were instructed to lift 2 cups, stack 2 checkers, pick up 2 small dried beans with a diameter of 0.5 to 1 cm, fold 2 towels, turn 2 large screws, manipulate 2 coins simultane-ously by each hand, or use both hands to hold a sprinkler can to water plants.
Control intervention group. With equivalent intensity and
duration, participants in the control group also received the treatment program for 2 hours per day, 5 days a week, for 3 weeks. This group received standard occupational therapy treatment that also focused on UE training and included neurodevelopmental techniques, trunk–arm control (ie, practice UE tasks during standing), weight bearing by the affected arm, fine motor tasks practice, and practice on compensatory strategies for daily activities.
Outcome Measures
Changes in motor control, motor performance, and func-tional ability were evaluated using kinematic analysis and
clinical evaluation, which were administered within 7 days before and after the 3-week intervention. During evalua-tion, the participants took rest breaks whenever they requested. Four occupational therapists blind to group allo-cation were trained to provide the evaluations. The training for the clinical assessment tools included careful examina-tion of written instrucexamina-tions and repeated practice. The training for conducting the kinematic analysis was based on standardized procedures described as follows. Rater com-petence for performing kinematic and clinical evaluations was assessed by the senior authors. After the 4 raters fin-ished the clinical evaluation of all participants, we calculated the post hoc interrater reliability for FMA and Functional Independence Measure (FIM) and obtained high reliability (intraclass correlation coefficients23 >.95 for both
mea-sures).24 Patients were advised not to indicate their treatment
assignment to the evaluator.
Kinematic analysis. Experimental tasks used in the
kine-matic analysis included 1 unilateral task and 1 bimanual task. The unilateral task involved pressing a desk bell, and the bilat-eral task involved opening a box to retrieve a sticky note. During the tasks, each participant sat on a height-adjustable chair with seat height set to 100% of the lower leg length, measured from the lateral knee joint to the floor, with the par-ticipant standing. The table height was adjusted to 2 inches. below the elbow. The participant rested his or her hands on the edge of the table. The trunk was harnessed to the chair back to minimize trunk flexion and rotation.
The target object (desk bell or box) was located along the participant’s midsagittal plane, and the reaching dis-tance was standardized to the participant’s functional arm length. Functional arm length was defined as the distance
Figure 1. Flow diagram of the randomization procedure.
Assessed for eligibility (n = 381)
Randomized (n = 33)
Excluded (n = 348) Not meeting inclusion criteria
(n = 287)
Refused to participate (n = 61)
Bilateral Arm Training (n = 16)
Control Intervention (n = 17)
from the medial border of the axilla to the distal wrist crease.25 If the maximum distance the patient could reach
was less than the functional arm length, the reaching dis-tance to the target was adjusted to the maximum reachable distance.
For the unilateral task, participants reached to press a desk bell 3 cm in length and width and 0.5 cm in height. The par-ticipant was instructed to use the index finger of the affected hand to reach and press the task bell as fast as possible. For the bilateral task, participants used the affected hand to open a 10 × 10 × 8 cm (length × width × height) box by holding the lid and used the unaffected hand to retrieve a sticky note (7 × 3 × 6 cm) inside the box at a comfortable speed. Only reaching movements of the affected hand were recorded during this task. After a practice trial, 3 data-producing trials were performed.
During the 2 tasks, an 8-camera motion analysis system (VICON MX; Oxford Metrics Inc, Oxford, UK) was used in conjunction with a personal computer to capture the move-ment of a marker attached on the ulnar styloid. The system was calibrated to have averaged residual errors not exceeding 0.5 mm for each camera before data acquisition. For the uni-lateral task, 1 channel of analog signals was collected to signal the end of the movement when the bell was pressed.
The experimenter said “ready’ to remind the participant to prepare performing the experimental task and then pressed the desk bell to indicate the start of the task. The time between the warning and start signal was not a fixed length. Movement onset was defined as a rise of tangential wrist velocity above 5% of its peak value for both testing tasks. This practice was based on the proposals of previous reports.26-28 During the unilateral task, end of movement
was defined as the time when the participant pressed the desk bell. During the bilateral task, movement offset was defined as a fall of tangential wrist velocity below 5% of its peak value. Movements were recorded at 120 Hz and digitally low-pass filtered at 5 Hz using a second-order But-terworth filter with forward and backward passes.
Clinical assessment. We used the 66-point, UE section of
the FMA to evaluate motor impairment of the affected UE. This instrument assesses several dimensions of motor impairment using a 3-point ordinal scale: 0 = cannot per-form; 1 = can perform partially; 2 = can perform fully.29 A
higher FMA score indicates less motor impairment. Test– retest reliability, interrater reliability, and construct validity of the test are well established in stroke patients.30,31
We used the FIM instrument to measure the functional ability of participants. It consists of 18 items grouped into 6 subscales measuring self-care, sphincter control, transfer, locomotion, communication, and social ability.32 Each item
is rated with a score from 1 to 7, for a maximum score of 126: 1 = complete assistance to perform basic activities of daily living; 2 = maximal assistance; 3 = moderate assis-tance; 4 = minimal assisassis-tance; 5 = supervision; 6 = modified
independence; and 7 = complete independence in perform-ing basic daily livperform-ing. The FIM has established good interrater reliability, construct validity,33,34 and discriminate
validity.35 We also used the Motor Activity Log (MAL), a
semistructured interview of patients, to assess the amount of use (AOU) and quality of movement (QOM) of the affected UE in 30 important daily activities using a 6-point ordinal scale. Higher scores indicate better performance. This scale shows good reliability and validity in stroke patients.36-38
Data Reduction for Kinematic Variables
An analysis program coded by LabVIEW (National Instru-ments, Inc, Austin, TX) language was used to process kinematic data. Kinematic variables for reaching included normalized movement time (nMT), normalized total dis-tance (nTD), and the percentage of movement time where peak velocity occurs (PPV):
1. Normalized movement time (nMT): Movement time (MT) refers to the time for execution of the reaching movement. It is the interval between movement onset and movement offset, representing temporal efficiency.25,39,40 Because
the task distance varied across subjects, MT was normalized to correct for variations in reaching distance.
2. Normalized total distance (nTD): Spatial effi-ciency was characterized by nTD, which refers to the path of hand in 3-dimensional space and normalized by the direct distance between hand at start position and target in each participant. The smaller the nTD was the more direct the movement path would be.40
3. The percentage of movement time at which
peak velocity occurs (PPV): This variable was
used to characterize the control strategy of reaching. It reflects the percentage of move-ment time used for the acceleration phase. The acceleration phase represents the major pre-planned aspect of the movement.41 A higher
PPV value indicates a longer acceleration phase, suggesting less online error correction and more preplanned control of the reaching movement.42-44
Data Analysis
Analysis of covariance (ANCOVA), controlling for pretreat-ment differences, was used to compare the 2 groups’ improvement for each outcome variable (kinematic values and FMA, FIM, and MAL scores). Pretest performance was the covariate, group was the independent variable, and posttest
46 Neurorehabilitation and Neural Repair 24(1)
performance was the dependent variable. Statistical signifi-cance was determined based on 1-tailed tests with an a of .05. To index the magnitude of group differences in performance, h2 = SS
b/SStotal was calculated for each outcome variable. The
value of h2 represents the variability in the dependent variable
(posttest performance) that can be explained or accounted for by the independent variable (group).20 A large effect is
repre-sented by an h2 of at least .138, a moderate effect by an h2 of
.059, and a small effect by an h2 of .01.45
Results
Characteristics of Participants
After being randomly assigned, 16 participants received BAT intervention, and 17 were in the control group. Table 1 summarizes the demographic and clinical characteristics of the participants in the 2 groups. Baseline characteristics were comparable across these 2 groups. There were no sig-nificant differences between the groups for age, months since stroke, side of stroke lesion (left/right), or Brunnstrom stage of proximal/distal part.
Kinematic Analysis
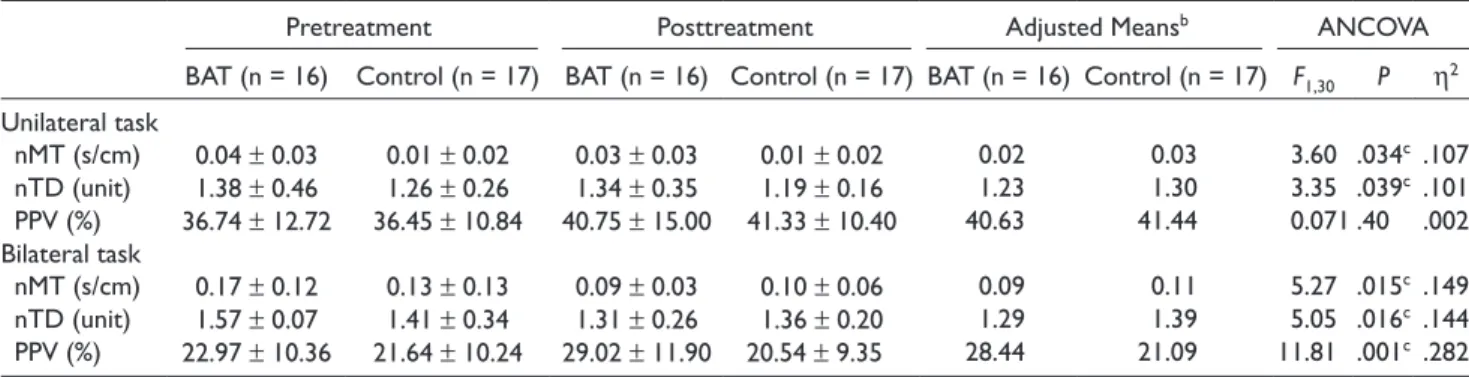
Table 2 summarizes the descriptive statistics and the results of the ANCOVA that tested the effects of BAT relative to CI on kinematic variables during the unilateral and bilateral tasks.
For kinematic variables in the unilateral functional task, the ANCOVA results showed that nMT and nTD were sig-nificantly less in the BAT group than in the control group (F1,30 = 3.601, P = .034, h2 = .107; F
1,30 = 3.352, P = .039,
h2 = .101, respectively). A nonsignificant and small effect
was found for the kinematic variable of PPV (F1,30 = .071,
P = .396, h2 = .002). The results of the kinematic analysis
indicated that the BAT group showed higher efficiency in the temporal and spatial aspects after treatment than the control group during the unilateral task, but there was no difference in relative time for online error correction.
The effects of BAT on reaching kinematics of the affected UE were significant and moderate for the bilateral functional task. The results of ANCOVA showed significantly lower nMT (F1,30 = 5.268, P = .015, h2 = .149), lower nTD (F
1,30 =
5.046, P = .016, h2 = .144), and higher PPV (F
1,30 = 11.811,
P = .001, h2 = .282) after BAT relative to CI. The BAT group
showed higher efficiency in the temporal and spatial aspects and less online error correction control after treatment than the control group during the bilateral task.
Motor Performance
Table 3 summarizes the descriptive statistics and the results of the ANCOVA that tested the effects of BAT relative to CI on
the FMA (ie, degree of motor impairment). The BAT group demonstrated a significantly greater improvement on the FMA than the control group (F1,30 = 3.257, P = .041, h2 = .098).
Functional Ability
Table 3 also presents the descriptive statistics and the results of the ANCOVA in functional ability as measured by the FIM and the MAL. No significant differences were noted in FIM total scores (F1,30 = 0.839, P = .18, h2 = .027) or in any
domains of the FIM between the 2 groups. No significant
differences were found in the AOU or QOM (AOU: F1,30 =
1.436, P = .12, h2 = .046; QOM: F
1,30 = 1.495, P = .17, h2 =
.047) between the 2 groups.
Discussion
Only a few randomized controlled trials3,19 have studied the
effects of BAT without robot assistance on motor control, motor impairment, and functional abilities. However, these previous studies involved very low intensity of training (eg, 30 minutes per session). The present study used a more intensive BAT program (ie, 2 hours per day) for comparison with a dose-matched control intervention. The results are consistent in part with the a priori hypotheses that patients with BAT would have better motor control, show less motor impairment, and obtain a greater gain in functional abilities than the control group. The BAT group showed a greater efficiency of reaching in the temporal and spatial aspects (less nMT and less nTD) during both the unilateral and bilateral tasks. The BAT group also showed better (more preplanned) control strategy (ie, higher PPV) than the con-trol group in the bilateral task. In addition, the BAT group achieved greater gains in FMA scores than did the control group but not in the FIM and MAL.
Kinematic Analysis
Consistent with previous studies,4,7,17 the BAT group
par-ticipants performed the reaching task more efficiently (less nMT) and more directly (less nTD) with the affected arm in both unilateral and bilateral tasks. This study extended pre-vious research9,10 by using test tasks different from the
training tasks. The possibility that the therapeutic effects could be specific to the task being trained is ruled out in the present study. The beneficial effects might be attributed to bilateral simultaneous movements promoting interhemi-spheric disinhibition and allowing for cortical reorganization by sharing normal movement commands from the undam-aged hemisphere.9,46 When the intact arm performed the
same spatiotemporal pattern as the affected arm, the “tem-plate” from the undamaged hemisphere would be generated, which could be applied to both limbs in bilateral actions9,10,47
Table 1. Demographic and Clinical Characteristics of the Participantsa
BAT (n = 16) Control (n = 17) Statistic Pb
Gender (male/female) 10/6 9/8 .041 .839
Age (years) 52.08 ± 9.60 55.50 ± 13.17 -.842 .406
Months since stroke 13.94 ± 12.73 13.12 ± 8.13 .222 .826
Side of stroke lesion (right/left) 7/9 9/8 .279 .732
Brunnstrom stage of proximal part of UE (median [range]) 5 (3-5) 5 (4-5) 111.50 .297 Brunnstrom stage of distal part of UE (median [range]) 4 (3-6) 5 (4-6) 90.00 .080
Abbreviations: BAT, bilateral arm training; UE, upper extremity.
aValues are mean ± standard deviation or as otherwise indicated.
bP associated with the c2 test for categorical variables, with the independent t test for continuous variables, and with the Mann–Whitney U test for
ordinal variables.
Table 2. Descriptive and Inferential Statistics for Analysis of Reaching Kinematicsa
Pretreatment Posttreatment Adjusted Meansb ANCOVA
BAT (n = 16) Control (n = 17) BAT (n = 16) Control (n = 17) BAT (n = 16) Control (n = 17) F1,30 P h2 Unilateral task nMT (s/cm) 0.04 ± 0.03 0.01 ± 0.02 0.03 ± 0.03 0.01 ± 0.02 0.02 0.03 3.60 .034c .107 nTD (unit) 1.38 ± 0.46 1.26 ± 0.26 1.34 ± 0.35 1.19 ± 0.16 1.23 1.30 3.35 .039c .101 PPV (%) 36.74 ± 12.72 36.45 ± 10.84 40.75 ± 15.00 41.33 ± 10.40 40.63 41.44 0.071 .40 .002 Bilateral task nMT (s/cm) 0.17 ± 0.12 0.13 ± 0.13 0.09 ± 0.03 0.10 ± 0.06 0.09 0.11 5.27 .015c .149 nTD (unit) 1.57 ± 0.07 1.41 ± 0.34 1.31 ± 0.26 1.36 ± 0.20 1.29 1.39 5.05 .016c .144 PPV (%) 22.97 ± 10.36 21.64 ± 10.24 29.02 ± 11.90 20.54 ± 9.35 28.44 21.09 11.81 .001c .282
Abbreviations: ANCOVA, analysis of covariance; BAT, bilateral arm training; nMT, normalized movement time; nTD, normalized total displacement; PPV, percentage of movement time to peak velocity.
aValues are mean ± standard deviation or as otherwise indicated.
bAdjusted means (ie, adjusted posttreatment means) are usually part of ANCOVA output and obtained from altering the original means to control for
the covariates (ie, pretreatment values).23,24 cP < .05.
arm. The postulated mechanism for the effects of BAT requires further study using functional brain imaging tech-niques. A second possible explanation for the beneficial effects lies in the limb-coupling mechanism for symmetric tasks. During the bilateral symmetric tasks of daily living, the temporal and spatial parameters of the 2 arms are sub-stantially similar, and the coupling mechanism is maximally operative.18 Accordingly, the performance of the affected
arm improved in the spatial and temporal aspects (move-ment straightness and efficiency) for both bilateral and unilateral tasks.
However, differences in the control strategy between the BAT and control group were found in the bilateral task but not in the unilateral task, consistent with a previous study.3
The BAT group used less online correction control strate-gies to implement the bilateral task than the control group. Both groups used less online control in the unilateral task posttreatment with no significant between-group differ-ences.44 The possible explanation for the differential
performance of the unilateral versus bilateral task pertains to the concept of interhemispheric disinhibition triggered
by bilateral movements. When the intact arm was resting during the unilateral task, the undamaged hemisphere was not activated to generate the “template” of firing organiza-tion to guide the affected arm during skilled acorganiza-tions.9,10,47
Another possible account is that the unilateral and bilateral experimental tasks involved different task objects (ie, target size and shape) that may affect the control strategy.48 The
bilateral task involved movement sequencing and might be easier to induce different strategies between the 2 groups than the unilateral task.
Motor Performance
The results showed that BAT demonstrated greater reduc-tion of motor impairment as measured by the FMA than the control treatment. Simultaneous activation of both hands may have rebalanced interhemispheric activation and inhi-bition, causing an additional facilitation in the affected hemisphere and positive after effects for reducing the motor impairment of the affected UE.18,46,47 In addition, a variety
48 Neurorehabilitation and Neural Repair 24(1)
BAT program in the study that allowed repetitive practice on skilled movements. Our findings are consistent with pre-vious studies5,6,12,15,17 that found improvements after BAT.
In contrast, some studies8,11,16 did not corroborate the
ben-efits of BAT, possibly because these studies were based on a small trial with heterogeneous characteristics of study subjects (eg, time after onset of stroke).
Functional Ability
Consistent with some previous research,5,8,13,16 this study
found no significant differences between the treatment groups in daily function as measured by the FIM and the MAL. This result might be due to the measurement tool that we used for assessing ADL. Although the FIM is a com-monly used evaluation tool in the efficacy research of stroke rehabilitation, it is a measure of level of assistance and is not specific to use and function of the paretic limb. The FIM might not be sensitive to detect changes for high functioning patients of stroke as studied in this present research. Further-more, patients in the BAT group did not perceive increased paretic UE use or better quality of UE movements as mea-sured by the MAL than those in the CI group. It is possible that we did not specifically recruit patients with the learned nonuse phenomenon who demonstrated to habitually use the unaffected UE although the affected UE has the potential to perform the task. Furthermore, the BAT program did not emphasize mass practice of the affected UE as constraint-induced therapy does, but involved bilateral symmetrical activities. In daily life, many bimanual tasks are bilateral
complementary tasks that require a differentiated role for each hand, for example, opening a jam jar.18 Practice on
sym-metric bilateral tasks might not be sufficient for improving functional performance. The findings suggest that other activities of daily living (ADL) instruments that measure specific daily functional abilities that depend on the improved UE function (eg, the Klein-Bell ADL scale49) should be
employed in future research to study the effects of BAT on daily functions. In addition, stroke patients may need to relearn various types of bimanual skills, including bilateral symmetric and complementary tasks, to achieve improve-ment in the wide variety of daily activities. Whitall et al12
reported greater gains in functional ability after bilateral training. Of note, their study involved no control group for comparison and thus failed to provide compelling evidence to support the benefits of BAT for improving daily function.
The overall findings of this study suggest that BAT might be a better approach than conventional rehabilitation to improve motor impairment and enhance motor control, especially during bilateral arm movements. However, the benefits of BAT for improving functional ability need fur-ther examination using appropriate measurements that may specifically detect changes in functional performance of the UE as a result of the treatment.
A few limitations of the present study warrant consider-ations when generalizing the results beyond the study. First, there is a lack of follow-up data to address whether the ben-efits of BAT persist. The advantages of BAT for motor control and motor performance over CI might be reversed over time, and follow-up study is required to examine this
Table 3. Descriptive and Inferential Statistics for Analysis of Motor Impairments (FMA) and Functional Ability (FIM and MAL)a
Pretreatment Posttreatment Adjusted Meansb ANCOVA
BAT (n = 16) Control (n = 17) BAT (n = 16) Control (n = 17) BAT (n = 16) Control (n =17) F1,30 P h2 FMA 48.00 ± 12.35 53.53 ± 7.58 57.63 ± 1.03 54.99 ± 1.00 57.63 54.99 3.26 .041c .098 FIM Self-care 37.38 ± 5.49 36.94 ± 7.30 38.38 ± 5.48 37.41 ± 6.69 38.36 37.39 0.97 .17 .031 Sphincter 13.37 ± 2.03 14.00 ± 0.00 13.50 ± 2.00 14.00 ± 0.00 13.49 13.99 0.61 .22 .020 Transfer 20.06 ± 2.38 20.47 ± 1.07 19.94 ± 2.35 20.35 ± 1.06 19.93 20.34 0.03 .43 .001 Locomotion 13.13 ± 1.78 13.24 ± 0.90 13.19 ± 1.83 13.00 ± 1.32 13.19 13.01 1.21 .14 .039 Communication 13.63 ± 0.72 13.00 ± 1.84 13.69 ± 0.60 13.06 ± 1.78 13.70 13.07 0.17 .34 .006 Social cognition 19.81 ± 1.52 19.00 ± 3.50 20.19 ± 1.38 19.35 ± 2.40 20.18 19.35 0.79 .19 .026 Total 117.38 ± 12.09 116.65 ± 10.82 118.88 ± 11.75 117.18 ± 9.63 118.84 117.14 0.84 .18 .027 MAL AOU 1.06 ± 0.83 1.27 ± 0.91 1.25 ± 0.92 1.70 ± 1.04 1.34 1.61 1.44 .12 .046 QOM 1.18 ± 0.80 1.54 ± 1.18 1.42 ±0.97 1.99 ± 1.10 1.56 1.86 1.50 .17 .047
Abbreviations: FMA, Fugl-Meyer Assessment; FIM, Functional Independence Measure; BAT, bilateral arm training; ANCOVA, analysis of covariance; MAL, Motor Activity Log; AOU, Amount of Use; QOM, Quality of Movement.
aValues are mean ± standard deviation.
bAdjusted means (ie, adjusted posttreatment means) are usually part of ANCOVA output and obtained from altering the original means to control for
the covariates (ie, pretreatment scores).23,24 cP < .05.
possibility. Second, this study only used a bilateral comple-mentary task to evaluate the changes in motor control after intervention. Whether the effects of BAT on such a bilateral task can be generalized to bilateral symmetrical tasks remains to be scrutinized. Third, PPV used in this study may not fully represent the control strategy of movement. Future research may measure the number of acceleration and deceleration phases to study the control strategy.39
Finally, although we used a sample larger than previous trials,7,8,11,13,16,19 our study subjects were higher functioning
patients and represented only a small percentage of all eli-gible patients. Further research should recruit a larger sample and study patients differing in severity of motor impairment. Future research also needs to identify factors that may affect study findings, including premorbid hand dominance and cognitive function relevant for stroke reha-bilitation such as divided attention50 and sequencing.
Future work should also address optimal dose and training characteristics for BAT and use outcome measures relevant for daily life situations (eg, the accelerometry system51,52 that
monitors activity of the bilateral UEs53 in the community). In
addition, brain imaging research is needed to verify the sup-positions regarding brain plastic change after BAT.
Conclusion
Extending previous research on BAT after stroke, this study incorporated a variety of bilateral symmetrical and functional tasks into a BAT program in chronic stroke patients who are not expected to show spontaneous recovery. The study showed the effects of the BAT program for improving some aspects of motor control strategies of the affected arm in both bilateral and unilateral tasks and reducing motor impairments. The findings suggest that the BAT should be a favorable approach if improving motor control and motor performance of the affected UE in stroke patients is the primary treatment goal. Future research might consider coupling bilateral with unilateral approaches to further improve the control strategy of the affected arm in unilateral functional tasks.
Authors’ Note
No commercial party having a direct financial interest in the results of research supporting this article has or will confer a ben-efit on the authors or on any organization with which the authors are associated.
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
This project was supported in part by the National Health Research Institutes (NHRI-EX98-9742PI) and the National Science
Council (NSC 96-2320-B-182-029, NSC 96-2628-B-002-033-MY2, and NSC 97-2314-B-002-008-MY3 and NSC 98-2811-B-002-073) in Taiwan.
References
1. Schaechter JD. Motor rehabilitation and brain plasticity after hemiparetic stroke. Prog Neurobiol. 2004;73:61-72.
2. Pomeroy V, Tallis R. Neurological rehabilitation: a science struggling to come of age. Physiother Res Int. 2002;7:76-89. 3. Mudie MH, Matyas TA. Responses of the densely
hemiple-gic upper extremity to bilateral training. Neurorehabil Neural
Repair. 2001;15:129-140.
4. Platz T, Winter T, Muller N, Pinkowski C, Eickhof C, Maurita KH. Arm ability training for stroke and traumatic brain injury patients with mild arm paresis: a single-blind, randomized, controlled trial. Arch Phys Med Rehabil. 2001;82:961-968. 5. Luft A, McCombe Waller S, et al. Repetitive bilateral arm
training and motor cortex activation in chronic stroke. A ran-domized controlled trial. JAMA. 2004;292:1853-1861. 6. Hesse S, Werner C, Pohl M, Rueckriem S, Mehrholz J,
Ling-nau ML. Computerized arm training improves the motor con-trol of the severely affected arm after stroke. A single-blinded randomized trial in two centers. Stroke. 2005;36:1960-1966. 7. Summers JJ, Kagerer FA, Garry MI, Hiraga CY, Loftus
A, Cauraugh JH. Bilateral and unilateral movement training on upper limb function in chronic stroke patients: a TMS study.
J Neurol Sci. 2007;252:76-82.
8. Morris JH, van Wijck F, Joice S, Ogston SA, Cole I, MacWalter RS. A comparison of bilateral and unilateral upper-limb task training in early poststroke rehabilitation: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89:1237-1245.
9. Mudie MH, Matyas TA. Upper extremity retraining follow-ing stroke: effects of bilateral practice. J Neurol Rehabil. 1996;10:167-184.
10. Mudie MH, Matyas TA. Can simultaneous bilateral move-ment involve the undamaged hemisphere in reconstruction of neural networks damaged by stroke? Disabil Rehabil. 2000;22:23-37.
11. Lewis GN, Byblow WD. Neurophysiological and behavioral adaptations to a bilateral training intervention in individuals following stroke. Clin Rehabil. 2004;18:48-59.
12. Whitall J, McCombe Waller S, Silver KHC, Macko RF. Repetitive bilateral arm training with rhythmic auditory cue-ing improves motor function in chronic hemiparetic stroke.
Stroke. 2000;31:2390-2395.
13. Richards LG, Senesac CR, Davis SB, Woodbury ML, Nadeau SE. Bilateral arm training with rhythmic auditory cueing in chronic stroke: not always efficacious. Neurorehabil Neural
Repair. 2008;22:180-184.
14. McCombe Waller S, Liu W, Whitall J. Temporal and spatial control following bilateral versus unilateral training. Hum
50 Neurorehabilitation and Neural Repair 24(1)
15. Stinear JW, Byblow WD. Rhythmic bilateral movement train-ing modulates corticomotor excitability and enhances upper limb motricity poststroke: a pilot study. J Clin Neurophysiol. 2004;21:124-131.
16. Lum PS, Burgar CG, van der Loos M, Shor PC, Majmundar M, Yap R. MIME robotic device for upper-limb neurorehabili-tation in subacute stroke subjects: a follow-up study. J Rehabil
Res Dev. 2006;43:631-642.
17. Chang JJ, Tung WL, Wu WL, Huang MH, Su FC. Effects of robot-aided bilateral force-induced isokinematic arm train-ing combined with conventional rehabilitation on arm motor function in patients with chronic stroke. Arch Phys Med
Reha-bil. 2007;88:1332-1338.
18. McCombe Waller S, Whitall J. Bilateral arm training: why and who benefits? NeuroRehabilitation. 2008;23:29-41. 19. Platz T, Bock S, Prass K. Reduced skillfulness of arm motor
behaviour among motor stroke patients with good clini-cal recovery: does it indicate reduced automaticity? Can it be improved by unilateral or bilateral training? A kinematic motion analysis study. Neuropsychologia. 2001;39:687-698. 20. Carr JH, Shepherd RB. Reaching and manipulation. In: Carr
JH, Shepherd RB, eds. Stroke Rehabilitation: Guidelines for
Exercise and Training to Optimize Motor Skill. Edinburgh,
UK: Butterworth Heinemann; 2003:159-191.
21. Brunnstrom S. Movement Therapy in Hemiplegia. New York, NY: Harper & Row; 1970.
22. Bohannon RW, Smith MB. Interrater reliability of a Modified Ashworth Scale of muscle spasticity. Phys Ther. 1987;67: 206-207.
23. Rosenthal R, Rosnow RL. Essentials of Behavioral Research:
Methods and Data Analysis. 2nd ed. New York, NY:
McGraw-Hill; 1991.
24. Portney LG, Watkins MP. Foundations of Clinical Research:
Application to Practice. 3rd ed. Upper Saddle River, NJ:
Prentice Hall Health; 2009.
25. Wu CY, Chen CL, Tang SF, Lin KC, Huang YY. Kinematic and clinical analyses of upper-extremity movements after constraint-induced movement therapy in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2007;88:964-670.
26. Michaelsen SM, Levin MF. Short-term effects of practice with trunk restraint on reaching movements in patients with chronic stroke. Stroke. 2004;35:1914-1919.
27. Michaelsen SM, Dannenbaum R, Levin MF. Task-specific training with trunk restraint on arm recovery in stroke: ran-domized control trial. Stroke. 2006;37:186-192.
28. Tunik E, Poizner H, Levin MF, et al. Arm-trunk coordination in the absence of proprioception. Exp Brain Res. 2003;153: 343-355.
29. Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-strike hemiplegia patient. I. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13-31.
30. Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cere-brovascular accident. Phys Ther. 1983;63:1601-1610. 31. DiFabio RP, Badke RB. Relationship of sensory organization
to balance function in patients with hemiplegia. Phys Ther. 1990;70:542-548.
32. Hamilton BB, Granger CV, Sherwin FS, Zielezny M, Tash-man JS. A uniform national data system for medical rehabili-tation. In: Fuhrer MJ, ed. Rehabilitation Outcomes: Analysis
and Measurements. Baltimore, MD: Brookes; 1987:137-147.
33. Chau N, Daler S, Andre JM, Patris A. Inter-rater agreement of two functional independence scales: the Functional Indepen-dence Measure (FIM) and a subjective uniform continuous scale. Disabil Rehabil. 1994;16:63-71.
34. Hamilton BB, Laughlin JA, Fiedler RC, Granger CV. Interra-ter reliability of the 7-level Functional Independence Measure (FIM). Scand J Rehabil Med. 1994;26:115-119.
35. Gosman-Hedstrom G, Svensson E. Parallel reliability of the Functional Independence Measure and the Barthel ADL index. Disabil Rehabil. 2000;22:702-715.
36. Sterr A, Elbert T, Berthold I, Kolbel S, Rockstroh B, Taub E. Longer versus shorter daily constraint-induced movement therapy of chronic hemiparesis: an exploratory study. Arch
Phys Med Rehabil. 2002;83:1374-1377.
37. Uswatte G, Taub E, Morris D, Light K, Thompson PA. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67:1189-1194.
38. van der Lee JH, Beckerman H, Knol DL, de Vet HCW, Bouter LM. Clinimetric properties of the Motor Activity Log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35:1410-1414.
39. Lin KC, Wu CY, Wei TH, Lee CY, Liu JS. Effects of modified constraint-induced movement therapy on reach-to-grasp move-ments and functional performance after chronic stroke: a ran-domized controlled study. Clin Rehabil. 2007;21:1075-1086. 40. Wu CY, Lin KC, Chen HC, Chen IH, Hong WH. Effects of
modified constraint-induced movement therapy on movement kinematics and daily function in patients with stroke: a kine-matic study of motor control mechanisms. Neurorehabil
Neu-ral Repair. 2007;21:460-466.
41. Georgopoulos AP. On reaching. Annu Rev Neurosci. 1986;9: 147-170.
42. Kamper DG, McKenna AN, Kahn LE, Reinkensmeyer DJ. Alterations in reaching after stroke and their relationship to movement direction and impairment severity. Arch Phys Med
Rehabil. 2002;83:702-707.
43. Haaland KY, Prestopnik J, Lee RR, Adair J. Hemispheric asymmetries for kinematic and positional aspects of reaching.
Brain. 2004;127:1145-1158.
44. Elliott D, Helsen, WF, Chua R. A century later: Woodworth’s (1899) two-component model of goal-directed aiming.
45. Cohen J. Statistical Power Analysis for the Behavioral
Sci-ences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988.
46. Cauraugh JH, Summers JJ. Neural plasticity and bilateral movements: a rehabilitation approach for chronic stroke. Prog
Neurobiol. 2005;75:309-320.
47. Stewart KC, Cauraugh JH, Summers JJ. Bilateral movement training and stroke rehabilitation: a systematic review and meta-analysis. J Neurol Sci. 2006;244:89-95.
48. Adam JJ. The effects of objectives and constraints on motor control strategy in reciprocal aiming movements. J Mot
Behav. 1992;24:173-185.
49. Smith RO, Morrow ME, Heitman JK, Rardin WJ, Powelson JL, Von T. The effects of introducing the Klein-Bell ADL Scale in a rehabilitation service. Am J Occup Ther. 1986;40: 420-424.
50. McDowd JM, Filion DL, Pohl PS, Richards LG, Stiers W. Attentional abilities and functional outcomes following stroke. J Gerontol B Psychol Sci Soc Sci. 2003;58:P45-P53. 51. Uswatte G, Giuliani G, Winstein C, Zeringue A, Hobbs L,
Wolf SL. Validity of accelerometry for monitoring real-world arm activity in patients with subacute stroke: evidence from the extremity constraint-induced therapy evaluation trial.
Arch Phys Med Rehabil. 2006;87:1340-1345.
52. Choquette S, Hamel M, Boissy P. Accelerometer-based wire-less body area network to estimate intensity of therapy in post-acute rehabilitation. J Neuroeng Rehabil. 2008;5:20. 53. Mehrholz J, Wagner K, Rutte K, Meissner D, Pohl M.
Predic-tive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch Phys Med