404
* To whom correspondence and reprint requests should be addressed. Tel: 886-2-27899549. Fax: 886-2-27858059.
E-mail: cac@gate.sinica.edu.tw
R
ibosomal DNA (rDNA) has long been used as a potential marker for phylogenetic studies (reviewed in Avise 2004). rRNA genes are orga-nized in clusters of tandemly repeated units, each of which consists of coding regions (18S, 5.8S,and 28S) and 2 internal transcribed spacers (ITS) and 1non-transcribed spacer (NTS) region. While the coding regions are evolutionarily conserved and have been utilized for phylogenetic inferences for major phyla (reviewed in Hills and Dixon 1990),
Analyses of the Ribosomal Internal Transcribed Spacers (ITS) and the
5.8S Gene Indicate that Extremely High rDNA Heterogeneity is a Unique
Feature in the Scleractinian Coral Genus Acropora (Scleractinia;
Acroporidae)
Nu-Wei Vivian Wei1,2, Carden C. Wallace3, Chang-Feng Dai2, Kamla Ruby Moothien Pillay4, and
Chaolun Allen Chen1,2*
1Research Center for Biodiversity, Academia Sinica, Nankang, Taipei, Taiwan 115, R.O.C. 2Institute of Oceanography, National Taiwan University, Taipei, Taiwan 106, R.O.C. 3Museum of Tropical Queensland, Townsville, Queensland Q4810, Australia
4Mauritius Oceanography Institute, France Centre, Victoria Avenue, Quatre-Bornes, Mauritius (Accepted September 28, 2005)
Nu-Wei Vivian Wei, Carden C. Wallace, Chang-Feng Dai, Kamla Ruby Moothien Pillay, and Chaolun Allen Chen (2006) Analyses of the ribosomal internal transcribed spacers (ITS) and the 5.8S gene indicate that
extremely high rDNA heterogeneity is a unique feature in the scleractinian coral genus Acropora (Scleractinia; Acroporidae). Zoological Studies 45(3): 404-418. One characteristic of ribosomal DNA (rDNA) sequences in staghorn corals, Acropora spp., is the extremely high levels of intragenomic heterogeneity and interspecific variation. This high genomic diversity is ascribed to incomplete lineage sorting that predated the divergence of species or to recent introgressive hybridization. In order to elucidate whether the high heterogeneity of rDNA is a unique feature of Acropora or a general pattern applicable to scleractinian corals, we examined the molecular evolution of the internal transcribed spacers (ITS) and 5.8S rDNA sequences from 78 species, representing 28 genera, and 12 families of scleractinian corals. Genetic distances (measured by p-distances) and frequency distribution analyses revealed that both extremely high intra- and interspecific heterogeneities of the ITS-5.8S rDNA are specific to the genus Acropora. The 5.8S rDNA phylogeny clearly showed a significantly long branch length leading to the cluster containing the genus Acropora. The molecular-clock hypothesis tested using the likelihood ratio test indicated a highly significant difference in the global evolutionary rate of scleractinian 5.8S rDNA. The relative rate tests showed that the rDNA of Isopora, Caribbean Acropora, and Indo-Pacific Acropora all evolved at constant tempos, indicating that the highly divergent rDNA was present in Acropora before it split into these three lineages. In contrast, rate constancy was rejected for most comparisons between
Acropora/Isopora and other coral genera, suggesting that the rates of evolution of 5.8S differed between Acropora/Isopora and the other lineages, and that the evolutionary rate of Acropora/Isopora has accelerated
since divergence from the common ancestor of scleractinian corals. http://zoolstud.sinica.edu.tw/Journals/45.3/404.pdf
the 2 ITS regions are appropriate for detecting dif-ferences between conspecific individuals and are hence potentially useful markers to study the rela-tionships of populations and closely related species in fungal, plant, and animal taxa due to their relatively rapid evolutionary rates (Baldwin 1992, Schlötterer et al. 1994, Mai and Coleman 1997, Weekers et al. 2001, Oliverio et al. 2002, Chen et al. 2002 2004).
One of the major concerns with the use of the rDNA locus in phylogenetic analyses is the exis-tence of polymorphisms among repeated units, which may cause extensive differentiation even within a single individual. However, concerted evolution, a process resulting in the homogeniza-tion of individual repeats, is assumed to produce a mostly uniform sequence in all repeats of a given species (Li 1997). Two mechanisms, unequal crossover and gene conversion, have been pro-posed for the process of concerted evolution. Unequal crossing-over is caused by a recombina-tion among tandem repeats either within (homolo-gous) or between (heterolo(homolo-gous) chromosomes, resulting in the stochastic elimination of variations in individuals and populations. In contrast, nonsto-chastic processes such as directed gene conver-sion assume that selection drives the homogeniza-tion of tandem repeats (Dover 1982, Hillis et al. 1991).
Phylogenetic studies based on fragments of rDNA ITS-5.8S have provided novel insights into scleractinian coral evolution (Hunter et al. 1997, Lopez and Knowlton 1997, Odorico and Miller 1997, Medina et al. 1999, van Oppen et al. 2000 2002 2004, Diekmann et al. 2001, Forsman 2003, Forsman et al. 2005a b, Lam and Morton 2003, Marquez et al. 2003, Chen et al. 2004, Fukami et al. 2004, Vollmer and Palumbi 2004, Moothien Pillay et al. 2005). Some of those studies indicat-ed that individual coral colonies host a high degree of intragenomic variation, and coral ITS phyloge-nies in several cases are polyphyletic among closely related congeners (Odorico and Miller 1997, Hatta et al. 1999, Medina et al. 1999, Fukami et al. 2000, van Oppen et al. 2000 2002 2004, Diekmann et al. 2001, Marquez et al. 2003). Most of these conclusions were derived from stud-ies of the genus Acropora. For example, variation in the ITS-5.8S fragment was estimated to be as high as 40% (p-distance) at the interspecific level for Acropora spp. (Odorico and Miller 1997, van Oppen et al. 2001 2002) and as low as < 8% among species of Madracis in the Caribbean
(Diekmann et al. 2001). Despite the extreme dis-parity in the divergence patterns of the ITS-5.8S regions between these 2 coral groups, both phylo-genetic studies concluded that the evolutionary patterns of potentially hybridizing corals are con-sistent with reticulation. On the contrary, phyloge-netic analyses of the ITS-5.8S fragment demon-strated clear boundaries for species in the genera
Pavona, Platygyra, Porites, and Siderastrea
(Forsman 2003, Lam and Morton 2003, Forsman et al. 2005, Moothien Pillay et al. 2005). These contrasting results imply that the rate of concerted evolution (i.e., homogenization) among tandem repeats of ITS-5.8S is variable in different lineages of scleractinian corals.
Recent analyses of ITS-5.8S regions have revealed that the phylogenetic signature of recent introgressive hybridization is obscured in the Caribbean Acropora because they shared ancient rDNA lineages that predated divergence of the species (Vollmer and Palumbi 2004). It was con-cluded that nuclear rDNA should be abandoned as a species- and population-level phylogenetic mark-er for sclmark-eractinian corals due to its complicated and undistinguishable characteristics of molecular evolution (Vollumer and Palumbi 2004). Chen et al. (2004) reevaluated this proposal by examining the phylogenetic utility and secondary structure of the ITS2 from 54 species of scleractinian corals, representing 25 genera and 11 families. The com-parative analysis showed that the extremely high ITS intragenomic divergence of Acropora appears to be an exception rather than the rule for the evo-lutionary history of scleractinian corals, suggesting that ITS2 DNA sequences are still applicable, with adequate adjustment of the secondary structures, to the primary sequence alignment of different lev-els of phylogenetic analyses (from populations to genera) in scleractinian corals.
In this study, we extended the examination of the molecular divergence of complete rDNA ITS-5.8S regions from 78 species, representing 28 genera, and 12 families of scleractinian corals. We then constructed phylogenetic trees based on 5.8S rDNA and applied the likelihood ratio test (LRT) and relative rate test (RRT) to examine the molecular evolutionary rate constancy. The results indicate that acceleration of the rDNA ITS-5.8S region occurred in the Acropora lineage after diver-gence from a common ancestor of scleractinian corals, and that the extremely high ITS rDNA diversity is a unique molecular feature of the genus
Table 1. Taxonomic information, GenBank accession numbers, and data sources
Taxon No. of species No. of seq. Accession numbers onGenBank Codes of data sources
Order Scleractinia Family Acroporidae
Genus Acropora
Subgenus Acropora 19 156 AF228164-AF538598 van Oppen et al. (2002), Marquez et al.
(2003), van Oppen et al. (2000), and this study
Subgenus Isopora 4 14 AF538561-AF538567 Marquez et al. (2003) and this study
Genus Montipora 7 7 AY722772-AY722780 Chen et al. (2004)
Genus Astreopora 1 1 AY722742 Chen et al. (2004)
Genus Anacropora 1 1 AY722747 Chen et al. (2004)
Family Pocilloporidae
Genus Pocillopora 2 2 AY722785, AY139815 Chen et al. (2004) and Genbank data
(unpub-lished)
Genus Stylophora 1 1 AY722795 Chen et al. (2004)
Genus Seriatopora 1 1 AY722794 Chen et al. (2004)
Family Faviidae
Genus Montastraea 4 68 AB065299-AB065364, Chen et al. (2004) and Genbank data
(unpub-lished) AY722774, AY722774
Genus Oulastrea 1 1 AY722781 Chen et al. (2004)
Genus Platygyra 2 12 AF481893-AF481905 Lan and Morton (2003)
Genus Plesiastrea 1 11 AF483804-AF483813 Rodriguez-Lanetty and Hoegh-Guldberg
(2002)
Genus Cyphastrea 1 3 AY722749-AY722751 Chen et al. (2004)
Genus Favites 1 2 AY722755, AY722756 Chen et al. (2004)
Genus Goniastrea 2 6 AY722759-AY722763 Chen et al. (2004)
Genus Cladocora 1 3 AY722752-AY722749 Chen et al. (2004)
Family Merulinidae
Genus Hydnophora 1 3 AY722769-AY722771 Chen et al. (2004)
Family Poritidae
Genus Porites 9 110 AY720289-AY722788 Chen et al. (2004), Forsman et al. (2005)
Genus Alveopora 1 1 AY711746 Chen et al. (2004)
Family Agariciidae
Genus Pavona 2 38 AB217876-AB217913 Pillay et al. (2005)
Family Siderastreidae
Genus Psammocora 1 3 AY722782-AY722784 Chen et al. (2004)
Genus Pseudosiderastrea 1 2 AY722789-AY722790 Chen et al. (2004)
Genus Siderastrea 4 38 AY322575-AY322612 Forsman et al. (2005)
Family Dendrophylliidae
Genus Tubastraea 1 1 AY722796 Chen et al. (2004)
Family Astrocoeniidae
Genus Stylocoeniella 1 3 AY722793-AY722791 Chen et al. (2004)
Genus Madracis 5 88 AF251847-AF251936 Diekmann et al. (2001)
Family Fungiacyathidae
Genus Fungiacyathus 1 2 AY722757-AY722758 Chen et al. (2004)
Family Oculinidae
Genus Galaxea 1 2 AY722765-AY722764 Chen et al. (2004)
Family Mussidae
Genus Acanthastrea 1 1 AY722739-AY722740 Chen et al. (2004)
Order Alcyonacea Family Alcyoniidae
MATERIALS AND METHODS
DNA sequence database
The published complete DNA sequences con-taining the ITS-5.8S rDNA region were retrieved from GenBank based on either original publica-tions or unpublished sources (Table 1). In addi-tion, 47 new DNA sequences, mainly from
Acropora and Isopora, were obtained in this study.
Coral samples were stored in 90%-95% EtOH. DNA extraction, PCR, cloning, and DNA sequenc-ing were described in our previous works (Chen et al. 2000 2002 2003 2004). Target segments con-taining the ITS1-5.8S-ITS2 region were amplified using the“anthozoan-universal”primer pairs, 1S:
5'-GGTACCCTTTGTACACACCGCCCGTCGCT-3' and 2SS: 5'-GCTTTGGGCGGCAGTCCCAAG-CAACCCGACTC-3', as described in Odorico and Miller (1997). PCR was performed in a PC-9606 thermal sequencer (Corbett Research, Sydney, NSW, Australia) using the following thermal cycle: 1 cycle of 95
°
C for 4 min; 4 cycles of 94°
C for 30 s, 50°
C for 1 min, and 72°
C for 2 min; and 30 cycles of 94°
C for 30 s, 55°
C for 1 min, and 72°
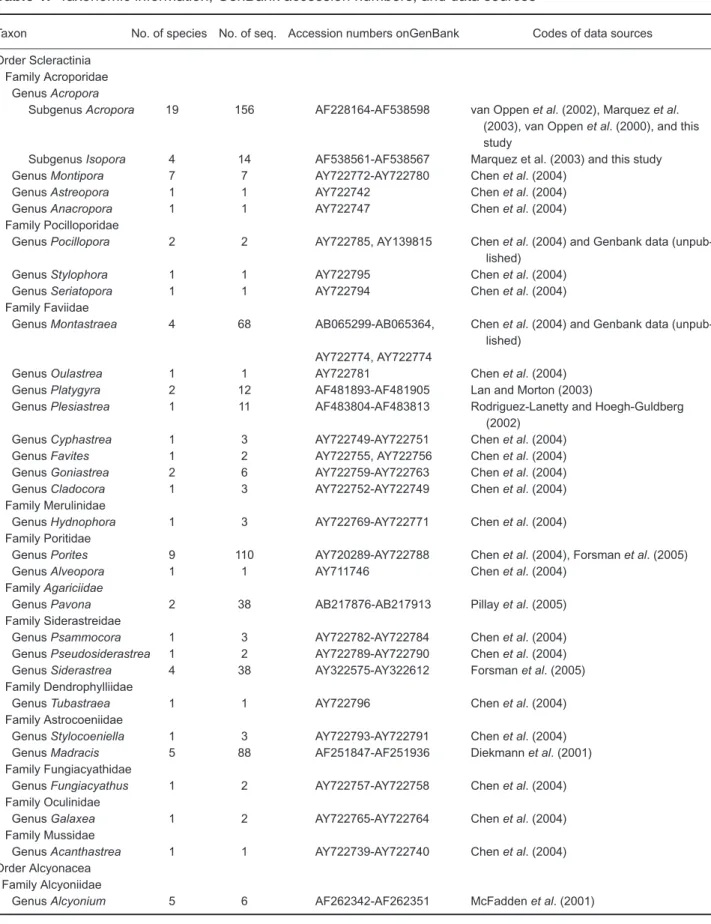
C for 2 min. The amplification reaction used 50-200 ng of template and BRL Taq polymerase in a 50 µl reaction volume, using the buffer supplied with the enzyme, under conditions recommended by the manufacturer. The PCR products were electrophoresed in a 1% agarose (FMC Bioproduct, Rockland, ME, USA) gel in 1X TAEa. Acropora (n = 12124) e. Porites (n = 5995) b. Isopora (n = 91) f. Pavona (n = 703) c. Montastrea (n = 2278) g. Siderastrea (n = 703) d. Platygyra (n = 66) 15 10 5 0 0 5 10 15 P-distance window (x 10-2) P ercentage (%) h. Madracis (n = 4005)
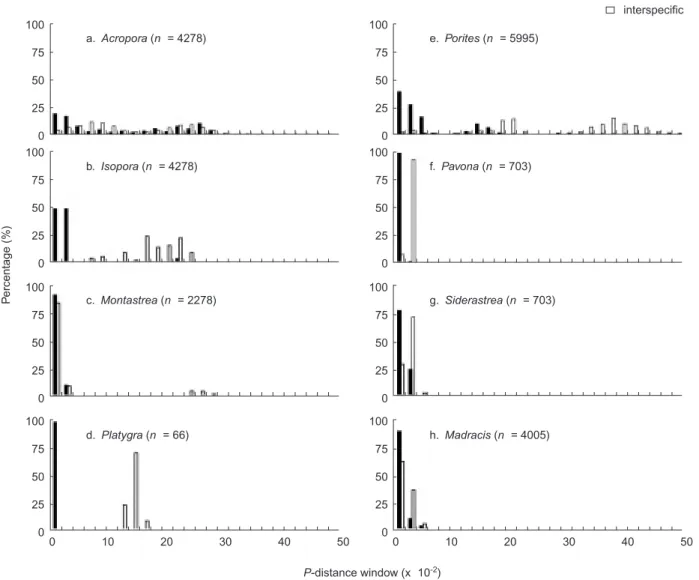
Fig. 1. Frequency distributions of intra- and interspecific genetic distances for 5.8S rDNA. The number of pairwise comparisons (n) is
buffer to assess the yield. Amplified DNA was extracted once with chloroform, precipitated with ethanol at -20
°
C, and resuspended in TE buffer. PCR products were cloned using the pGEM-T sys-tem (Promega, Madison, MI, USA) under condi-tions recommended by the manufacturer. Nucleotide sequences were determined for com-plementary strands of at least 2 clones from each sample using an ABI 377 Genetic Analyzer. The sequences obtained were submitted to GenBank under the accession numbers listed in table 1. Molecular evolutionary analysisDue to the extreme divergence of the 2 ITS regions between Acropora and non-Acropora corals (Chen et al. 2004), it is impossible to pro-duce a consistent alignment of ITS-5.8S among all scleractinian corals for molecular evolutionary analyses. However, reliable alignment of ITS can be obtained at inter- and intraspecific levels using the secondary structure as a guide. DNA sequences were initially aligned using CLUSTAL X (Thompson et al. 1994), and default gap and extension penalties were used followed by manual editing using SeqApp 1.9 (Gilbert 1994). Alignments were then adjusted by eye following guidance of the predicted secondary structure by Odorico and Miller (1997) and Chen et al. (2004). The uncorrected pairwise p-distances (Li 1997) were calculated for the alignments from the default options of CLUSTAL for 8 genera that contained sequence data from more than 2 species (Tables 2, 3). Genetic variations at the intraspecific and interspecific levels were visualized by frequency distributions of pairwise p-distances. Genetic dis-tances were divided into different categories, e.g., 1-2 x 10-2(Fig. 1). Genetic distances derived from
pairwise comparisons that fit into each category were counted. If the intraspecific variation is as high as the interspecific variation, the 2 distribu-tions will highly overlap. On the contrary, the fre-quency plots are separated into 2 clear distribu-tions when the former is smaller than the latter. Frequency distributions of pairwise p-distances were respectively conducted for ITS1, 5.8S, and ITS2 of 8 genera for which both intraspecific and interspecific variations were available. The signifi-cant difference of these 2 distributions was exam-ined by Chi-squared test using Statview 5.1. Phylogenetic trees based on 5.8S rDNA sequences were constructed using PAUP 4.0b10 (Swofford 2002). The LRT implemented in the program MODELTEST vers. 3.6 (Posada and
Crandall 1998) indicated that the Kimura 2-para-meter (K2P) model (Kimura 1980) was the best-fit model of sequence evolution for 5.8S rDNA under the criterion of the hierarchical LRT. Neighbor-join-ing (NJ) analysis was performed usNeighbor-join-ing the K2P model estimated by Modeltest. Branch lengths leading to the major clades in the NJ tree were cal-culated. The robustness of the NJ phylogeny was assessed by 1000 bootstrap replicates. A molecu-lar clock LRT, 2∆ = log Lno clock- Log Lclock, which is distributed as X2 with (n - 2) degrees of freedom
where n is the number of sequences (Felsenstein 1981, Muse and Weir 1992), was performed using TREE-PUZZLE 5.1 (Schmidt et al. 2002) to deter-mine whether there was a statistical difference in the global evolutionary rate for 5.8S rDNA. To examine the evolutionary rate constancy among the major clades in the 5.8S phylogeny, a modified RRT (Wu and Li 1985, Li and Bousquet 1992) introducing a phylogenetic weighting scheme (Robinson et al. 1998) was carried out using RRTree 1.1 (Robinson et al. 1998).
RESULTS
The complete sequence data set consisted of 587 sequences of ITS-5.8S from 78 species, rep-resenting 28 genera, and 12 families of scleractin-ian corals (Table 1). In addition, 6 sequences of 5
Alcyonium species were used as out groups in the
5.8S gene phylogenetic construction (see below). Eight genera/subgenera, including Acropora,
Isopora, Montastrea, Platygyra, Porites, Pavona, Siderastrea, and Madracis, for which rDNA
sequences were available for intraspecific and interspecific comparisons, were used for genetic distance estimation and the frequency distribution analysis.
Genetic distances
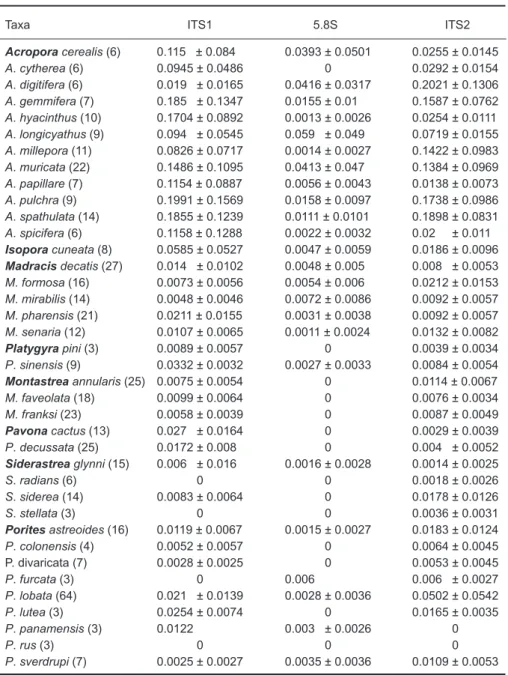
Intraspecific genetic distances (p-distances) for 5.8S, ITS1, and ITS2 were respectively calcu-lated from 38 species (Table 2). The 5.8S was rel-atively conserved with 14 species showing identi-cal (p-distance = 0) DNA sequences at the intraspecific level. The intraspecific genetic dis-tances of Acropora 5.8S were comparatively high-er than those of othhigh-er corals with the highest p-distance value found in A. longicyathus (0.059 ± 0.049). The ITS1 intraspecific variations of
Acropora were larger than those of the other
± 0.0165 in A. digitifera to 0.1991 ± 0.1569 in A.
pulchra. Similarly, ITS2 variations were
consis-tently higher in Acropora than in the other genera examined, with the largest genetic distance of 0.2021 ± 0.1306 in A. digitifera.
The interspecific genetic distances of the 5.8S varied from 0 in Pavona spp. to 0.0491 ± 0.0353 in
Acropora spp. Two Platygyra species were
mod-erately divergent in 5.8S with a genetic distance of
0.0196 ± 0.0026. Interspecific genetic distances of the ITS1 were also unusually higher in Acropora (0.2354 ± 0.1151) and Isopora (0.2109 ± 0.0614) compared to those of the other corals, except for large genetic distances observed in Porites (0.1942 ± 0.0805) and Platygyra (0.1955 ± 0.0183). Unexpectedly, the largest genetic dis-tance for the ITS2 was seen in Porites (0.3031 ± 0.1232), probably reflecting deeper divergence
Table 2. Intraspecific p-distances of the 3 ribosomal regions of ITS1, 5.8S, and ITS2 in scleractinian corals. For data sources, refer to table 1. The number of sequences used for the p-distance calculations is indicated. The number of sequences used for calculating the p-distances is indicated in parentheses Please insert the correct file
Taxa ITS1 5.8S ITS2
Acropora cerealis (6) 0.115 ± 0.084 0.0393 ± 0.0501 0.0255 ± 0.0145 A. cytherea (6) 0.0945 ± 0.0486 0 0.0292 ± 0.0154 A. digitifera (6) 0.019 ± 0.0165 0.0416 ± 0.0317 0.2021 ± 0.1306 A. gemmifera (7) 0.185 ± 0.1347 0.0155 ± 0.01 0.1587 ± 0.0762 A. hyacinthus (10) 0.1704 ± 0.0892 0.0013 ± 0.0026 0.0254 ± 0.0111 A. longicyathus (9) 0.094 ± 0.0545 0.059 ± 0.049 0.0719 ± 0.0155 A. millepora (11) 0.0826 ± 0.0717 0.0014 ± 0.0027 0.1422 ± 0.0983 A. muricata (22) 0.1486 ± 0.1095 0.0413 ± 0.047 0.1384 ± 0.0969 A. papillare (7) 0.1154 ± 0.0887 0.0056 ± 0.0043 0.0138 ± 0.0073 A. pulchra (9) 0.1991 ± 0.1569 0.0158 ± 0.0097 0.1738 ± 0.0986 A. spathulata (14) 0.1855 ± 0.1239 0.0111 ± 0.0101 0.1898 ± 0.0831 A. spicifera (6) 0.1158 ± 0.1288 0.0022 ± 0.0032 0.02 ± 0.011 Isopora cuneata (8) 0.0585 ± 0.0527 0.0047 ± 0.0059 0.0186 ± 0.0096 Madracis decatis (27) 0.014 ± 0.0102 0.0048 ± 0.005 0.008 ± 0.0053 M. formosa (16) 0.0073 ± 0.0056 0.0054 ± 0.006 0.0212 ± 0.0153 M. mirabilis (14) 0.0048 ± 0.0046 0.0072 ± 0.0086 0.0092 ± 0.0057 M. pharensis (21) 0.0211 ± 0.0155 0.0031 ± 0.0038 0.0092 ± 0.0057 M. senaria (12) 0.0107 ± 0.0065 0.0011 ± 0.0024 0.0132 ± 0.0082 Platygyra pini (3) 0.0089 ± 0.0057 0 0.0039 ± 0.0034 P. sinensis (9) 0.0332 ± 0.0032 0.0027 ± 0.0033 0.0084 ± 0.0054 Montastrea annularis (25) 0.0075 ± 0.0054 0 0.0114 ± 0.0067 M. faveolata (18) 0.0099 ± 0.0064 0 0.0076 ± 0.0034 M. franksi (23) 0.0058 ± 0.0039 0 0.0087 ± 0.0049 Pavona cactus (13) 0.027 ± 0.0164 0 0.0029 ± 0.0039 P. decussata (25) 0.0172 ± 0.008 0 0.004 ± 0.0052 Siderastrea glynni (15) 0.006 ± 0.016 0.0016 ± 0.0028 0.0014 ± 0.0025 S. radians (6) 0 0 0.0018 ± 0.0026 S. siderea (14) 0.0083 ± 0.0064 0 0.0178 ± 0.0126 S. stellata (3) 0 0 0.0036 ± 0.0031 Porites astreoides (16) 0.0119 ± 0.0067 0.0015 ± 0.0027 0.0183 ± 0.0124 P. colonensis (4) 0.0052 ± 0.0057 0 0.0064 ± 0.0045 P. divaricata (7) 0.0028 ± 0.0025 0 0.0053 ± 0.0045 P. furcata (3) 0 0.006 0.006 ± 0.0027 P. lobata (64) 0.021 ± 0.0139 0.0028 ± 0.0036 0.0502 ± 0.0542 P. lutea (3) 0.0254 ± 0.0074 0 0.0165 ± 0.0035 P. panamensis (3) 0.0122 0.003 ± 0.0026 0 P. rus (3) 0 0 0 P. sverdrupi (7) 0.0025 ± 0.0027 0.0035 ± 0.0036 0.0109 ± 0.0053
times or a broader taxonomic sampling (Forsman 2003, 2005b). Acropora and Isopora still had rela-tively high interspecific genetic distances (0.1507 ± 0.0911 and 0.188 ± 0.0444, respectively) com-pared to the other corals.
Frequency distribution analyses of intraspecif-ic and interspecifintraspecif-ic genetintraspecif-ic distances
Frequency distribution analyses of intraspecif-ic and interspecifintraspecif-ic genetintraspecif-ic distances of ITS-5.8S rDNA revealed that Acropora possesses relatively high heterogeneity of ITS-5.8S rDNA (Figs. 1-3, Table 4). Over 75% of the genetic distances of 5.8S rDNA were < 0.02 at both the intra- and inter-specific levels in Isopora, Montastrea, Platygyra,
Porites, Pavona, Siderastrea, and Madracis,
reflecting the conservative nature of this gene frag-ment (Fig. 1). For Acropora, the intraspecific genetic distances were dispersed and overlapped with those of the interspecific comparisons, although these 2 distributions statistically signifi-cantly differed (X2-test = 84.07, p < 0.001). In
con-trast, the distribution of intraspecific genetic
dis-tances in Platygyra 5.8S was clearly separated from that at the interspecific level (X2-test = 199, p < 0.001).
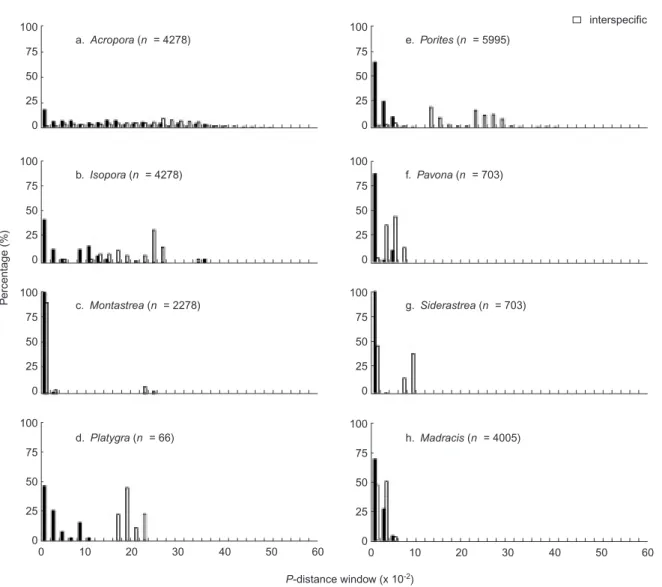
The frequency distribution of ITS1 intraspecif-ic genetintraspecif-ic distances (Fig. 2) signifintraspecif-icantly differed from those of the interspecific comparisons in
Isopora, Pavona, Platygyra, Porites, and Siderastrea (X2-test, p < 0.0001, Table 4),
sug-gesting that the ITS1 contains signals for species phylogeny. However, in Madracis and Montastrea, the difference in frequency distributions was less significant (X2-test, p < 0.05). In Acropora, the
tribution of intra- and interspecific genetic dis-tances highly overlapped and did not statistically significantly differ.
For ITS2, highly significant differences between intra- and interspecific genetic distances (Fig. 3) were detected in Isopora, Pavona,
Platygyra, Porites, Madracis, and Siderastrea (X2
-test, p < 0.001, Table 4), and separate distributions suggest phylogenetic signals. Highly overlapping frequency distributions between intra- and inter-specific genetic distances of both the 5.8S and ITS1 were similarly observed for the ITS2 of Table 3. Interspecific p-distances of the 3 ribosomal regions of ITS1, 5.8S, and
ITS2 in 8 major groups of scleractinian corals. For data sources, refer to table 1
Taxa ITS1 5.8S ITS2
Acroporoa 0.2354 ± 0.1151 0.0491 ± 0.0353 0.1507 ± 0.0911 Isopora 0.2109 ± 0.0614 0.0082 ± 0.0041 0.1880 ± 0.0444 Montastrea 0.0272 ± 0.0628 0.0013 ± 0.0043 0.0310 ± 0.0708 Platgyra 0.1955 ± 0.0183 0.0196 ± 0.0026 0.1460 ± 0.0074 Porites 0.1942 ± 0.0805 0.0054 ± 0.0058 0.3031 ± 0.1232 Pavona 0.0437 ± 0.0156 0 0.0231 ± 0.0042 Siderastrea 0.0465 ± 0.0363 0.0006 ± 0.0018 0.0218 ± 0.0123 Madracis 0.0204 ± 0.0088 0.0044 ± 0.0058 0.0195 ± 0.0102
Table 4. Chi-squared tests of intraspecific and interspecific variations based on the frequency distribution of figures. 1-3. The degree of freedom is indi-cated in parentheses. -, Chi-squared test not available
ITS1 5.8S ITS2
Taxon Chi-square P value Chi-square P value Chi-square P value
Acropora 35.40 (12) 0.05 84.07 (23) <0.001 32.25 (15) 0.006 Isopora 160.45 (14) <0.001 2.48 (1) 0.12 187.44 (10) <0.001 Pavona 138.15 (3) <0.001 - - 169.89 (1) <0.001 Porites 167.1 (13) <0.001 3.71 (2) 0.16 152.48 (19) <0.001 Siderastrea 73.97 (2) <0.001 - - 48.62 (2) <0.001 Madracis 11.61 (2) 0.003 0.04 (1) 0.84 21.26 (2) <0.001 Platygyra 200 (9) <0.001 199 (3) <0.001 199 (3) <0.001 Montastrea 9.53 (3) 0.02 8.33 (1) 0.003 9.3 (4) 0.05
Acropora, but the statistical test was significant
(X2-test, p < 0.05).
Molecular phylogenetic analysis and evolution-ary rate tests of the 5.8S phylogeny
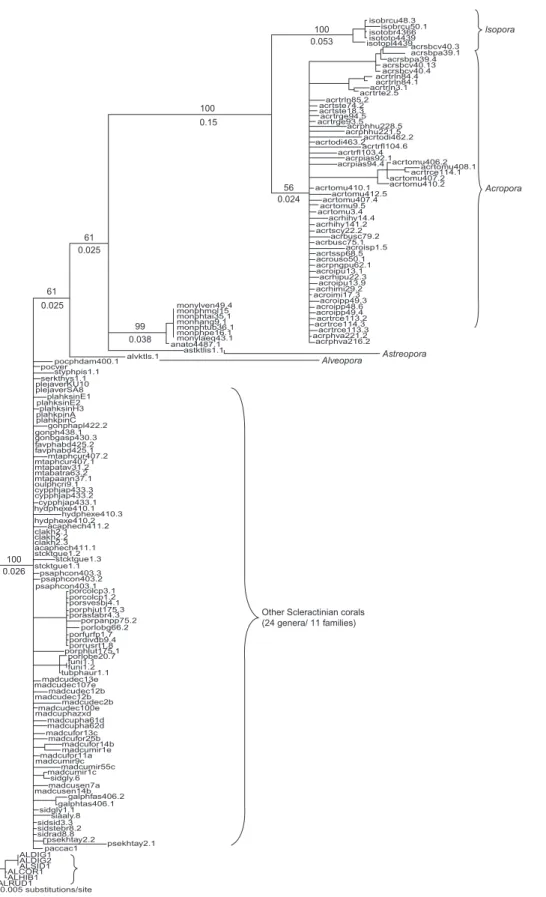
The NJ tree of 5.8S rDNA, estimated using the Kimura 2-parameter model, clearly showed a significantly long branch length leading to the clade of the genus Acropora, including both sub-genera (Fig. 4). In contrast, the 5.8S rDNA was highly conserved among the 24 genera and 11 families of scleractinian corals, thus forming an unresolved polytomy with a short branch length in the NJ tree. The branch length leading to the
Acropora/Isopora clade (0.15) was 4-6 times
longer than those of other branches leading to the major clades (0.024-0.026).
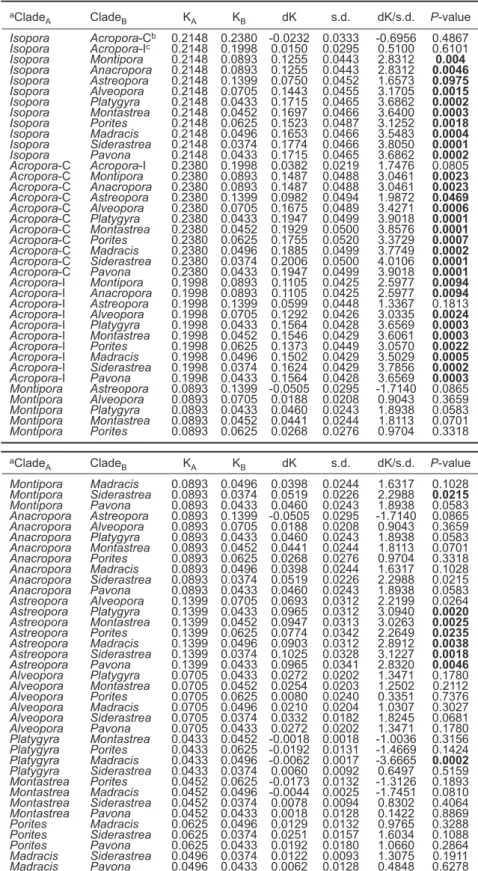
The molecular-clock hypothesis tested by the LRT was rejected for 5.8S rDNA (-log Lno clock = 858.29, -Log Lclock = 1016.12, d.f. = 154, p < 0.000001), indicating a highly significant difference in the global evolutionary rate for scleractinian 5.8S rDNA. The RRTs in table 5 show that the rDNA of Isopora, Caribbean Acropora, and Indo-Pacific Acropora evolved at constant tempos (Fisher,s exact test, p > 0.05), indicating that the divergent rDNA existed before these 3 lineages split. In contrast, rate constancy was rejected for most of the comparisons between
Acropora/Isopora and the other coral genera
(Fisher,s exact test, p < 0.01), suggesting that the
a. Acropora (n = 4278) b. Isopora (n = 4278) c. Montastrea (n = 2278) e. Porites (n = 5995) f. Pavona (n = 703) g. Siderastrea (n = 703) d. Platygra (n = 66) h. Madracis (n = 4005) P e rcentage (%) P-distance window (x 10-2)
Fig. 2. Frequency distributions of intra- and interspecific genetic distances for ITS1 rDNA. The number of pairwise comparisons (n) is
rates of evolution of 5.8S differ between
Acropora/Isopora and the other lineages, and that
the evolutionary rate of Acropora/Isopora acceler-ated after divergence from the common ancestor of scleractinian corals.
DISCUSSION
Molecular characteristics of the rDNA ITS-5.8S region in scleractinian corals
Our analyses confirm that the molecular evo-lutionary pattern of the rDNA ITS-5.8S region of
Acropora, including the subgenus Isopora, is the
most heterogeneous at the levels of both species
and individuals among scleractinian corals (Marquez et al. 2003, Chen et al. 2004). In addi-tion to the extreme divergence, 2 other molecular characteristics, namely the shortest length of the ITS and the unusual ITS2 secondary structure, make the rDNA of Acropora unique. Acropora has the shortest ITS (ITS1 of 70-94 bp and ITS2 of 107-141 bp) not only among scleractinian corals but also among all metazoans examined to date (Odorico and Miller 1997, Marquez et al. 2003, Chen et al. 2004). For the other scleractinian corals, the length of the ITS is relatively compara-ble among genera (Hunter et al. 1997, Lopez and Knowlton 1997, Medina et al. 1999, Diekmann et al. 2001, Rodriguez-Lanetty and Hoegh-Guldberg 2001, Forsman et al. 2003 2005, Lam and Morton
a. Acropora (n = 4278) e. Porites (n = 5995) b. Isopora (n = 4278) f. Pavona (n = 703) c. Montastrea (n = 2278) g. Siderastrea (n = 703) d. Platygra (n = 66) 50 30 20 40 10 0 h. Madracis (n = 4005) 50 30 20 40 10 0 P e rcentage (%) P-distance window (x 10-2)
Fig. 3. Frequency distributions of intra- and interspecific genetic distances for ITS2 rDNA. The number of pairwise comparisons (n) is
2003, Chen et al. 2004, Fukami et al. 2004, Moothien Pillay et al. 2005). The mechanism causing such short ITS sequences in species of
Acropora remains unknown. Furthermore, Acropora ITS2 forms a unique but stable 5-domain
secondary structure that differs from that of other scleractinian corals. Chen et al. (2004) examined the secondary structure of ITS2 from 54 species of scleractinian corals, representing 25 genera and 11 families of both the complex and robust clades previously defined in molecular phylogenetic analyses (Fukami et al. 2004). A standard of 4 domains was observed in 17 species of corals, while 23 species had a modified number of 5 domains with domain I divided into 2 subdomains. These 2 types of secondary structures were observed across 11 coral families. The 3rd type, 5 domains with domain III divided into 2 subdo-mains, was only seen in the genus Acropora. Extreme rDNA diversity in Acropora
The extreme rDNA diversity in Acropora may be due to the presence of pseudogenes that have been maintained through recent introgressive hybridization and slow concerted evolution as a result of frequent asexual propagation (Marquez et al. 2003). In Acropora, up to nine rDNA types may be present in a single colony (Odorico and Miller 1997), and distinct ITS types are often shared by species (van Oppen et al. 2000 2001 2002). Marquez et al. (2003) analyzed the RT-PCR of 5.8S rDNA expressed in A. millepora, examined the pattern of methylation that may indicate silenc-ing caused by nucleolar dominance, and looked for mutations that could disrupt the secondary struc-ture and functionality of the rRNA. These analyses consistently indicated that 1 rDNA sequence type present in a broad range of Indo-Pacific Acropora is likely to consist primarily of pseudogenes. It was suggested that interspecific hybridization may have brought together divergent rDNA copies into a single genome, as high divergence in the ITS region may have suppressed recombination across the entire rDNA array, slowing down con-certed evolution (Muir et al. 2001). In addition, asexual reproduction, such as fragmentation by
Acropora, may also limit concerted evolution and
cause slow homogenization of divergent rDNA copies. Consequently, some of the rDNA types combined by hybridization may have been silenced by nucleolar dominance, causing them to evolve as pseudogenes, thus increasing sequence
heterogeneity (Marquez et al. 2003).
Although these patterns are thought to be consistent with the occurrence of interspecific hybridization, incomplete lineage sorting and incomplete concerted evolution may also be alter-native explanations (Vollmer and Palumbi 2002 2004). Vollmer and Palumbi (2004) analyzed sequence divergence rates between rDNA and single-copy nuclear genes of the Caribbean
Acropora and suggested that the Caribbean Acropora rDNA lineages were quite ancient and
predated the split of the species. By comparison, the most divergent ITS lineages in the Caribbean
Acropora occurred approximately 40 million years
ago (mya) (Vollmer and Palumbi 2004) which is roughly 6 times older than a conservative time of 6.6 mya for Acropora divergence in the Caribbean (Budd et al. 1994, van Oppen et al. 200, Miller and van Oppen 2003). Molecular evolutionary rate tests of the 5.8S conducted in our study clearly indicated that persistence of ancient rDNA could be the case in the Indo-Pacific Acropora, and that it is older than the split of the Caribbean and Indo-Pacific Acropora, or even older than the common ancestor of Acropora and Isopora. First, rate con-sistency was rejected by the LRT for the global molecular phylogeny of the scleractinian 5.8S. The significantly longer branch leading to the clade
Acropora/Isopora suggests that the unusually
high-ly divergent rDNA persisted in the common ances-tor of Acropora and Isopora after divergence from other scleractinian corals (Fig. 4). Second, the RRT showed that Isopora, Caribbean Acropora, and Indo-Pacific Acropora evolved at constant tempos, and that rDNA in each of these 3 lineages is highly divergent (Table 5). Interestingly, Isopora, a subgenus of Acropora, is composed of 4 described species (Wallace 1999). Unlike
Acropora, species of Isopora are distributed less
sympatrically. Moreover, they brood larvae which would considerably reduce the opportunity for cross-species hybridization. However, species of
Isopora still possess highly divergent rDNA which
evolved at a similar rate to that of Acropora, sup-porting the argument that recent introgressive hybridization alone cannot account for the diver-gence patterns found in Acropora (Vollmer and Palumbi 2002 2004). In plants, ancient divergent paralogous rDNA lineages and the persistence of these lineages predating speciation events are commonly observed in many lineages (Buckler et al. 1997, Muir et al. 2001, Alvarez and Wendel 2003).
100 0.053 0.024 56 0.15 0.025 0.025 0.038 0.026 100 99 61 61 100 isobrcu48.3 lsopora Acropora isotobr4366 isototo4439 isotopl4439acrsbcv40.3 acrsbpa39.1 acrsbpa39.4 acrsbcv40.13 acrsbcv40.4 acrtrln84.4 acrtrln84.1 acrtrln3.1 acrtrte2.5 acrtrln85.2 acrtste74.2 acrtste18.3 acrtrge94.5 acrtrge93.5 acrphhu228.5 acrphhu221.5 acrtodi462.2 acrtodi463.2acrtrfl104.6 acrtrfl103.4 acrpias92.1 acrpias94.4 acrtomu406.2 acrtomu408.1 acrtrce114.1 acrtomu407.2 acrtomu410.2 acrtomu410.1 acrtomu412.5 acrtomu407.4 acrtomu9.5 acrtomu3.4 acrhihy14.4 acrhihy141.2 acrtscy22.2 acrbusc79.2 acrbusc75.1 acroisp1.5 acrtssp68.5 acrouso50.1 acrpngpu62.1 acroipu13.1 acrhipu22.3 acroipu13.9 acrhimi29.2 acroimi17.3 acroipp49.3 acroipp48.6 acroipp49.4 acrtrce113.2 acrtrce114.3 acrtrce113.3 acrphva221.2 acrphva216.2 monylven49.4 monphmol15 monphtai35.1 monhang9.1 monphtub36.1 monphpe16.1 monylaeq43.1 anato4487.1 astktlis1.1 alvktls.1 pocphdam400.1 pocver styphpis1.1 serkthys1.1 plejaverKU10 plejaverSA8 plahksinE1 plahksinE2 plahksinH3 plahkpinA plahkpinC gonphapl422.2 gonph438.1 gonbgasp430.3 favphabd425.2 favphabd425.1 mtaphcur407.2 mtaphcur407.1 mtapatav31.2 mtabatra63.2 mtapaann37.1 oulphcri9.1 cypphjap433.3 cypphjap433.2 cypphjap433.1 hydphexe410.1 hydphexe410.3 hydphexe410.2 acaphech411.2 clakh2.1 clakh2.2 clakh2.3 acaphech411.1 stcktgue1.2 stcktgue1.3 stcktgue1.1 psaphcon403.3 psaphcon403.2 psaphcon403.1 porcolcp3.1 porcolcp1.2 porsvesbj4.1 porphjut175.3 porastabr4.3 porpanpp75.2 porlobg66.2 porfurfp1.7 pordivdb9.4 porrusrt1.8 porphlut175.1 porlobe20.7 funi1.1 funi1.2 tubphaur1.1 madcudec13e madcudec107e madcudec12b madcudec12b madcudec2b madcudec100e madcuphazxd madcupha61d madcupha62d madcufor13c madcufor25b madcufor14b madcumir1e madcufor11a madcumir9c madcumir55c madcumir1c sidgly.6 madcusen7a madcusen14b galphfas406.2 galphtas406.1 sidgly1.1 siaaly.8 sidsid3.3 sidstebr8.2 sidrad8.8 psekhtay2.2 psekhtay2.1 -0.005 substitutions/site paccac1 ALDIG1 ALDIG2 ALSID1 ALCOR1 ALHIB1 ALRUD1 Astreopora Alveopora isobrcu50.1
Fig. 4. Phylogenetic analysis derived from the Neighbor-joining (NJ) algorithms of scleractinian 5.8S rDNA. Numbers above and below
the branches indicate the bootstrap values for the NJ (1000 replicates) analysis and the branch length of each major clade. The sam-ple codes are the abbreviation of the genus name, locality, species name, samsam-ple number, and the clone that was sequenced (e.g., acrophva216.2 is the number 2 clone of sample 216 of Acropora valida collected from Penghu). The complete 5.8S dataset is available upon request from the senior author.
Table 5. Relative-rate test results for 5.8S clades in the Neighbor-join-ing phylogeny aClade A CladeB KA KB dK s.d. dK/s.d. P-value Isopora Acropora-Cb 0.2148 0.2380 -0.0232 0.0333 -0.6956 0.4867 Isopora Acropora-Ic 0.2148 0.1998 0.0150 0.0295 0.5100 0.6101 Isopora Montipora 0.2148 0.0893 0.1255 0.0443 2.8312 0.004 Isopora Anacropora 0.2148 0.0893 0.1255 0.0443 2.8312 0.0046 Isopora Astreopora 0.2148 0.1399 0.0750 0.0452 1.6573 0.0975 Isopora Alveopora 0.2148 0.0705 0.1443 0.0455 3.1705 0.0015 Isopora Platygyra 0.2148 0.0433 0.1715 0.0465 3.6862 0.0002 Isopora Montastrea 0.2148 0.0452 0.1697 0.0466 3.6400 0.0003 Isopora Porites 0.2148 0.0625 0.1523 0.0487 3.1252 0.0018 Isopora Madracis 0.2148 0.0496 0.1653 0.0466 3.5483 0.0004 Isopora Siderastrea 0.2148 0.0374 0.1774 0.0466 3.8050 0.0001 Isopora Pavona 0.2148 0.0433 0.1715 0.0465 3.6862 0.0002 Acropora-C Acropora-I 0.2380 0.1998 0.0382 0.0219 1.7476 0.0805 Acropora-C Montipora 0.2380 0.0893 0.1487 0.0488 3.0461 0.0023 Acropora-C Anacropora 0.2380 0.0893 0.1487 0.0488 3.0461 0.0023 Acropora-C Astreopora 0.2380 0.1399 0.0982 0.0494 1.9872 0.0469 Acropora-C Alveopora 0.2380 0.0705 0.1675 0.0489 3.4271 0.0006 Acropora-C Platygyra 0.2380 0.0433 0.1947 0.0499 3.9018 0.0001 Acropora-C Montastrea 0.2380 0.0452 0.1929 0.0500 3.8576 0.0001 Acropora-C Porites 0.2380 0.0625 0.1755 0.0520 3.3729 0.0007 Acropora-C Madracis 0.2380 0.0496 0.1885 0.0499 3.7749 0.0002 Acropora-C Siderastrea 0.2380 0.0374 0.2006 0.0500 4.0106 0.0001 Acropora-C Pavona 0.2380 0.0433 0.1947 0.0499 3.9018 0.0001 Acropora-I Montipora 0.1998 0.0893 0.1105 0.0425 2.5977 0.0094 Acropora-I Anacropora 0.1998 0.0893 0.1105 0.0425 2.5977 0.0094 Acropora-I Astreopora 0.1998 0.1399 0.0599 0.0448 1.3367 0.1813 Acropora-I Alveopora 0.1998 0.0705 0.1292 0.0426 3.0335 0.0024 Acropora-I Platygyra 0.1998 0.0433 0.1564 0.0428 3.6569 0.0003 Acropora-I Montastrea 0.1998 0.0452 0.1546 0.0429 3.6061 0.0003 Acropora-I Porites 0.1998 0.0625 0.1373 0.0449 3.0570 0.0022 Acropora-I Madracis 0.1998 0.0496 0.1502 0.0429 3.5029 0.0005 Acropora-I Siderastrea 0.1998 0.0374 0.1624 0.0429 3.7856 0.0002 Acropora-I Pavona 0.1998 0.0433 0.1564 0.0428 3.6569 0.0003 Montipora Astreopora 0.0893 0.1399 -0.0505 0.0295 -1.7140 0.0865 Montipora Alveopora 0.0893 0.0705 0.0188 0.0208 0.9043 0.3659 Montipora Platygyra 0.0893 0.0433 0.0460 0.0243 1.8938 0.0583 Montipora Montastrea 0.0893 0.0452 0.0441 0.0244 1.8113 0.0701 Montipora Porites 0.0893 0.0625 0.0268 0.0276 0.9704 0.3318 aClade A CladeB KA KB dK s.d. dK/s.d. P-value Montipora Madracis 0.0893 0.0496 0.0398 0.0244 1.6317 0.1028 Montipora Siderastrea 0.0893 0.0374 0.0519 0.0226 2.2988 0.0215 Montipora Pavona 0.0893 0.0433 0.0460 0.0243 1.8938 0.0583 Anacropora Astreopora 0.0893 0.1399 -0.0505 0.0295 -1.7140 0.0865 Anacropora Alveopora 0.0893 0.0705 0.0188 0.0208 0.9043 0.3659 Anacropora Platygyra 0.0893 0.0433 0.0460 0.0243 1.8938 0.0583 Anacropora Montastrea 0.0893 0.0452 0.0441 0.0244 1.8113 0.0701 Anacropora Porites 0.0893 0.0625 0.0268 0.0276 0.9704 0.3318 Anacropora Madracis 0.0893 0.0496 0.0398 0.0244 1.6317 0.1028 Anacropora Siderastrea 0.0893 0.0374 0.0519 0.0226 2.2988 0.0215 Anacropora Pavona 0.0893 0.0433 0.0460 0.0243 1.8938 0.0583 Astreopora Alveopora 0.1399 0.0705 0.0693 0.0312 2.2199 0.0264 Astreopora Platygyra 0.1399 0.0433 0.0965 0.0312 3.0940 0.0020 Astreopora Montastrea 0.1399 0.0452 0.0947 0.0313 3.0263 0.0025 Astreopora Porites 0.1399 0.0625 0.0774 0.0342 2.2649 0.0235 Astreopora Madracis 0.1399 0.0496 0.0903 0.0312 2.8912 0.0038 Astreopora Siderastrea 0.1399 0.0374 0.1025 0.0328 3.1227 0.0018 Astreopora Pavona 0.1399 0.0433 0.0965 0.0341 2.8320 0.0046 Alveopora Platygyra 0.0705 0.0433 0.0272 0.0202 1.3471 0.1780 Alveopora Montastrea 0.0705 0.0452 0.0254 0.0203 1.2502 0.2112 Alveopora Porites 0.0705 0.0625 0.0080 0.0240 0.3351 0.7376 Alveopora Madracis 0.0705 0.0496 0.0210 0.0204 1.0307 0.3027 Alveopora Siderastrea 0.0705 0.0374 0.0332 0.0182 1.8245 0.0681 Alveopora Pavona 0.0705 0.0433 0.0272 0.0202 1.3471 0.1780 Platygyra Montastrea 0.0433 0.0452 -0.0018 0.0018 -1.0036 0.3156 Platygyra Porites 0.0433 0.0625 -0.0192 0.0131 -1.4669 0.1424 Platygyra Madracis 0.0433 0.0496 -0.0062 0.0017 -3.6665 0.0002 Platygyra Siderastrea 0.0433 0.0374 0.0060 0.0092 0.6497 0.5159 Montastrea Porites 0.0452 0.0625 -0.0173 0.0132 -1.3126 0.1893 Montastrea Madracis 0.0452 0.0496 -0.0044 0.0025 -1.7451 0.0810 Montastrea Siderastrea 0.0452 0.0374 0.0078 0.0094 0.8302 0.4064 Montastrea Pavona 0.0452 0.0433 0.0018 0.0128 0.1422 0.8869 Porites Madracis 0.0625 0.0496 0.0129 0.0132 0.9765 0.3288 Porites Siderastrea 0.0625 0.0374 0.0251 0.0157 1.6034 0.1088 Porites Pavona 0.0625 0.0433 0.0192 0.0180 1.0660 0.2864 Madracis Siderastrea 0.0496 0.0374 0.0122 0.0093 1.3075 0.1911 Madracis Pavona 0.0496 0.0433 0.0062 0.0128 0.4848 0.6278 Siderastrea Pavona 0.0374 0.0433 -0.0060 0.0092 -0.6497 0.5159 Note- KAand KBare mean Kimura 2-parameter distances; dK= KA- KB; s. d.: standard deviation Fisher,s exact test: significant p values are labeled in bold. aOcotorals were
assigned as outgroups in all calculations. bCaribbean Acropora. cIndo-West Pacific Acropora.
Phylogenetic utility of coral rDNA ITS-5.8S regions: Acropora is an exception
Our study confirms the previous hypothesis that the extremely high diversity of rDNA is unique to Acropora and is not a common feature of all scleractinian corals (Chen et al. 2004). The sug-gestion that ITS rDNA should be abandoned as a species- and population-level phylogenetic marker due to its complicated and undistinguishable char-acteristics of molecular evolution (Vollmer and Palumbi 2004) should be treated with caution since Acropora has several atypical and unusual characteristics that are significantly distinct from other scleractinian corals (Chen et al. 2004, this study). Both genetic distance and frequency distri-bution analyses of the rDNA clearly showed that the extremely high and overlapping heterogeneity is characteristic only of the subgenus Acropora. Even though high genetic diversity was observed in the subgenus Isopora, frequency analyses of ITS1 and ITS2 showed significantly non-overlap-ping genetic distance distributions between intra-and interspecific comparisons (Figs. 2b, 3b), sug-gesting that phylogenetic signals are still informa-tive, at least for specific phylogenetic inferences (Chen et al. unpubl. data). Similar patterns were also observed in Pavona, Platygyra, Porites, and
Siderastrea, but their specific phylogenies were
successfully resolved using the ITS-5.8S region (Lam and Morton 2003, Forsman et al. 2003 2005a b, Moothien Pillay et al. 2005). For
Madracis and Montastrea, the ITS1 and ITS2 were
not as informative as for those coral genera described above, although they could be used to resolve the phylogenetic relationship of each lin-eage to a certain extent (see Diekmann et al. 2001, Fukami et al. 2004). In addition, guided by the homologous secondary structure, the aligned ITS2 successfully provided a concordant pattern (Chen et al. 2004) with the phylogenies based on mitochondrial, nuclear ribosomal, and protein-cod-ing genes which showed the families Faviidae, Merulindae, and Mussidae to be monophyletic within the suborder Faviina (except for Oulastrea), although relationships at the family level are apparently not monophyletic (Romano and Cairns 2000, Chen et al. 2002, Fukami et al. 2004).
In summary, genetic distance and frequency distribution analyses demonstrated that the extremely high diversity of the rDNA ITS-5.8S region is unique to Acropora. Molecular phyloge-netic inferences and evolutionary rate tests sup-port the scenario that the divergent rDNA has been
maintained since ancient times and probably pre-dated the speciation of the common ancestor of
Acropora.
Acknowledgments: Many thanks are given to the staff of the Penghu Aquarium, a facility of the Taiwan Fishery Research Institute, for providing hospitality during our field trips. We thank mem-bers of the Evolution and Ecology Discussion Group, Research Center for Biodiversity, Academia Sinica (RCBAS) and 4 anonymous ref-erees for constructive comments. N.V. Wei was supported by a predoctoral fellowship from the National Science Council, Taiwan (NSC). This work was supported by grants from RCBAS and NSC to C.A.C. This is the Evolution and Ecology Group, RCBAS contribution no. 36.
REFERENCES
Alverez I, JF Wendel. 2003. Ribosomal ITS sequences and plant phylogenetic inference. Mol. Phylogenet. Evol. 29: 417-434.
Avise JC. 2004. Molecular markers, natural history, and evolu-tion, 2nd ed. Sunderland, MA: Sinauer Associates. Baldwin BG. 1992. Phylogenetic utility of the internal
tran-scribed spacers of nuclear ribosomal DNA in plants: an example from the Compositae. Mol. Phylogenet. Evol. 1: 3-16.
Buckler ES IV, TP Holtford. 1996. Zea systematics: ribosomal ITS evidence. Mol. Biol. Evol. 13: 612-622.
Budd AF, TA Stemann, KG Johnson. 1994. Stratigraphic distri-butions of neogene to recent Caribbean reef corals: a new compilation. J. Paleontol. 68: 951-959.
Chen CA, CC Chang, NV Wei, CH Chen, YT Lein, HE Lin, CF Dai, CC Wallace. 2004. Secondary structure and phylo-genetic utility of the ribosomal internal transcribed spacer 2 (ITS2) in scleractinian corals. Zool. Stud. 43: 759-771. Chen CA, CC Wallace, J Wolstenholme. 2002. Analysis of
mitochondrial 12S RNA gene supports a two-clade hypothesis of the evolutionary history of scleractinian corals. Mol. Phylogenet. Evol. 23: 137-149.
Chen CA, JK Yu, NW Wei. 2000. Strategies for amplification by polymerase chain reaction of the complete sequence of nuclear large subunit ribosomal RNA-encoding gene in corals. Mar. Biotechnol. 6: 558-570.
Diekmann OE, RPM Bak, WT Stam, JL Olsen. 2001. Molecular genetic evidence for probable reticulate specia-tion in the coral genus Madracis from a Caribbean fringing reef slope. Mar. Biol. 139: 221-223.
Dover GA. 1982. Molecular drive, a cohesive model of species evolution. Nature 299: 111-117.
Felsenstein J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 17: 368-376.
Forsman ZH. 2003. Phylogeny and phylogeography of Porites and Siderastrea (Scleractinia: Cnidaria) species in the Caribbean and eastern Pacific; based on the nuclear ribo-somal ITS region. PhD dissertation. Univ. of Houston, Houston, TX.
Forsman ZH, HM Guzman, CA Chen, GE Fox, GM Wellington. 2005. An ITS region phylogeny of Siderastrea (Cnidaria: Anthozoa): Is S. glynni endangered or introduced? Coral Reefs 24: 343-347.
Forsman ZH, CL Hunter, GE Fox, GM Wellington. Is the ITS region the solution to the“species problem”in corals? Intragenomic variation and alignment permutation in
Porites, Siderastrea and outgroup taxa. Proceedings of
the 10th International Coral Reef Symposium. Okinawa, Japan. (in press)
Fukami H, AF Budd, G Paulay, A Sole-Cava, CA Chen, K Iwao, N Knowlton. 2004. Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature 427: 832-835.
Fukami H, M Omori, M Hatta. 2000. Phylogenetic relation-ships in the coral family Acroporidae, reassessed by infer-ence from mitochondrial genes. Zool. Sci. 17: 689-696. Gilbert DC. 1994. SeqApp 1.9. A biological sequence editor
and analysis program for Macintosh computers. Available via an anonymous ftp at ftp://bio.indiana.edu.
Hillis DM, MT Dixon. 1991. Ribosomal DNA: molecular evolu-tion and phylogenetic inference. Q. Rev. Biol. 66: 411-453.
Hillis DM, C Moritz, CA Porter, RJ Baker. 1991. Evidence for biased gene conversion in concerted evolution of riboso-mal DNA. Science 251: 308-310.
Hunter CL, CW Morden, CM Smith. 1997. The utility of ITS sequences in assessing relationships among zooxanthel-lae and corals. Proc. 8th Int. Coral Reef Sym. 2: 1599-1602.
Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16: 111-120. Lam K, B Morton. 2003. Morphological and ITS1, 5.8S, and
partial ITS2 ribosomal DNA sequence distinctions between two species Playtygyra (Cnidaria; Scleractinia) from Hong Kong. Mar. Biotechnol. 5: 555-567.
Li P, J Bousquet. 1992. Relative-rate test for nucleotide substi-tutions between two lineages. Mol. Biol. Evol. 9: 1185-1189.
Li WH. 1997. Molecular evolution. Sunderland, MA: Sinauer Associates.
Lopez J, N Knowlton. 1997. Discrimination of species in the
Montastraea annularis complex using multiple genetic
loci. Proc. 8th Int. Coral Reef Sym. 2: 1613-1618. Mai JC, AW Coleman. 1997. The internal transcribed spacer 2
exhibits a common secondary structure in green algae and flowering plants. J. Mol. Evol. 44: 258-271.
Marquez LM, DJ Miller, JB MacKenzie, MJH van Oppen. 2003. Pseudogenes contribute to the extreme diversity of nuclear ribosomal DNA in the hard coral Acropora. Mol. Biol. Evol. 20: 1077-1086.
Medina M, E Weil, AM Szmant. 1999. Examination of the
Montastraea annularis species complex (Cnidaria:
Scleractinia) using ITS and COI sequences. Mar. Biotechnol. 1: 89-97.
Moothien Pillay KRM, T Asahida, CA Chen, H Terashima, H Ida. 2005. ITS ribosomal DNA distinctions and genetic structure of populations in two sympatric species of
Pavona (Cnidaria: Scleractinia) from Mauritius. Zool.
Stud. 45: 132-144.
Miller DJ, MJH van Oppen. 2003. A“fair go”for coral hybridization. Mol. Ecol. 12: 805-807.
Muir G, CC Fleming, C Schlotterer. 2001. Three divergent rDNA clusters predate the species divergence in Quercus
petraea (Matt.) Liebl. and Quercus robur L. Mol. Biol.
Evol. 18: 112-119.
Muse SV, BS Weir. 1992. Testing for equality of evolutionary rates. Genetics 132: 269-276.
Odorico DM, DJ Miller. 1997. Variation in the ribosomal inter-nal transcribed spacers and 5.8S rDNA among five species of Acropora (Cnidaria; Scleractinia): patterns of variation consistent with reticulate evolution. Mol. Biol. Evol. 14: 465-473.
Oliverio M, M Cervelli, P Mariottini. 2002. ITS2 rRNA evolution and its congruence with the phylogeny of muricid neogas-tropods (Caenogastropoda, Muricoidea). Mol. Phylogenet. Evol. 25: 63-69.
Posada D, KA Crandall. 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817-818.
Robinson M, M Gouy, C Gautier, D Mouchiroud. 1998. Sensitivity of the relative-rate test to taxonomic sampling. Mol. Biol. Evol. 15: 1091-1098.
Rodriguez-Lanetty M, O Hoegh-Guldberg. 2002. The phylo-geography and connectivity of the latitudinally widespread scleractinian coral Plesiastrea versipora in the Western Pacific. Mol. Ecol. 11: 1177-1189.
Romano SL, SD Cairns. 2000. Molecular phylogenetic hypotheses for the evolution of scleractinian corals. Bull. Mar. Sci. 67: 1043-1068.
Schlötterer C, M Hauser, A von Haeseler, D Tautz. 1994. Comparative evolutionary analysis of rDNA ITS regions in
Drosophila. Mol. Biol. Evol. 11: 513-522.
Schmidt HA, K Strimmer, M Vingron, A von Haeseler. 2002. TREE-PUZZLE: maximum likelihood phylogenetic analy-sis using quartets and parallel computing. Bioinformatics
18: 502-504.
Swofford DL. 2002. PAUP 4.0b10: Phylogenetic Analysis Using Parsimony (and other methods). Sunderland, MA: Sinauer Associates.
Thompson JD, DG Higgins, TJ Gibson. 1994. CLUSTAL X: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673-4680.
van Oppen MJH, EM Koolmees, JEN Veron. 2004. Patterns of evolution in the scleractinian coral genus Montipora (Acroporidae). Mar. Biol. 144: 9-18.
van Oppen MJH, BJ McDonald, BL Willis DJ Miller. 2001. The evolutionary history of the coral genus Acropora (Scleractinia, Cnidaria) based on a mitochondrial and a nuclear marker: reticulation, incomplete lineage sorting, or morphological convergence? Mol. Biol. Evol. 18: 1315-1329.
van Oppen MJH, BL Willis, T van Rheede, DJ Miller. 2002. Spawning times, reproductive compatibilities and genetic structuring in the Acropora aspera group: evidence for natural hybridization and semi-permeable species bound-aries in corals. Mol. Ecol. 11: 1363-1376.
van Oppen MJH, B Willis, HWJA van Vugt, DJ Miller. 2000. Examination of species boundaries in the Acropora
cervi-cornis group (Scleractinia, Cnidaria) using nuclear DNA
sequence analyses. Mol. Ecol. 9: 1363-1373.
Vollmer SV, SR Palumbi. 2002. Hybridization and the evolu-tion of reef coral diversity. Science 296: 2023-2025. Vollmer SV, SR Palumbi. 2004. Testing the utility of internally
transcribed spacer sequences in coral phylogenetics. Mol. Ecol. 13: 2763-2772.
Wallace CC. 1999. Staghorn corals of the world: a revision of the coral genus Acropora (Scleractinia; Astrocoeniina;
Acroporidae) worldwide, with emphasis on morphology, phylogeny and biogeography. Collingwood, Australia: CSIRO Publishing.
Weekers PHH, FJ de Jonckheere, HJ Dumont. 2001. Phylogenetic relationships inferred from ribosomal ITS sequences and biogeographic patterns in representative
of the genus Calopteryx (Insecta: Odonata) of the West Mediterranean and adjacent west European zone. Mol. Phylogenet. Evol. 20: 89-99.
Wu CI, WH Li. 1985. Evidence for higher rates of nucleotide substitution in rodents than in man. Proc. Natl. Acad. Sci. USA 82: 1741-1745.