Elsevier, and the attached copy is provided by Elsevier for the
author’s benefit and for the benefit of the author’s institution, for
non-commercial research and educational use including without
limitation use in instruction at your institution, sending it to specific
colleagues that you know, and providing a copy to your institution’s
administrator.
All other uses, reproduction and distribution, including without
limitation commercial reprints, selling or licensing copies or access,
or posting on open internet sites, your personal or institution’s
website or repository, are prohibited. For exceptions, permission
may be sought for such use through Elsevier’s permissions site at:
Author's personal copy
Removal of methyl tert-butyl ether (MTBE) with Nafion
Hsing-Lung Lien
a,∗, Wei-Xian Zhang
baDepartment of Civil and Environmental Engineering, National University of Kaohsiung, Kaohsiung 811, Taiwan, ROC bDepartment of Civil and Environmental Engineering, Lehigh University, Bethlehem, PA 18015, USA
Received 8 May 2006; received in revised form 3 October 2006; accepted 3 October 2006 Available online 13 October 2006
Abstract
A solid organic polymer, Nafion, is tested for the removal of methyl tert-butyl ether (MTBE) in water. Nafion with perfluorosulfonic acid backbone and terminal sulfonic acid groups has a surface acidity similar to 100% sulfuric acid, and has been commonly used as a strong-acid catalyst in many organic reactions. Sorption and subsequent transformation of MTBE were observed in batch experiments. The transformation of MTBE by porous nanocomposite Nafion SAC-13 to tert-butyl alcohol (TBA), acetone, isobutene and probably methanol was found. Subsequent transformation of TBA to acetone was also observed. Results suggest that transformational pathways may include hydrolysis, dehydrogenation and oxidation. Dissolved oxygen is needed for the oxidation of isobutene to acetone. As Nafion is insoluble in water, chemically stable, and regenerable, its use in packed-bed reactors for MTBE removal looks promising.
© 2006 Elsevier B.V. All rights reserved.
Keywords: Methyl tert-butyl ether (MTBE); tert-Butyl alcohol (TBA); Nafion; Superacid; Hydrolysis; Dehydrogenation; Sorption
1. Introduction
Methyl tert-butyl ether (MTBE) is used primarily as a gaso-line oxygenate that, when mixed with gasogaso-line, promotes more complete combustion, thereby reducing exhaust emissions of carbon monoxide and reactive organic compounds [1–3]. The increasing use of MTBE since 1990 has quickly turned it into one of the most frequently detected contaminants in groundwater and surface waters in the U.S.[4]. Incidents of both groundwa-ter and surface wagroundwa-ter contamination by MTBE have been widely reported[5–7]. Public concern about MTBE contamination has led to the phase out or ban of MTBE as a fuel additive.
MTBE is a comparatively unreactive compound in the envi-ronment. The ether linkage is stable toward bases, oxidizing agents, and reducing agents. In so far as the ether linkage itself is concerned, ethers undergo primarily one kind of reaction, cleav-age by acids; however, the cleavcleav-age reaction takes place only under quite vigorous conditions: concentrated acids and high temperature [8]. Although simple hydrolysis of MTBE under neutral conditions is unlikely, studies have shown that the
acid-∗Corresponding author. Tel.: +886 7591 9221; fax: +886 7591 9376. E-mail address:lien.sam@nuk.edu.tw(H.-L. Lien).
catalyzed hydrolysis of MTBE is a possible reaction pathway under acidic conditions[9].
The use of a water-soluble strong acid such sulfuric acid would not be applicable for drinking water and wastewater treat-ment due to obvious obstacles such as a strongly acidic effluent and corrosion. This work is aimed to explore the use of water-insoluble solid acids as a catalyst for MTBE transformation. Our preliminary results suggest that Nafion appears promising for this purpose.
Nafion, with average molecular weight greater than 1500, is a polymeric organic acid that consists of perfluorosulfonic acid resin with terminal sulfonic acid groups is as shown in Scheme 1 [10–12].The perfluorinated backbone gives the mechanical strength and chemical stability. The ether link-age between the side chain and polymer backbone leads to its flexibility [10]. Nafion in the acid form has a terminal –CF2CF2SO3H group. The fluorocarbon portion of the polymer
molecule has high electron-withdrawing capacity, which leaves the sulfonate–proton bond strongly polarized. The acid groups in Nafion have a Hammett acidity (−H0∼ 12) similar to 100%
sulfuric acid[10,11]. Accordingly, it has been often termed as a superacidic catalyst. The high acid strength and chemical inert-ness of the fluorocarbon make Nafion an attractive alternative for solid acid catalysts[13].
0304-3894/$ – see front matter © 2006 Elsevier B.V. All rights reserved. doi:10.1016/j.jhazmat.2006.10.004
Author's personal copy
Scheme 1. Chemical structure of Nafion.An important advantage of a solid catalyst used in a packed-bed reactor is the easy separation of treated water from the cat-alyst. Furthermore, deactivated Nafion can be regenerated with dilute acid solutions[10,11]. Therefore, no chemical is added during the treatment run. Objectives of this study are to test fea-sibility of Nafion as a solid catalyst for the MTBE removal from aqueous solutions and to investigate the removal processes and the MTBE transformation pathways. Experiments are designed to determine the sorption of MTBE, rate and extent of MTBE transformation, reaction intermediates and final products.
2. Experimental
2.1. Materials and chemicals
A composite of Nafion and silica, Nafion SAC-13 is used in this study. Obtained from Aldrich, Nafion SAC-13 is pro-duced by Du Pont using the sol–gel technique in which Nafion is fixed on amorphous silica surface during the solidification process[14]. Nafion SAC-13 has values of surface area, pore vol-ume, and pore diameter of >200 m2/g, >0.6 ml/g, and >10 nm, respectively[14]. The composite consists of 10–20% Nafion. The sample of Nafion SAC-13 was purchased from Aldrich. HPLC grade methyl tert-butyl ether (MTBE), tert-butyl alco-hol (TBA), tert-amyl alcoalco-hol, acetone, methanol, and isobutene were obtained from Sigma–Aldrich. tert-Amyl methyl ether (TAME) from Aldrich has a purity of 97%.
2.2. Batch experiments
The batch tests for MTBE removal were conducted with 100 ml serum vials with crimp top septa. Stock solutions were prepared by deionized water. Typically, 4 g of Nafion SAC-13 were charged to a 50 ml aqueous solution containing a sig-nal target reactant at 22± 1◦C. The reactant included MTBE (40–50 mg/l), TBA (40 mg/l) and TAME (60 mg/l). Then the serum vials were put in a rotator (50 rpm) and sampled at reg-ular intervals with a gastight syringe. The pH measurements at the beginning and end of the experiment indicated no significant change of the solution pH (6.6± 0.2) during the reaction. The experiments were conducted in duplicate to check the repro-ducibility of batch results.
2.3. Method of analyses
At selected time intervals, 0.5 ml of aqueous aliquot with-drawn by a gastight syringe was diluted with 4 ml of distilled
water for GC/MS analysis. A Shimadzu QP5000 GC/MS cou-pled to a Tekmar 3000 purge and trap concentrator was used. A VOCARB 3000 trap column (Supelco) was installed in the Tekmar 3000 to remove excessive water. The Tekmar default method was used except that the desorption time was shortened from 120 to 30 s. The GC was equipped with a DB-624 cap-illary column (30 m× 0.25 mm, 0.25 m film thickness). GC temperature was programmed at 50◦C for 5 min and increased at a rate of 5◦C/min to 100◦C. Injection and detector tempera-tures were set at 150 and 230◦C, respectively. Quadrupole mass spectrometer was set to scan from 20 to 150 m/z and data collec-tion every 0.1 s. Deteccollec-tion and qualificacollec-tion were performed by using the full scan mode while quantification was performed in the selected ion monitoring (SIM). Quantitative analysis was conducted by an external standard method, which is accept-able in GC/MS analysis[15]. Linear standard calibration curves were achieved for all compounds over the range 0.1–10 mg/l (R2> 0.99). The variability of calibration curves was checked daily before analysis (15%). The detection limit of MTBE and TBA was 50g/l.
3. Results and discussion
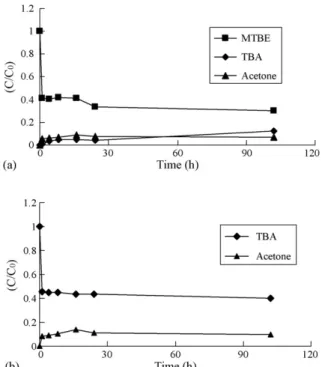
Fig. 1a shows the time courses of MTBE (50 mg/l) removal and measured transformational products in a 50 ml batch solution containing 4 g of Nafion SAC-13 under ambient conditions. Rapid sorption and slow transformation were observed. Approximately 58% of MTBE was removed within the first hour with instantaneous appearance of acetone. After approximately 6 h, TBA was detected in the solution. Concentrations of acetone and TBA increased slowly after that.
Fig. 1. (a) Transformation of MTBE over Nafion SAC-13. (b) Transformation of TBA over Nafion SAC-13. Initial concentrations of MTBE and TBA were 50 and 40 mg/l, respectively. Nafion loading was 4 g/50 ml.
Author's personal copy
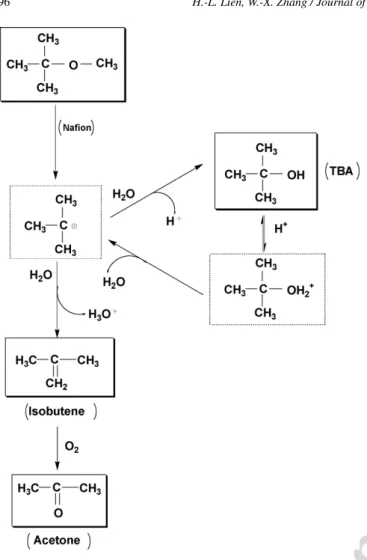
Fig. 2. A scheme of the reaction pathways for the transformation of MTBE withNafion SAC-13.
Yields on a molar basis of acetone and TBA after 100 h were about 6% and 12%, respectively. The overall MTBE removal efficiency was about 70%. The results for the transformation of TBA (40 mg/l) are shown inFig. 1b. Similar to the MTBE reaction, the disappearance of TBA occurred initially. About 46% of TBA was removed within 1 h; no further removal of TBA was observed after that time. Acetone was found as the only product accounting for 11% of the TBA lost.
Based upon the observed reaction products, a scheme of the MTBE transformational pathways is presented inFig. 2. The acid-catalyzed hydrolysis of MTBE takes place at the acidic surface of Nafion leading to form tert-butyl alcohol (TBA) and methanol:
(CH3)3COCH3+ H2O → (CH3)3COH + CH3OH (1)
Dehydrogenation of MTBE to isobutene and methanol is also thermodynamically favorable:
(CH3)3COCH3→ (CH3)2C CH2+ CH3OH (2)
Following the extensive work by Olah et al. [13,16], the above two reactions involve a common intermediate: a carboca-tion (tert-butyl carbonium ion). For example, protonated s-butyl methyl ether can be cleaved to protonated methanol and
tert-butyl cation[13,16]:
(3) Computational studies have also suggested that acid-catalyzed hydrolysis of MTBE proceeding via tert-butyl car-bonium ion formation is a likely reaction pathway for MTBE degradation[9].
The carbocation formed from MTBE is extremely unstable and undergoes rapid hydrolysis and dehydrogenation. As a mat-ter of fact, hydrolysis of carbocations by wamat-ter is a classical nucleophilic substitution reaction[17]. Dehydrogenation of car-bocations, on the other hand, can be termed as an elimination reaction. For MTBE transformation, hydrolysis of tert-butyl car-bonium ion yields TBA while the dehydrogenation leads to the formation of isobutene.
Subsequently, isobutene undergoes oxidation to acetone
[18,19]. A competition between the hydrolysis and dehydro-genation reactions in the transformation of MTBE is therefore expected.
Isobutene is the key intermediate to explain the observations and hypothesized reaction pathways. However, the quantifica-tion of isobutene was restricted by the presence of water. Even though a VOCARB 3000 trap column was installed in the Tekmar 3000 to remove excessive water, water vapor caused a large peak masking the peak of isobuene. In general, the coeluting peaks may still be identified or even quantified by GC/MS using selected ion mode (SIM) if their reference and indicator ions are different. We have positively identified the existence of isobutene (base peak of m/z 41) in the coeluting peak using SIM. However, for routinely quantitative analyses, the solvent cut time was set at 3 min to protect the mass spec-trometer that inevitably scarified the opportunity for isobutene analysis.
To overcome this limitation, tert-amyl methyl ether (TAME) was used as a probe molecule. The structural difference between MTBE and TAME is substitution of an ethyl group for a methyl group on the tertiary carbon. If isobutene is formed during MTBE transformation, then a-isoamylene should be produced during the TAME degradation:
(CH3)2(C2H5)COCH3→ (CH3)2C CH(CH3) + (CH3)OH
(4) Formation of tert-amyl alcohol (TAA) and acetone are also expected.
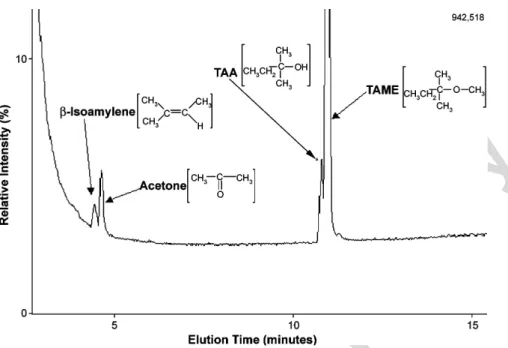
Fig. 3presents a GC/MS spectrum of TAME transformation (60 mg/l) with 4 g of the Nafion in a 50 ml aqueous solution under ambient conditions. TAA, acetone and-isoamylene were pos-itively identified. The appearance of-isoamylene from TAME supports the theory that the dehydrogenation reaction occurs in the transformation of MTBE. A scheme of transformation of
Author's personal copy
Fig. 3. A GC/MS chromatogram of TAME and products formed after a 12-h contact time.TAME over Nafion is shown inFig. 4and is consistent with that of MTBE as shown inFig. 2.
Production of acetone can be rationalized by the oxidation of isobutene[17,20]. The above-described batch experiments were
Fig. 4. A proposed reaction scheme for acid-catalyzed destruction of TAME over Nafion SAC-13.
conducted under ambient conditions under which the aqueous phase contained substantial dissolved oxygen and may act as the oxidant. To determine the role of oxygen, an experiment with oxygen-free solution was subsequently conducted (Fig. 5). The solution was first purged with N2gas for 60 min in a serum
bottle containing a 50 ml aqueous solution and 4 g of Nafion. MTBE (40 mg/l) was then injected after the purge. As compared toFig. 1a, only trace acetone (<1%) was produced as shown in
Fig. 5; however, TBA was found in a reactively large amount (22%). These results are consistent with the reaction scheme shown inFig. 2that the formation of acetone is via an oxida-tion reacoxida-tion and the oxygen is the oxidant. The accumulaoxida-tion of TBA suggests that the hydrolysis rather than the dehydro-genation of tert-butyl carbonium ion is the dominant pathway for MTBE degradation under anoxic conditions (Fig. 2). Fur-thermore, this study indicates that anoxic removal of MTBE by Nafion is an effective method to minimize the production of undesirable acetone.
Because Nafion SAC-13 has a large surface area (>200 m2/g) and high pore volume (>0.6 ml/g), it may be possible that the
Fig. 5. Transformation of MTBE with Nafion SAC-13 in a N2-purged solution. Initial concentrations of MTBE was 40 mg/l. Nafion loading was 4 g/50 ml.
Author's personal copy
Table 1Selected technologies for MTBE removal Technologies Specific (vendor/product
or materials)
Initial MTBE concentration (mg/l) Removal capacity (mg/g) or efficiency (%) Reaction byproducts Experimental conditions References GAC adsorption
Calgon/PCB 0.02 0.14–0.22 mg/g None Column test [23]
US filter/CC602 0.02 0.11–0.19 mg/g Column test [23]
Calgon/F400 0.02–0.05 0.46–0.69 mg/g Batch test [24]
Calgon/F600 0.02–0.05 0.56–1.43 mg/g Batch test [24]
AOPs
Fenton’s reagent 88.2 99% a, b, e, f Batch test [25]
O3/H2O2 0.012 >99% a, b Batch test [26]
UV/H2O2 81.1 >99% a–f Batch test [27]
Bifunctional Al0 14.2 90–99% a, b, e, f Batch test [28,29]
Nafion Nafion SAC-13 50 70%; sorption likely b, e, g Batch test This study
Byproducts: a, tert-butyl formate (TBF); b, tert-butyl alcohol (TBA); c, 2-methoxy-2-methyl propionaldehyde (MMP); d, formaldehyde; e, acetone; f, methyl acetate; g, isobutene.
sorption of MTBE and its reaction products occurred at the sur-face of Nafion SAC-13. The fast initial removal of MTBE and TBA followed by a slow transformation process showed an indi-rect evidence for the sorption of MTBE and TBA to Nafion (Fig. 1). In a heterogeneous system, removal of reactants essen-tially undergoes three stages: (1) mass transfer of reactants to the surface, (2) sorption or chemical reaction of reactants at the surface, and (3) desorption of reactants or products from the sur-face. For example, the reaction of MTBE at the Nafion surface has been confirmed at this stage; yet the products accounted for only 18% of the MTBE lost. A large unaccounted for fraction of MTBE lost (−50%) may be attributed to the sorption of MTBE and its products (e.g. TBA and acetone) at the Nafion surface. The detail sorption behavior that may involve either simple or competitive sorption between MTBE and its products is not clear now. However, it has been found that Nafion is capable of sorb-ing organic solvents such as methanol and propanol[21,22]. In addition, the presence of ether bonds in both Nafion and MTBE may favor the sorption and accumulation of MTBE over the Nafion surface.
This study offers a preliminary understanding of the unique properties of Nafion for MTBE removal. It may serve as a catalyst for MTBE degradation and a sorbent for MTBE sorp-tion. Many physicochemical processes for MTBE treatment are related to the sorption or chemical reactions such as granular activated carbon (GAC) adsorption and advanced oxidation pro-cesses (AOPs) [23–29].Table 1 compares these technologies with Nafion. With the dual functionality of Nafion, its appli-cation for MTBE-contaminated water treatment, however, still requires more work to conclude the feasibility. These include: (1) to determine the MTBE and TBA sorption capacity, (2) to under-stand the sorption behavior, (3) to evaluate the MTBE removal effectiveness at lower level (e.g. <0.1 mg/l), (4) to enhance the MTBE degradation, and (5) to obtain basic parameters for a reactor design by column tests.
4. Conclusions
Removal of MTBE over a superacidic organic polymer, Nafion was studied in batch experiments. MTBE was degraded to tert-butyl alcohol (TBA), acetone, isobutene and possibly
methanol. Transformation of TBA to acetone was also detected. Results suggest that possible transformation pathways may include hydrolysis, dehydrogenation and oxidation reactions. The hydrolysis reaction of MTBE leads to the formation of TBA while the dehydrogenation reaction of MTBE yields isobutene. The observation of -isoamylene from the TAME transfor-mation further supports the possibility for dehydrogenation of MTBE. Our test also demonstrates that dissolved oxygen is needed for the oxidation of isobutene to acetone. Because of its high surface area and pore volume, Nafion may have the sorption capacity of MTBE and its reaction products. Further investiga-tion is needed to fully understand the capacity for environmental applications of Nafion.
References
[1] U.S. Environmental Protection Agency, Technical information review, methyl tertiary-butyl ether (MTBE) (Case 1634-04-4), Office of Pollution Prevention and Toxics, Washington, DC, 1993.
[2] U.S. Environmental Protection Agency, Assessment of potential risks of gasoline oxygenated with methyl tertiary butyl ether (MTBE), report no. EPA/600/R-93/206, Office of Research and Development, Washington, DC, November 1993.
[3] U.S. Environmental Protection Agency, Oxygenates in water: critical infor-mation and research needs, EPA/600/R-98/048, Office of Research and Development, 1998.
[4] P.J. Squillace, J.S. Zogorski, W.G. Wilber, C.V. Price, Preliminary assess-ment of the occurrence and possible sources of MTBE in groundwater in the United States, 1993–1994, Environ. Sci. Technol. 30 (1996) 1721–1730. [5] G.C. Delzer, J.S. Zogorski, T.J. Lopes, R.L. Bosshart, Occurrence of the
gasoline oxygenate MTBE and BTEX compounds in urban stormwater in the United States, 1991–1995, U.S. Geological Survey, Water Resources Investigations Report 96-4145, 1996.
[6] Y.-J. An, D.H. Kampbell, M.L. Cook, Co-occurrence of MTBE and ben-zene, toluene, ethylbenben-zene, and xylene compounds at Marinas in large reservoir, J. Environ. Eng. 128 (2002) 902–906.
[7] J.E. Reuter, B.C. Allen, R.C. Richards, J.F. Pankow, C.R. Goldman, R.L. Scholl, J.S. Seyfriend, Concentration, sources, and fate of the gasoline oxygenate methyl tert-butyl ether (MTBE) in a multiple-use lake, Environ. Sci. Technol. 32 (1998) 3666–3672.
[8] R.T. Morrison, R.N. Boyd, Organic Chemistry, 6th ed., Prentice Hall, Englewood Cliffs, 1992.
[9] K.L. Bhat, W.H. Brendley Jr., C.W. Bock, Thermodynamics and kinetics of MTBE degradation: a density functional theory study, Soil Sediment Contam. 13 (2004) 267–281.
Author's personal copy
[10] F.J. Waller, R.W. Van Scoyoc, Catalysis with Nafion, Chemtech 17 (1987)438–441.
[11] M. Misono, T. Okuhara, Solid superacid catalysts, Chemtech 23 (1993) 23–29.
[12] Q. Sun, M.A. Harmer, W.E. Farneth, An extremely active solid acid cat-alyst, Nafion resin/silica composite, for the Friedel-Crafts benzylation of benzene and p-xylene with benzyl alcohol, Ind. Eng. Chem. Res. 36 (1997) 5541–5544.
[13] G.A. Olah, G.K.S. Prakash, J. Sommer, Superacids, John Wiley & Sons Inc., 1985.
[14] M.A. Harmer, W.E. Farneth, Q. Sun, High surface area Nafion resin/silica nanocomposites: new class of solid acid catalyst, J. Am. Chem. Soc. 118 (1996) 7708–7715.
[15] E. Hoffmann, V. Stroobant, Mass Spectrometry: Principles and Applica-tions, 2nd ed., John Wiley & Sons Inc., 2001.
[16] G.A. Olah, P.S. Lyer, G.K. Surya Prakash, Perfluorinated resinsul-fonic acid (Nafion-H) catalysis in synthesis, Synthesis (1986) 513– 531.
[17] M. Matouq, S. Goto, Kinetics of liquid phase synthesis of methyl tert-butyl ether from tert-butyl alcohol and methanol catalyzed by ion exchange resin, Int. J. Chem. Kinet. 25 (1993) 825–831.
[18] O.C. Feeley, Q. Sun, R.G. Herman, M. Johansson, L. Lietti, K. Klier, Selective isotopic oxygen incorporation into C5 and C6 ethers via solid acid-catalyzed reaction of methanol and ethanol with isobutanol, Catal. Lett. 35 (1995) 13–22.
[19] J.G. Nunan, K. Klier, R.G. Herman, Methanol and 2-methyl-1-propanol (isobutanol) coupling to ethers and dehydration over Nafion H: selectivity, kinetics, and mechanism, J. Catal. 139 (1993) 406–420.
[20] R.D. Barreto, K.A. Gray, K. Anders, Photocatalytic degradation of methyl-tert-butyl ether in TiO2slurries: a proposed reaction scheme, Water Res. 29 (1995) 1242–1248.
[21] L.-X. Sun, T. Okada, Studies on interactions between Nafion and organic vaporous by quartz crystal microbalance, J. Membr. Sci. 183 (2001) 213–221.
[22] A. Sungpet, Reduction of alcohol permeation through Nafion®by polypyr-role, J. Membr. Sci. 226 (2003) 131–134.
[23] T.C. Shih, M. Wangpaichitr, M. Suffet, Evaluation of granular activated carbon technology for the removal of methyl tertiary butyl ether (MTBE) from drinking water, Water Res. 37 (2003) 375–385.
[24] L. Yu, C. Adams, D. Ludlow, Adsorption isotherms for methyl tert-butyl ether and other fuel oxygenates on two bituminous-coal activated carbons, J. Environ. Eng. 131 (2005) 983–987.
[25] X.-R. Xu, Z.-Y. Zhao, X.-Y. Li, J.-D. Gu, Chemical oxidative degrada-tion of methyl tert-butyl ether in aqueous soludegrada-tion by Fenton’s reagent, Chemosphere 55 (2004) 73–79.
[26] C. Baus, F. Sacher, H.-J. Brauch, Efficiency of ozonation and AOP for methyl-tert-butylether (MTBE) removal in waterworks, Ozone Sci. Eng. 27 (2005) 27–35.
[27] M.I. Stefan, J. Mack, J.R. Bolton, Degradation pathways during the treat-ment of methyl tert-butyl ether by the UV/H2O2 process, Environ. Sci. Technol. 34 (2000) 650–658.
[28] H.-L. Lien, R. Wilkin, Reductive activation of dioxygen for degradation of methyl tert-butyl ether by bifunctional aluminum, Environ. Sci. Technol. 36 (2002) 4436–4440.
[29] H.-L. Lien, W. Zhang, Novel bifunctional aluminum for oxidation of MTBE and TAME, J. Environ. Eng. 128 (2002) 791–798.