J. CHEM. SOC. PERKIN TRANS. I 1994 603
Reactions of 1,3-Dithiolane 1,3-Dioxides with Nucleophiles
Wen-Chih Chou, Sheng-Ann Yang and Jim-Min Fang*Department of Chemistry, National Taiwan University, Taipei, Taiwan 1061 7, Republic of China
Reaction of benzenethiol with the 1,3-dithioIane 1,3-dioxides 3a-f gave the 1,4-dithiane 1 -oxides 4-5, the ct,P-unsaturated sulfoxides 6-9, the ring-opening products 10-1 1 and various reduction products. Addition of benzenethiol, malononitrile, diethyl malonate or 2-mercapto-4,5-dihydrothiazole to the double bonds of the 2-alkenyl-l,3-dithiolane 1,3-dioxides 3g-h was performed with methylmagnesium chloride in methanol. Addition of methanol or an ally1 group to the dioxide 3h occurred regioselectively to give the 1.3-dithiolane 1 -oxide 19 or the 1,3-dithioIane 20.
Sulfoxides are versatile reagents in organic synthesis,' being used as acyl nucleophile equivalents, in dehydrosulfinylation to give alkenes, in the Pummerer rearrangement to give carbonyl compounds and in [2,3] sigmatropic rearrangements to give allylic alcohols. Sulfoxides are intrinsically chiral reagents and their use in asymmetric control is well documented.' In contrast, there has been little work reported with dis~lfoxides,~ although compounds such as the trans-l,3-dithiolane 1,3- dioxides, which have C,-symmetry, are potential auxiliaries for enantioselective reactions. For example, Aggarwal and co- workers have shown both stereoselective addition reactions of trans- 1,3-dithiane 1,3-dioxides with aromatic aldehydes and Diels-Alder reactions of trans-2-methylene-l,3-benzodithiole
1,3-dioxide with dienes. Khiar and his co-workers have demonstrated the use of C,-symmetric disulfoxides as chiral ligands in asymmetric Diels-Alder reactions. We report herein the addition and ring-expansion of 1,3-dithiolane 1,3-dioxides 3a-h in the presence of benzenethiol and other nucleophiles.
?-
0-0 - 0 -
1 a-h 2a-h 3a-h 3b
*-
Results and Discussion
Preparation of Disulfoxides 3a-h.-Alt hough 1,3-dithiolanes can be oxidized directly to their corresponding 1,3-dioxides by use of 2 equiv. of Na104,4 we carried out this transformation in two successive oxidation steps, as shown in Table 1. This method provided an ambiguous assignment of the stereostruc- tures of the disulfoxides 3a-h. Oxidation of the 1,3-dithiolanes la-h with 1 equiv. of NaIO, gave the corresponding mono- sulfoxdes 2a-h in favour of the trans-isomers.' The dithiolane Id which had 2-methyl and 2-ethoxycarbonyl substituents afforded only the trans-isomer 2d, where the sulfinyl oxygen and ethoxycarbonyl group were on opposite faces of the molecule.' In the 'H NMR spectra of compounds 2, the 2-H or 2-Me resonances of the trans-isomers usually occurred at lower field than those of the ~is-isomers.~ The monosulfoxides 2a-h were then oxidized with 1 equiv. of m-chloroperbenzoic acid (m- CPBA)8 to give the corresponding disulfoxides 3a-h. The presence of C==C double bonds did not interfere with the oxidation process. Since the trans- and cis-isomers of 2b were both converted into the disulfoxide 3b, the product must have the trans-configuration. Similarly, the disulfoxides 3d-h must also have the trans-configuration. The disulfoxides 3a and 3c exist as a mixture of two isomers, where the cis-isomers have the two oxygen atoms and the methyl group on the same face to
Table 1
via the monosulfoxides 2a-h
Conversion of the dithiolanes la-h to the disulfoxides 3a-h
Monosulfoxide" Disulfoxide (yield %; ratio of
trans : cis isomers)
(yield %; ratio of trans : cis isomers) Dithiolane R', R2 ~~ ~ l a Ph, Me l b CH=CHCMe,CH2CH2 l c MeCCH,],, Me Id EtO,C, Me l e MeCH,CH,, H If PhCH=CH, Me l g PhCH=CH, H lh MeCH=CH, H 2a (66; 84 : 16) 2b (54; 59: 41) 2c (76; 66 : 34) 2d (42; 1OO:O) 2e (37; 72 : 28) 2f (24; 63 : 27) 2g (86; 75 : 25) 2h (80; 75 : 25) 3a (64; 25 : 75) 3b (80; 1OO:O) 3c (87; 80: 20)' 3d (84; 1OO:O) 3e (44; 100 : 0) 3f (60; 100 : 0) 3g (55; 1OO:O) 3h (100; 1OO:O)
a Oxidant, NaIO, (1 equiv.). Oxidant rn-CPBA (1 equiv.). ' The cis-
isomer of 3a (or 3c) had the two oxygen atoms and the methyl group on the same face.
form a C,-symmetric molecule, as inferred by the coalescence of the C-4 and C-5 resonances. These results indicate that oxidation of the 1,3-dithiolane 1-oxides 2b and 2d-h occurred exclusively on the face opposite to the sulfinyl oxygen, whereas oxidation of the 1,3-dithiolane l-oxides 2a and 2c were less selective, owing to the steric effect of the phenyl or the pentyl group.
Thermal Reactions of the Disulfoxides 3a+ in the Presence
of Benzenethiol-A methanolic solution of dioxide 3a and benzenethiol(1 equiv.) was heated at reflux to give the dithiane monooxide 4 in 72%, as a mixture of the trans- and cis-isomers (86: 14). The 2-H and C-2 resonances in the trans isomer of 4 appeared at low field (6 3.97 and 68.7), compared to the signals for the cis isomer (6 3.85 and 61.3). The C-5 resonance occurs at 6 25.5 for the trans isomer, indicating an equatorial sulfinyl oxygen, whereas it resonates at 6 16.1 for the cis isomer, indicating an axial configuration. Thermal ring expansion of the 2-methyl-l,3-dithiolane 1-oxide l o has been
shown to involve an H-shift from the methyl group to the sulfinyl oxygen, accompanied by a concurrent ring opening to give an intermediate vinylthioethylsulfenic acid. The product 5,6-dihydro- 1,4-dithiine is subsequently obtained by a ring- closure process presumably uia the sulfur-stabilized carbonium ion intermediate. Accordingly, ring-expansion of the dioxide 3a to compound 4 was probably initiated by formation of the sulfenic acid intermediate A shown in Scheme 1. However, the intermediate A containing an electron deficient C==C double bond should act differently from the electrophilic vinyl sulfide species described above. The sulfenic acid A might undergo an intramolecular Michael addition l 1 to give a disulfoxide B, in which the less hindered sulfoxide group is selectively reduced.
604 J. CHEM. SOC. PERKIN TRANS. 1 1994 3a A
1
Ph\/S\ 0 -phfj
t 0 - B?I
1
PhvS\
B 4Scheme 1 Reagents and conditions: PhSH, MeOH, 70 O C , 36 h (72%)
5 6 Y = H 7 Y=SPh 8 9 Y = H Y=SPh Me SY +S
-d
'SPh C D 10 Y = H 11 Y=SPhAlternatively, reduction of the sulfenic acid A to the thiol B', followed by an intramolecular Michael addition would lead to the same product. The 1,3-dithiolane 1,3-dioxide 3b, generated from 4,4-dimethylcyclohex-2-enone, underwent a similar ring expansion to afford the 1,4-dithiane 1-oxide 5 (73%). Since the ring-junction protons (1-H and 6-H) in compound 5 showed a small coupling constant (3.2 Hz), this bicyclic compound was determined to have a cis fused ring-junction and the resonance of C-4 at 6 21.7 was taken to indicate an equatorial sulfinyl oxygen.' The reaction of 2-methyl-2-pentyl-l,3-dithiolane 1,3- dioxide 3c under similar conditions gave the a, P-unsaturated sulfoxides 6 and 8 accompanied by a significant amount of the disulfide 7. This reaction was considered to involve the sulfenic acid intermediates generated from deprotonation of either the methyl or the pentyl group, similar to that shown in Scheme 1. Subsequent disproportionation of these sulfenic acid inter- mediates with benzenethiol would lead to the observed products. From the distribution of products, (6
+
7) : 8 = 7: 1, deprotonation of the methyl group appeared to be the more favoured process. In order to promote the ring closure of compounds 6 and 8, the reaction temperature was raised to 110°C for a prolonged period. However, such conditions yielded mainly the disulfides 7 and 9, but no cyclization product. A 16% nuclear Overhauser enhancement of the vinyl proton(S
6.18) in the sulfoxide 8 was observed by irradiation of the adjacent methyl group (6 1.83), indicating the Z-configur- ation for compound 8. Due to the deshielding effect of the sulfoxide group, the vinyl proton on the P-carbon of E-isomer usually resonates at lower field (about S 6.4) than that of the 2-isomer. The configuration of 9 was assigned as 2-, due to the vinyl resonance at 6 6.12 which was close to the valueobserved for compound 8. The stereochemical outcome was in agreement with a concerted H-shift as shown in C.
Treatment of the dithiolane dioxide 3d with benzenethiol in refluxing methanol gave the thiol 10 and the corresponding phenyl disulfide 11. These products were characterised by characteristic methyl doublets in the 'H NMR spectra. The reaction sequence was presumably initiated by direct attack
'
of benzenethiol on one of the sulfoxide groups to give the intermediate anion D, stabilized by the other adjacent ester and the other sulfoxide group.2-Propyl-1,3-dithiolane 1,3-dioxide 3e was heated to 110 "C in a sealed tube in the presence of benzenethiol to give the partially reduced product 2e. Partial deoxygenation of the disulfoxide 3f gave the monosulfoxide 2f on treatment with lithium ethanethiolate (1 equiv.) and complete deoxygenation gave the dithiolane If on treatment with lithium dimethyl- cuprate (2 equiv.). These results were consistent with a previous report on the reduction of vinyl sulfoxides with EtMgBr-CuI.12
Addition to the
G==
Double Bond of the Disulfoxides 3g and3h.-Ally1 sulfoxides are known to undergo [2,3] sigmatropic rearrangements to form intermediates, which can be reduced with benzenethiol to give ally1 alcohol^.'^ However, it was noted that benzenethiol adds to the C=C double bond of 2- styryl-l,3-dithiolane 1,3-dioxide 3g in MeOH upon mediation with a Grignard reagent to give the disulfoxide 12 in 91% yield. Use of malonotrile as the nucleophile gave the addition product 13 as a diastereoisomeric mixture (67 : 33). Similar reactions of 2-prop-1 -enyl-l,3-dithiolane 1,3-dioxide 3h with the nucleo- philes benzenethiol, malononitrile, diethyl malonate and 2- mercapto-4,5-dihydrothiazole in the presence of MeMgCl gave the addition products 14, 15, 16 and 17 respectively. In one case, addition of malononitrile to the disulfoxide 3h was mediated by Al,03.'
6-
12 R = S P h , Y = H [2H]-12 R = SPh, Y = D 13 R = CH(CN)2, Y = H b6 -
18 0 - 14 R = S P h , Y = H [*H]-14 R = SPh, Y = D 15 R = CH(CN)2. Y = H [2H]-15 R = CH(CN)2, Y = D 16 R = CH(COZEt)z, Y = H 17 R=S*N) Y = H S 0 -d?
18aTwo possible mechanisms were considered to account for the observed additions (Scheme 2). Path a evoked direct addition of the nucleophile to the C==C double bond; the intermediate might be stabilised by coordination to the sulfinyl oxygen as shown in
E. Path b involved a primary isomerisation of compound 3g (or 3h) to the conjugated disulfoxide F, which then underwent a Michael-type reaction l 6 with the nucleophile to give the
observed product. Path a seemed less likely since the addition of EtSH to 2-methyl-2-styryl-l,3-dithiolane 1,3-dioxide 3f, con- taining no hydrogen atom at C-2 failed (Table 2, entry 7). We thought the addition performed in MeOD would afford doubly deuteriated products G via path b. To our surprise, the monodeuteriated addition product C2H]-12 was obtained from
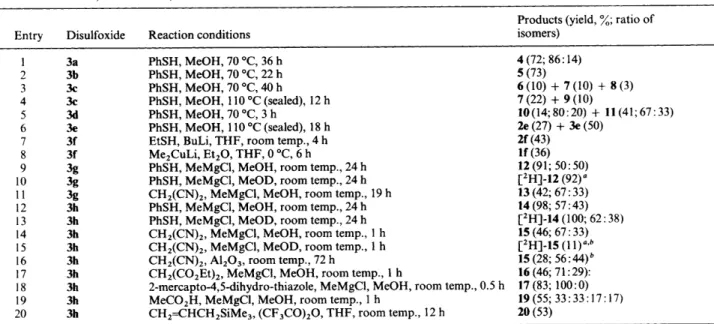
J. CHEM. SOC. PERKIN TRANS. 1 1994 605 Table 2 Reactions of 1,3-dithiolane 1,3-dioxides 3a-h
~~~~ ~
Products (yield, %; ratio of
Entry Disulfoxide Reaction conditions isomers)
1 3a PhSH, MeOH, 70 "C, 36 h 4 (72; 86: 14)
2 3b PhSH, MeOH, 70 "C, 22 h 5 (73)
3 3c PhSH, MeOH, 70 "C, 40 h 6(10)
+
7(10)+
8(3)4 3c PhSH, MeOH, 110 "C (sealed), 12 h 7(22)
+
9(10)6 3e PhSH, MeOH, 110 "C (sealed), 18 h 2e(27)
+
3e(50)7 3f EtSH, BuLi, THF, room temp., 4 h 2i (43)
12 (91; 50: 50)
9 3g
13 (42; 67 : 33)
10 3g
1 1 3g CH,(CN),, MeMgC1, MeOH, room temp., 19 h
12 3h PhSH, MeMgCl, MeOH, room temp., 24 h 14 (98; 57 : 43)
13 3h PhSH, MeMgC1, MeOD, room temp., 24 h ['HI-14 (1 00; 62 : 38)
14 3h CH,(CN),, MeMgCI, MeOH, room temp., 1 h 15 (46; 67 : 33)
16 3h CH,(CN),, AI,O,, room temp., 72 h 15 (28; 56 : 44)
17 3h CH2(C02Et),, MeMgC1, MeOH, room temp., 1 h 16 (46; 71 : 29):
18 3h 2-mercapto-4,5-dihydro-thiazole, MeMgC1, MeOH, room temp., 0.5 h 17 (83; 100 : 0)
19 3h MeCO'H, MeMgCI, MeOH, room temp., 1 h
5 3d PhSH, MeOH, 70 "C, 3 h 10 (14; 80 : 20)
+
11 (41; 67 : 33)8 3f Me'CuLi, Et,O, THF, 0 "C, 6 h If (36)
PhSH, MeMgC1, MeOH, room temp., 24 h
PhSH, MeMgC1, MeOD, room temp., 24 h ['HI-12 (92)"
15 3h CH,(CN),, MeMgC1, MeOD, room temp., 1 h ['H]-15 (1
19 (55; 33 : 33 : 1 7 : 1 7)
20 3h CH,=CHCH,SiMe,, (CF,CO),O, THF, room temp., 12 h 20 (53)
" Ratio of isomers was not determined. Starting material 3h was recovered.
0 - I
j
H $+ path a 3g R = Ph 3h R = M e path b\
0 - F E ['HF12 R = Ph ['H]-12 R = M e iii/
RA5
D D s+ I 0 -1
GScheme 2 Reagents and conditions: PhSH, MeMgC1, MeOD, room temp. 24 h. Path a: direct addition. Path b: i, isomerisation; ii, Michael addition; iii, work-up with aq. NH,Cl.
the reaction of the disulfoxide 3g with benzenethiolate ion in MeOD. Similarly, the monodeuteriated product ['HI-14 or C2H]-15 was also obtained from the reaction of the sulfoxide 3h with either benzenethiol or malononitrile with the Grignard MeMgCl in MeOD. Since there was no deuterium atom incorporated at C-2 in the final product, we assumed a rapid exchange of the C,-D, even in the presence of aqueous NH4Cl. From l i t e r a t ~ r e , ~ the acidity of 2-H in 1,3-dithiane 1,3- dioxide has been measured to have a p K , value of 25.0. To support our assumption, the addition of the conjugated disulfoxide 18 with PhSMgCl in MeOD, followed by work-up with aqueous NH,Cl, gave the adduct 14 containing no deuterium atom.
Treatment of 2-prop- 1 -enyl- 1,3-dithiolane 1,3-dioxide 3h with chloromagnesium acetate in MeOH gave 2-(2-methoxy- propy1idene)- 1,3-dithiolane 1 -oxide 19 as a mixture of four diastereoisomers ( a : b : c : d = 33:33:17:17). In the 'H NMR spectrum, the vinyl resonances of the major 2-isomers (a
+
b) occurred at 6 5.94 and 5.99, respectively, whereas the minorE-isomers (c and d) resonated at lower field (6 6.40 and 6.44 respectively). The reaction presumably proceeded with an intermediate a,P-unsaturated dithiolanium ion H (Scheme 3) derived from a Pummerer rearrangement similar to that proposed for the reactions of sulfoxides with Grignard reagents or allyltrimethylsilane.
'
Efficient trapping with methanol of the intermediate H, which presumably adopts the favourable trans-conformation at C(l')-C(2), leads to the formation of the 2-isomers. Similarly, the reaction of the dioxide 3h with allyltrimethylsilane in the presence of trifluoroacetic anhydride, gave the diallylated product 20. In both reactions, the nucleophile added selectively at the y-carbon (C-2') of the a,P- unsaturated dithiolanium intermediate. The second ally1 group was introduced by a subsequent Michael-type reaction to give the intermediate I, which then underwent isomerisation to furnish the final product 20.Experimental
General information concerning instrumentation and materials has been described previously.'* J Values are given in Hz.
n- r 1
3
'
6 -
I
L -II
3h H 19 R=MeO 20a R = CH2=CHCH2 1 -I 20 1Scheme 3 Reagents and conditions: MeCO'H, MeMgCI, MeOH, room temp., 1 h, giving 19 (5573, or CH,=CHCH,SiMe,, (CF,CO,),O, THF, room temp., 12 h, giving 20 (53%)
606 J. CHEM. SOC. PERKIN TRANS. 1 1994
1,3-Dithiolanes l a 4 were prepared from the corresponding carbonyl compounds by literature methods.
'
General Procedure for Oxidation of the Dithiolanes la-h to the Disulfoxides 3a-h via the MonosuIfoxides 2a-h.-A solution of 2-methyl-2-phenyl-l,3-dithiolane l a (1.26 g, 6.1 mmol) in MeOH (70 cm3) was stirred with aqueous NaIO, (1.3 g, 6.1 mmol in 15 cm3 water) at 0 "C for 12 h. The yellow solid was filtered off and the filtrate was concentrated and then extracted with EtOAc (3 x 30 cm3). The extracts were combined, washed with brine (3 x 30 cm3), dried (Na,SO,) and filtered. The filtrate was concentrated and chromatographed on silica gel with EtOAc-hexane (1 : 1) to give the monosulfoxide 2a (0.85 g, 66%) as a mixture of the trans- and cis-isomers (84:16). The monosulfoxide 2a in CH,Cl, (40 cm3) was treated with a solution of m-CPBA (0.68 g, 4 mmol) in CH,Cl, (10 cm3) at 0 "C for 2 h. The white solid was filtered off and the filtrate washed with aqueous NaOH (20%), dried (Na2S04), concen- trated and the residue chromatographed on silica gel with EtOAc to give the disulfoxide 3a (0.58 g, 64%) as a white solid. This was obtained as a mixture of the trans- and cis- isomers (25:75), when the cis-sulfoxides are also cis to the methyl group.
2-Methyl-2-phenyl-l,3-dithiolane 1 -oxide 2a. A mixture of trans- and cis-isomers (66%; 84: 16); oil: [ R , 0.49, hexane- EtOAc (1:4)]; m/z 212 (M', 78%), 136 (68) and 121 (100); 6,(CDC13) 1.92 (s, Me cis), 1.98 (s, Me trans), 2.63-2.79 (1 H, m), 3.02-3.14 (1 H, m, dithiolane ring H), 3.21-3.33 (1 H, m), 3.61-3.75 (1 H, m), 7.21-7.38 (3 H, m) and 7.58-7.64 (2 H, m); Gc(CDC1,) 23.0 (4, Me trans)/25.0 (4, Me cis), 31.9 (t, C-4), 52.6 (t, C-5 trans)/52.0 (t), 82.9 (s, C-2 trans)/84.0 (s), 127.35 (d, 2 C), 128.1 (s), 128.4 (d) and 128.4 (d, 2 C) (Found: M + , 212.0313. Calc. for CloH,,0S2: M, 212.0330).
8,s-Dimethyl- 1,4-dithiaspiro[4.5]dec-6-ene 1 -oxide 2b. Two diastereoisomers (54%; 59:41) were separated by HPLC on a p-Porsasil column with EtOAc as eluent. Major isomer: oil; HPLC(Et0Ac) t, 7.7 min; v,,,(neat)/cm-' 1054; m/z 216 (M', H, s), 1.68-1.76 (2 H, m), 1.94-2.08 (1 H, m), 2.44-2.54 (1 H, m), 3.10-3.22 (1 H, m), 3.28-3.41 (2 H, m), 3.62-3.76 (1 H, m), 5.27 (1 H, d, J 9.9) and 5.69 (I H, d, J 9.9); 6,(CDC13) 24.3 (t), 28.5 (q), 29.3 (l), 30.3 (t), 31.7 (s), 35.5 (t), 52.8 (t), 77.0 (s), 123.3 (d) and 143.5 (d) (Found: M f , 216.0636. Calc. for CloHl,OS,: M, 216.0642). Minor isomer: white solid, m.p. 90-91 "C; HPLC (EtOAc) t , 9.5 min; v,,,(KBr)/cm-' 1054; m/z 216 (M', 7579, 199 (42) and 140 (100); 6,(CDC13) 1.03 (3 H, s), 1.04 (3 H, s), 1.64-2.05 (4 H, m), 3.05-3.21 (1 H, m), 3.33-3.49 (2 H, m), 3.80-3.94 (1 H, m), 5.73 (1 H, d, J 10.0, H-b) and 5.88 (1 H, d, J 10.0); 6,(CDC13) 28.3 (q), 29.1 (q), 31.35 (s), 31.65 (t), 32.7 (t), 34.3 (t), 54.8 (t), 76.4 (s), 119.5 (d) and 144.0 (d) (Found: M', 216.0642. Calc. for CloH,,OS,: M , 216.0642).
2-Methyl-2-pentyl- 1,3-dithiolane 1 -oxide 2c. A mixture of trans- and cis-isomers (76%; 66: 34); oil; TLC (hexane-EtOAc, 1 : 4) R, 0.34; v,,,(neat)/cm-' 1449 and 1052; m/z 206 (M
'
,76%) and 55 (100); G,(CDCl,) 0.80 (3 H, t, J 6.9), 1.20-1.29 (4 H, m), 1.40 (s, Me cis), 1.55 (s, Me trans), 1.35-1.59 and 1.70- 1.95 (4 H, m), 3.07-3.29 (3 H, m) and 3.45-3.64 (1 H, m); 6,(CDC13) 13.75 (q), 19.0 (4, C-1" truns)/24.3 (q), 22.1 (t)/22.2 (t), 24.95 (t)/25.95 (t), 29.6 (t)/30.9 (t), 31.7 (t)/31.8 (t), 38.8 (t)/34.3 (t), 54.1 (t)/53.6 (t) and 75.6 (s)/77.1 (s) (Found: M', 206.081 1. Calc. for CgHl80S2: M, 206.0799).2-Ethoxycarbonyl-2-methyl- 1,3-dithiolane 1 -oxide 2d. trans- Isomer: oil (42%); TLC (EtOAc) R , 0.49; v,,,(neat)/cm-' 1722, 1251 and 1052; m/z 209 (M'
+
1, 41%) and 59 (100); 3.55-3.66 (1 H, m), 3.79-3.87 (1 H, m) and 4.22 (2 H, q, J7.1); G,(CDCl,) 13.9 (q), 17.6 (q), 34.6 (t), 53.3 (t), 62.6 (t), 79.5 (s) 7973, 199 (38) and 140 (100); &(CDCl,) 1.04 (3 H, s), 1.05 (3&(CDCl3) 1.31 (3 H, t, J7.1), 1.80 (3 H, s), 3.39-3.46 (2 H, m),
and 170.4 (s) (Found: M + , 208.0230. Calc. for C7H1203S2, M, 208.0228).
2-Propyl- 1,3-dithiolane 1 -oxide 2e. A mixture of trans- and cis-isomers (37%; 72 : 28): oil; TLC (hexane-EtOAc, 1 :4) R, 0.20; v,,,(neat)/cm-' 1458 and 1039; m/z 165 (M'
+
1, 100%) and 2.18 (4 H, m), 2.66-2.81 (1 H, m), 3.19-3.34 (2 H, m), 3.48-3.60 (1 H, m) and 4.02 (t, J 7.3, 2-H trans)/3.98 (t, J 7.3); Gc(CDC13) 13.3 (9)/13.6(@,21.2(t)/22.2(t), 31.0(t)/29.7(t), 34.9(t)/ 30.7 (t), 54.3 (t)/55.7 (t) and 73.1 (d)/68.3 (d) (Found: M', 164.0333. Calc. for C,Hl,0S2: M, 164.0330).2-Methyl-2-(2-phenylvinyl)-l,3-dithiolane 1-oxide 2f. A mix- ture of trans- and cis-isomer (24%; 63 : 27): TLC (EtOAc-hexane, 1 : 1) R, 0.2; v,,,(KBr)/cm-' 2967, 1598, 1445, 1100 and 1053; m/z 238 (M+, 73%) and 161 (100); 6,(CDC13) 1.87 (s, Me trans)/1.78 (s), 3.14-3.86 (4 H, m), 6.24 (1 H, d, J 16)/6.53 (d, J 16), 6.88 (1 H, d, J 15.6)/6.85 (d, J 16) and 7.29-7.48 ( 5 H, m); 79.2 (s)/74.1 (s), 126.6 (d, 2 C)/127.6 (d), 126.7 (d)/124.1 (d), 128.3 (d), 128.5 (d, 2 C), 134.8 (d)/133.4 (d) and 135.2 (s)/135.6 (s) (Found: M', 238.0480. Calc. for Cl2H1,OS,: M, 238.0486).
2-(2-Phenylvinyl)- 1,3-dithiolane 1 -oxide 2g. A mixture of trans- and cis-isomers (86%; 75 : 25): oily solid, TLC (EtOAc- hexane, 1:1) R, 0.16; v,,,(neat)/cm-' 2931, 1635, 1446, 1309 and 1049; m/z 224 (M+, 11%) and 115 (100); d~(cDC13) 2.84- 3.95(4H,m),5.03(d, J8,2-H)/4.96(d, J9),6.05(dd, J14and8, 1-H)/6.33 (dd, J 16 and 9), 6.84 (d, J 14,2'-H)/6.98 (d, J 16) and 7.29-7.46 (5 H, m); &(CDCl,) 31.9 (t, C-4)/32.2 (t), 53.7 (t, 126.8 (d)/126.9 (d), 128.5 (d), 128.6 (d), 128.7 (d), 135.5 (s)/135.6 (s) and 136.4(d, C-2')/136.7 (d) (Found: M', 224.0323. Calc. for C1 lH1,OS,: M , 224.0339).
2-Prop- 1 -enyl- 1,3-dithiolane 1 -oxide 2h. trans-Isomer: colour- less crystals (80%), m.p. 108-109 "C, HPLC (EtOAc-hexane, 4: 1) t, 7.2 min; v,,,(KBr)/cm-' 2993, 1655, 1035 and 926; m/z (M', 9%), 147 ( 5 ) , 129 (8) and 108 (100); 6,(CDC13) 1.70 (3 H, d, J 6 , Me), 2.68-3.02 (4 H, m), 4.75 (d, J9,2-H), 5.32 (dd, J 15 and 9, 1'-H) and 6.04 (dq, J 15 and 6, 2'-H) (Found: M + , 162.0182. Calc. for C,HloOS,: M , 162.0173). Cis-isomer: liquid, HPLC (EtOAc-hexane, 4: 1) t, 8.4 min; v,,,(neat)/m-' 2912, 1656, 1423, 1036 and 926; G,(CDCl,) 1.75 (d, J 6, Me), 2.80-3.90 (4 H, m), 4.70 (d, J 9, H-2), 5.50 (dd, J 15 and 9, 1 '-H) and 5.90 (dq, J 6 and 15,2'-H).
2-Methyl-2-phenyl- 1,3-dithiolane 1,3-dioxide 3a. A mixture of trans- and cis-isomers (64%; 25 : 75) inseparable by chromato- graphy, m.p. 1 1 6 1 18 "C; TLC (MeOH-EtOAc, 1 : 9) R, 0.25; v,,,(KBr)/cm-' 1591, 1057 and 694; m/z 229 (M'
+
1, 3%), 108 (92) and 84 (loo); dH(CDC1,) 1.77 (s, Me truns)/l.95 (s, Me cis), 3.463.72 (4 H, m) and 7.16-7.38 (5 H, m); 6,- (CDC1,) 13.8 (q)/15.8 (q), 50.05 (t, C-4 and C-5, cis), 50.9 (t, C-5, trans), 53.9 (t, C-4, trans), 86.3 (s, (2-2, cis), 91.1 (s, C-2, trans), 126.9 (d), 128.0 (d), 128.6 (d), 129.0 (d), 129.35 (d) and 135.35 (s), (Found: M', 228.0270. Calc. for CloHl,0,S2: M ,228.0279).
8,8-Dimethyl- 1,4-dithiaspiro[4.5]dec-6-ene 1,4-dioxide 3b. White solid (80%), m.p. 131-132 "C; TLC (EtOAc) R , 0.25; v,,,(KBr)/cm-' 1043; m/z 232 (M+, 5%) and 125 (100);
m), 1.81-1.95 (1 H, m), 2.28-2.42 (1 H, m), 3.55-3.82 (4 H, m), 5.43 (1 H, d, J 10.1) and 6.09 (1 H, d, J 10.1); G,(CDCl,) 20.6 (t), 28.4 (q), 28.7 (q), 31.6 (s), 34.8 (t), 50.9 (t), 51.9 (t), 88.5 (s), 114.2 (d) and 146.8 (d) (Found: M', 232.0597. Calc. for C10H1602S2: M, 232.0592).
2-Methyl-2-pentyl- 1,3-dithiolane 1,3-dioxide 3c. A mixture of trans- and cis-isomers (87%; 80:20) were separated by chromatography on silica gel (MeOH-EtOAc, 1 : 9). trans- Isomer: white solid, m.p. 56-57 "C; TLC (MeOH-EtOAc, 1 : 9) 164 (M', 97); d~(cDC13) 0.85 (t, J 7.3)/0.89 (t, J 7.3), 1.34-
Gc(CDC13) 14.0 (q)/23.5 (q), 32.0 (t)/29.0 (t), 53.2 (t)/52.9 (t),
C-5)/56.4 (t), 75.2 (d, C-2)/70.1 (d), 121.5 (d, C-1')/119.0 (d),
J. CHEM. SOC. PERKIN TRANS. 1 1994 607 R, 0.30; v,,,(KBr)/cm-' 1460 and 1035; m / z 223 (M+
+
1,27%) and 108 (100); G,(CDCl,) 0.87 (3 H, t, J 629, 1.22-1.40 (4 H, m), 1 .3 8 (3 H, s), 1.46-2
.OO
(4 H, m) and 3.44-3.8 1 (4 H, m); G,(CDCl,) 13.8 (q), 13.9 (q), 22.3 (t), 25.3 (t), 30.75 (t), 31.9 (t), 49.95 (t), 51.0 (t) and 90.0 (s) (Found: M + , 222.0766. Calc. for C,H,@,S,: M, 222.0748). cis-Isomer: white solid, m.p. 60- 61 "C; TLC (MeOH-EtOAc, 1 : 9) R, 0.24; v,,,(KBr)/cm-' 1438 and 1035; m/z 223 (M'+
1, 15%), 206 (40) and 108 (100); 1.40-1.53 (4 H, m) and 3.34-3.64 (4 H, m); G,(CDCl,) 11.3 (q), 13.6 (q), 22.0 (t), 24.3 (t), 31.6 (t), 34.0 (t), 48.6 (t, 2 C) and 82.7 (s) (Found: M + , 222.0738. Calc. for C,H,,O,S,: M , 222.0748).2-Ethoxycarbonyl-2-methyl- 1,3-dithiolane 1,3-dioxide 3d. White solid (84%), m.p. 91-92OC; TLC (EtOAc) Rf 0.27; v,,,(KBr)/cm-' 1733, 1246 and 1045; m/z 225 (M'
+
1, s), 3.63-3.84 (3 H, m), 3.994.16 (1 H, m) and 4.29 (2 H, q, J7.2); and 163.9 (s) (Found: M + , 224.0176. Calc. for C7H1204S2: M, 224.0 177).2-Propyl-l,3-dithiolane 1,3-dioxide&. White solid (44%), m.p. 95-96 "C; TLC (MeOH-EtOAc, 1 : 9) R, 0.23; v,,,(KBr)/cm-' Me), 1.73 (2 H, sexet, J 7.2), 1.94-2.06 (2 H, m), 3.57-3.80 (4 H, m) and 3.84 (1 H, d, J 7); Gc(CDCl,) 13.7 (t), 22.0 (t), 25.4 (t), 50.7 (t), 51.8 (t) and 91.0 (d) (Found: M + , 180.0274. Calc. for
2-Methyl-2-(2-phenylvinyl)- 1,3-dithiolane 1,3-dioxide 3f. White solid (60%), m.p. 109-1 10 "C; TLC (EtOAc) Rf 0.17; v,,,(KBr)/cm-' 1636,1492,1400,1089 and 1039; m/z 254 (M', 7%) and 129 (100); G,(CDCI,) 1.67 (3 H, s), 3.46-3.86 (4 H, m), 6.35 (d, J 16, 1'-H), 6.75 (d, J 16,2'-H) and 7.30-7.43 (5 H, m); Gc(CDC1,) 13.8 (9, 2 C), 50.5 (t), 51.3 (t), 88.2 (s), 118.9 (d), 126.3(d,2C), 128.1 (d,2C), 128.2(d), 134.8(d)and 134.9(s) (Found: M +, 254.0436. Calc. for C12H,,02S,: M, 254.0435).
2-(2-Phenylvinyl)-l,3-dithiolane 1,3-dioxide 3g. White crystal (5579, m.p. 155-157 "C (from EtOAc); TLC (EtOAc) Rf 0.17; v,,,(KBr)/cm-' 2927, 1662, 1389, 1102 and 1031; m / z 240 (d, J 10, 2-H), 6.15 (dd, J 16 and 10, 1'-H), 6.98 (d, J 16, 2'-H) and 7.30-7.48 (5 H, m); Gc(CDCl,) 52.0 (t), 52.7 (t), 93.1 (d), 112.5 (d), 127.1 (d, 2 C), 128.8 (d, 2 C), 129.2 (d), 135.1 (s) and 140.2 (d) (Found: C, 68.9; H, 5.0; S, 18.5. Calc. for C,,H1202S2: C, 68.84; H, 4.95; S, 18.50%).
2-Propenyl-l,3-dithiolane 173-dioxide 3h. Oil (loo%), TLC (MeOH-EtOAc, 1 : 9) R, 0.25; v,,,(KBr)/cm-' 2967, 1652, 1395, 1085 and 1027; m/z 179
(M+
+
1, 1%) and 108 (100); GH(CDCI,) 1.85 (d, J 6, Me), 3.50-3.86 (4 H, m), 4.50 (d, J 9, 2-H), 5.44 (dd, J 15 and 9, 1'-H) and 6.10 (1 H, dq, J 15 and 6, 2'-H) (Found: M + , 178.0119. Calc. for C6Hl00,S,: M, 178.0122). &(CDCI,)0.80(3H,t, J6.7), 1.17-1.28(4H,m), 1.51 ( ~ H , s ) , 100%) and 21 1 (43); &(CDCI,) 1.32 (3 H, t, J 7.2), 1.75 (3 H, Gc(CDCI3) 12.7 (q), 13.9 (q), 51.6 (t), 53.3 (t), 63.1 (t), 94.1 (s) 1455 and 1021; m / z 180 (M+, 100%); dH(CDCI3) 1.07 (t, J 7.2, C6H1202S2: M , 180.0278). (M', 14%) and 147 (100); d~(cDC1,) 3.68-3.92 (4 H, m), 4.722- Phenyl- 1,4-dithiane 1 -Oxide 4.-A mixture of the dithiolane disulfoxide 3a (1 14 mg, 0.5 mmol) and benzenethiol(O.05 cm3, 0.5 mmol) in MeOH (10 cm3) was heated to 70 "C at reflux for 36 h. The mixture was cooled to room temperature, concen- trated under reduced pressure and chromatographed on silica gel by elution with hexane-EtOAc (1 :4) to give the trans- and cis-isomers of 4 in 62 and 10% yields, respectively. trans-Isomer: white solid, m.p. 180-181 "C; TLC (hexane-EtOAc, 1:4) Rf 0.16; v,,,(KBr)/cm-' 1579, 1035 and 699; m / z 212 (M', 34%) and 104 (100); G,(CDCI,) 3.05-3.26 (5 H, m), 3.60-3.70 (1 H, m), 3.97 (dd, J 10.3 and 2.4. 2-H), 7.32-7.38 (5 H, m); Gc- 128.5 (d, 2 C), 128.75 (d), 129.1 (d, 2 C) and 135.4 (s) (Found: M + , 212.0315. Calc. for CloH120S2: M, 212.0330). cis-Isomer: white solid, m.p. 135-1 36 "C; TLC (hexane-EtOAc, 1 : 4) R,
0.35; v,,,(KBr)/cm-' 1597, 1026 and 696; m/z 212 (M+, 65%) (CDC1,) 25.5 (t, C-5), 31.4 (t, C-3), 52.6 (t, C-6), 68.7 (d, C-2),
and 104 (100); dH(CDCl,) 2.43 (1 H, br d, J 12.9), 2.48 (1 H, br d, J 1 1.8), 3.01 (1 H, ddd, J 14.0, 12.7 and 2.8), 3.35 (1 H, ddd, J
14.0,2.8and2.1),3.73(1H,ddd,J12.9,12.7and2.1),3.85(1 H, dd, J 11.8 and 2.0), 4.02 (1 H, dd, J 11.8 and 11.8) and 7.25- 7.38 (5 H, m); d,(CDCI,) 16.1 (t, C-5), 22.6 (t, C-3), 47.8 (t, and 135.4 (s) (Found: M', 212.0314. Calc. for CloHl,0S2: M, 212.0330).
C-6), 61.3 (d, C-2), 128.05 (d, 2 C), 128.6 (d), 129.0 (d, 2 C)
8,8-Dimethyl-2,5-dithiabicyclo[4.4.O]dec-9-ene 2-oxide 5.- Thermolysis of the disulfoxide 3b by a procedure similar to that of 3a described above gave 73% yield of the bicycle 5 as a white solid, m.p. 108-109°C; m / z 216 (M', 50%) and 107 (100); G,(CDCI,) 1.08 (s, Me), 1.1 1 (s, Me), 1.74 (br d, J 13.4, 7P-H), 2.04 (dd, J 13.4 and 13.4, 7a-H), 2.83 (dd, J 4 . 1 and 4.1, 4-H), 3.02-3.18 (m, 3-H and 4-H), 3.39 (dt, J 13.4 and 3.2, 6-H), 3.47 (dd, J5.2and3.2, lO-H),3.60(dd, J9.2and4.5,3-H),5.82(d, J 10.0,9-H) and 6.01 (dd, J 10.0 and 5.2, 10-H);Gc(CDC1,) 21.7 (t, C-4), 28.8 (4, Me), 30.3 (9, Me), 35.1 (s, C-8), 38.6 (t, C-7), 43.3 (d, C-6), 52.9 (t, C-3), 63.3 (d, C-1), 120.8 (d, C-9) and 143.0 (d, C- 10) (Found: M + , 216.0645. Calc. for CloH160S2: M , 216.0642).
Hept- 1 -en-2-yl 2-Mercaptoethyl Sulfoxide 6, Hept- 1 -en-2-y1 2-Phenylthioethyl Sulfoxide 7, Hept-2-en-2-yl2-Mercaptoethyl Sulfoxide 8 and Hept-2-en-2-yl2-Phenylthioethyl Sulfoxide 9.- Compounds 6-9 were obtained by thermolysis of the disul- foxide 3c at 70 "C or 110 "C (Table 2) by a procedure similar to that of 3a described above. 6: Oil, TLC (hexane-EtOAc, 1 : 1) R,
0.34; v,,,(neat)/cm-' 1622 and 1048; m/z 206 (M+, 8%), 129 (48) and 61 (100); G,(CDCI,) 0.88 (t, J 6.7, 3, Me), 1.27-1.36 (4 H, m), 1.61-1.68 (2 H, m), 2.00-2.32 (2 H, m), 2.69-3.02 (4 H, m), 5.64 (1 H, d, J 1.8) and 5.83 (1 H, d, J 1.8); Gc(CDCl,) 13.9 (q), 17.1 (t), 22.3 (t), 27.6 (t), 28.4 (t), 31.2 (t), 54.9 (t), 116.3 (t) and 152.0 (s) (Found: M + , 206.0792. Calc. for C9Hl8OS2: M, 206.0799). 7: Oil, TLC (hexane-EtOAc, 1 : 1) R, 0.54; v,,,(neat)/ cm-' 1623,1434,1052 and 740; m/z (20 eV) 315 (M'
+
1,973, 168 (61) and 141 (100); d,(CDCI,) 0.90 (t, J 6.5, Me), 1.25- 1.35(4H,m), 1.40-1.60(2H,m), 1.90-2.22(2H,m),2.81-3.13 (4H,m),5.62(1 H,s),5.79(1H,s),7.21-7.37(3H,m)and7.50-7.56 (2 H, m); G,(CDCI,) 13.9 (q), 22.3 (t), 27.5 (t), 28.35 (t), 30.2(t),31.2(t),49.8(t), 116.4(t), 127.35(d), 128.0(d,2C), 129.2 (d, 2 C), 136.6 (s) and 151.8 (s) (Found: M', 314.0815. Calc. for C,H,,S,: M, 314.0832). 8: Oil, TLC (hexane-EtOAc, 1:l) R,
0.28; v,,,(neat)/cm-' 1650 and 1048; m/z 206 (M', 10%) and 103 (100); d,(CDCl,) 0.89 (3 H, t, J 6.9), 1.24-1.48 (4 H, m), 1.83 (3 H, s), 2.13-2.25 (2 H, m), 2.72-2.96 (4 H, m) and 6.18 (t), 30.8 (t), 54.6 (t), 135.2 (d) and 136.9 (s) (Found: M + , 206.0793. Calc. for C,H,,0S2: M, 206.0799). 9: Oil; TLC (30% EtOAc-hexane, 3: 7) R, 0.26; v,,,(neat)/cm-' 1574 and 1048; m/z (20 eV) 315 (M'
+
1, 16%), 169 (69) and 151 (100); 2.12-2.19 (2 H, m), 2.88-3.05 (4 H, m), 6.12 (1 H, t, J 7.3), 7.22- 7.36 (3 H, m) and 7.50-7.55 (2 H, m); G,(CDCl,) 9.2 (q), 13.8 (q), 22.3 (t), 27.7 (t), 30.5 (t), 30.7 (t), 49.5 (t), 127.3 (d), 128.05 (d, 2 C), 129.1 (d, 2 C), 135.2 (d) and 136.6 (s, 2 C) (Found: M + , 314.0836. Calc. for C15H2,0S,: M, 314.0832). (1 H, t, J7.5); Gc(CDCI3) 9.2 (q), 13.8 (q), 17.5 (t), 22.3 (t), 27.8 d~(cDC1,) 0.90 (3 H, t, J7.1), 1.25-1.42 (4 H, m), 1.77 (3 H, s),1 -Ethoxycarbonylethyl 2-Mercaptoethyl Sulfoxide 10 and 1- Ethoxycarbonylethyl2-Phenylthioethyl Sulfoxide 1 1 .-Therm- olysis of the disulfoxide 3d by a procedure similar to that described for compound 3a, gave the monosulfoxides 10 (14%) and 11 (41%). Either compound 10 or 11 existed as a mixture of diastereoisomers. 10, a mixture of two isomers (80 : 20): oil, TLC (EtOAc) R, 0.34; v,,,(neat)/cm-' 1723 and 1020; m/z 211 (M+
+
1, 11%) and 150 (100); G,(CDCl,) 1.32 (t, J 7.2, Me), 1.58 (d, J 7.3, Me major)/l.53 (d, J 7.3, minor), 2.92-3.12 (4 H, m), 3.62 (1 H, q, J 7.3)/3.78 (9, J 7.3) and 4.25 (4, J 7.2,608 J. CHEM. SOC. PERKIN TRANS. 1 1994
OCH,)/4.26 (d, J 7.2); Gc(CDCl3) 10.9
(a,
14.1 (q), 17.9 (t), 55.0 (t, major)/52.6 (t, minor), 60.5 (d)/59.2 (d), 62.1 (t, OCH,) and 168.65 (s) (Found: M + , 210.0270. Calc. for C,H,,O,S,: M, 210.0384). 11, a mixture of two isomers (67:33): oil, TLC (EtOAc) R, 0.53; v,,,(neat)/m-' 1723, 1049 and 1045; m/z319 (M'
+
1, 76%) and 168 (100); G,(CDCI,) 1.28 (t, J 7.0, Memajor)/1.32(t,J7.0, Meminor), 1.52(d, J7.3, Me)/1.59(d, J 7.3, Me), 3.02-3.25(4H,m), 3.56(q, J7.3)/3.64(q, J7.3),4.15- 4.30(2H,m,0CH2),7.21-7.37(3 H,m)and7.47-7.56(2H,m); Gc(CDC13) 10.7 (q, Me major)/lO.8 (q), 14.1 (q), 30.8 (t)/31.2 (t), 50.1 (t)/50.3 (t), 60.45 (d), 62.0 (t), 127.1 (s), 127.4 (d), 128.1 (d), 129 (d, 2 C) and 168.5 (s) (Found: M + , 318.0442. Calc. for C13H1803S3: M, 318.0418).2-(2-PhenyI- 1 -phenylthioethyl)- 1,3-dithiolane 1,3-Dioxide 12.-To a solution of benzenethiol(O.1 cm3, 1 mmol) in MeOH (2 cm3) was added dropwise a solution of MeMgCl (0.8 cm3, 1.25 mol drn-,, diethyl ether). The mixture was stirred for 20 min, after which a solution of the disulfoxide 3a (228 mg, 1 mmol) in THF (2 cm3) was added to it. The mixture was stirred at room temperature for a further 24 h to complete the addition (TLC analysis). Saturated aqueous NH,CI (1 cm3) was then added to the mixutre and MeOH and T H F removed by rotary evaporation. The residue was extracted with EtOAc (3 x 30 cm3) and the combined extracts were dried (Na,SO,) and filtered. The filtrate was concentrated and the residue chromatographed on silica gel to give the title compound 12 (31 8 mg, 91%) containing two isomers (50: 50). Isomer a was crystallised from EtOAc, m.p. 151.5-153.5 "C; v,,,(KBr)/cm-' 2983,1472,1392,1230,1141 and 1026; m/z 350 (M', 36%), 223
(100) and 148 (21); b,(CDCI,) 2.944.03 (8 H, m) and 7.28- 7.36 (10 H, m); SC(CDCl3) 40.4 (t), 48.7 (d), 51.2 (t), 52.1 (t), 96.0 (d, C-2), 127.0, 128.2, 129.1, 129.3, 129.7, 132.8 and 136.8 (Found: M', 350.0463. Calc. for C17H180,S3: M , 350. 0469). Isomer b was isolated from the mother liquor by chromato- graphy with EtOAc as eluent. G,(CDCl3) 40.0 (t), 47.4 (d), 50.7 (t), 52.2 (t), 97.9 (d), 128.3, 128.4, 129.1, 129.3, 133.2, 133.4, 133.5 and 137.0. The reaction in MeOD gave 92% yield of ["HI- 12. G,(CDCl,) 3.244.05 (7 H, m) and 7.26-7.42 (10 H, m); m/z351 (Mf,7%),316(11),242(19),223(39), 148(40)and 110 (100) (Found: M + , 351.0525. Calc. for C17H17D02S3: M, 35 1.053 1).
2-[ 1 -(Dicyanomethyl)-2-phenylethyI-J- 1,3-dithiolane 1,3-Di- oxide 13.-Addition of 3g with malononitrile by a procedure similar to that described for the dithiolane dioxide 12 gave compound 13 (42%) as a mixture of two diastereoisomers (67:23). The major isomer was crystallised from EtOAc, colourless crystals, m.p. 183-184.5 "C; v,,,(KBr)/cm-' 2991, 2252,1388,1181 and 1021; m/z 306 (M', l%), 241 (99) and 115 [d, J 5.1, CH(CN),] and 7.26-7.41 ( 5 H, m); G,(CDCl,) 25.6 (d), 37.6 (t), 40.2 (d), 91.7 (d), 128.5 (d), 129.2 (d, 2 C), 129.6 (d, 2 C), 134.6 (s) and 167.0 (s, CN) (Found: M', 306.0481. Calc. for C,,H,,0,N2S,: M , 306.0496). Minor isomer: d,(CDCl,) 3.263.37 (3 H, m), 3.74-3.99 ( 5 H, m), 4.96 (1 H, d, J 4 ) and 7.29-7.43 ( 5 H, m).
(100); d~(cDC13) 3.263.37 (3 H, m), 3.74-3.99 (5 H, m), 4.38
2-( l-Phenylthiopropyl)-1,3-dithiolane 1,3-Dioxide 14.-Addi- tion of benzenethiol with the dithiolane dioxide 3h in a similar manner to that described for compound 12 gave the title compound 14 (98%) containing two diastereoisomers (57 : 43). Two isomers were separated by HPLC on a p-Porasil column by elution with EtOAc-Me,CO (50 : 50). Major isomer: white solid, m.p. 1 18-120 "C; HPLC (EtOAc-acetone, 1 : 1) t, 10.9 min; v,,,(KBr)/cm-l 2966, 1436, 1393, 1088 and 1032; m/z 288 7.2, Me), 1.87-1.98,2.11-2.20 (2 H, m), 3.42 (td, J 10,3.5, 1'-H), (M', 73%), 212 (56) and 180 (100); GH(CDC13) 1.26 (3 H, t, J
3.62-3.90 (5 H, m), 7.32-7.38 and 7.54-7.59 (5 H, m); 6,- 128.8 (d), 129.2 (d, 2 C), 131.5 (s) and 134.5 (d, 2 C) (Found: M + , 288.0294. CaIc. for C12H1602S3: M , 288.0312). Minor isomer: oil, HPLC (EtOAc-acetone, 1 : 1) t, 1 1.4 min; v,,,(neat)/ cm-' 2965, 1577, 1434, 1087, 1030 (s, S O ) , 750 and 692; d,(CDCI,) 1.24 (t, J 7.1, Me), 1.71-1.79, 1.90-2.02 (2 H, m), 3.38 (td, J 9.1 and 3.1, 1 '-H), 3.54-3.94 (5 H, m), 7.30-7.38 and 7.54-7.60 (5 H, m); G,(CDCl,) 11.2 (q), 26.6 (t), 47.0 (d), 50.4 (t), 52.3 (t), 98.6 (d), 128.5 (d), 129.2 (d) 131.8 (s) and 134.1 (d, 2 C) (Found: M + , 288.0302. Calc. for C1,Hl6O,S3: M ,
28 8.03 1 2).
(CDCI,) 11.2 (q), 26.7 (t), 46.9 (d), 50.6 (t), 52.1 (t), 97.5 (d),
2-[I -(Dicyanomethyl)Jpropyl- 1,3-dithiolane 1,3-Dioxide15.- Method A: Addition of the dithiolane dioxide 3h with malononitrile by a procedure similar to that described for compound 12 gave the title compound 15 (46%) containing two diastereoisomers (67 : 33). Method B: Neutral alumina (0.5 g) was activated by heating at 140 "C for 2 h, cooled and added to a solution of malononitrile (33 mg, 0.5 mmol) in T H F (1 cm3). The mixture was stirred for 0.5 h after which a solution of the dithiolane dioxide 3h (89 mg, 0.5 mmol) in T H F (1 cm3) was added to it and the whole stirred for 72 h under an atmosphere of argon. After removal of the THF, the residue was extracted with EtOAc (2 x 10 cm3). The combined extracts were washed with brine, dried (Na,SO,) and filtered. The filtrate was concentrated and the residue chromatographed on silica gel by elution with EtOAc to give the title compound 15 (34 mg, 28%) as a mixture of two isomers (56 : 44); colourless solid, m.p. 125- 127OC; TLC (EtOAc) Rf 0.25; v,,,(KBr)/cm-' 2971, 2168 (CN), 1628, 1094 and 1026; m/z 244 ( M + , 4%) and 108 (100); dH(CDC1,/CD3OD) 1.21 (t, J 7.4, Me), 2.05-2.19 (2 H, m, 2'-H),2.81-2.92(1 H,m, lf-H),3.67-3.90(5H,m),4.49(d,J5.8, CH(CN), major)/4.99 (d, J 3.0, minor) (Found: M', 244.0341. Calc. for C9H12N20,S,: M, 244.0339). When the reaction was conducted in MeOD, C2H]-15 was obtained, accompanied by recovery of the starting material 3h; m/z 245 (M', 1%) and 108
(100); bH(CDC13/CD30D) 1.28 (d, J 7.2, Me), 2.18-2.28 (1 H, m, 2'-H), 2.89-3.01 (1 H, m, 1'-H), 3.74-3.95 (5 H, m), 4.54 [d, J 5.9, CH(CN),, major] and 5.07 (d, J 3.0, minor) (Found: M', 245.0401. Calc. for C9Hl ,DN2O2S2: M, 245.0402).
2-{ 1 -[Bis(ethoxycarbonyl)methyl]}propyl- 1,3-dithiolane 1,3- Dioxide 16.-Addition of the dithiolane dioxide 3h with diethyl malonate by a procedure similar to that described for compound 12 gave the title compound 16 (46%) containing two diastereomers (71 : 29). Liquid, TLC (MeOH-EtOAc, 1 : 9) R,
0.4; v,,,(neat)/cm-' 2975, 1722 (s, C--O), 1231, 1093 and 1032; 7.3, Me major)/l.05 (t, J 7.3, Me minor), 1.27-1.37 (6 H, m), 1.541.74 (2 H, m), 2.85-2.94 (1 H, m), 3.50-3.98 (7 H, m) and 4.194.39 (3 H, m) (Found: M', 338.0855. Calc. for m / z 339 (M+
+
1, 2%) and 108 (100); dH(CDC13) 1.02 (t, Jc,
3H2206S2: M , 338.0858).2-{ 1 -[(4,5-Dihydro- 1,3-thiazol-2-yI)thio])propyl- 1,3-dithiol- ane 1,3-Dioxide 17.-Addition reaction of the dithiolane dioxide 3h with 2-mercapto-4,5-dihydrothiazole by a procedure similar to that described for compound 12 gave the title compound 17 (83%). Liquid, TLC (MeOH-EtOAc, 1:9); R,
0.1; v,,,(neat)/cm-' 1625 and 1030; m/z 297 (M', 1%) and 119 2.15(1 H,m), 3.35-3.49(2H,m), 3.60-3.99(6H,m)and4.11-- 4.22 (2 H, m);Gc(CDC13) 10.2 (q), 25.7 (t), 28.0 (t), 51.2 (t), 53.0 (t), 53.4 (t), 53.6 (t), 94.9 (d, C-2) and 199.4 (s) (Found: M + , 296.9968. Calc. for C9H1 ,NO,S,: M, 296.9986).
(100); &(CDCl,), 1.03 (t, J 7.2, Me), 1.82-1.94 (I H, m), 2.02-
2-Propylidene- 1,3-dithiolane 1,3-Dioxide 18.-By a similar procedure to that described above for the dithiolane dioxide 3a,
J. CHEM. SOC. PERKIN TRANS. 1 1994 609
2-propylidene- 1,3-dithiolane 2 o was oxidized with NaIO, (1
equiv.) to give the 2-propylidene- 1,3-dithiolane 1 -oxide 18a
(59%) as a mixture of E- and 2-isomers (79 : 21); pale yellow oil, TLC (EtOAc-hexane, 1 : 1) R, 0.13; v,,(neat)/cm-' 2965, 1676, 1397, 1171 and 1045; m/z 162 (M', 47%) and 145 (100); 3.4C3.64, 3.984.12 (4 H, m) and 6.46 (t, J 7.1, 1'-H E-
isomer)/6.09 (t, J 7.8, l'-H Z-isomer); &(CDCl,) 12.4 (4, C-3' E-isomer)/l4.2 (9, C-3' 2-isomer), 25.6 (t, C-2')/26.5 (t), 31.9 (t, C-4)/31.4 (t), 54.9 (t, C-5)/55.6 (t), 135.3 (d, C-1')/134.6 (d) and 145.5 (s, C-2)/144.5 (s) (Found: M + , 162.0176. Calc. for C6HloOS2: M , 162.0173). Further oxidation of the mono- sulfoxide 18a with m-CPBA (1 equiv.) gave 42% yield of the dioxide 18: oil; v,,,(neat)/cm-' 2973, 1607, 1459, 1327 and 7.5, Me), 2.69 (2 H, qd, J 7.7 and 7.9, 3.58-3.86 (4 H, m) and (t), 155.7 (s) and 156.7 (d) (Found: M', 178.0115. Calc. for &(CDCl,) 1.06 (t, J 7.5, Me), 2.12-2.27 (2 H, m), 2.57-2.78,
1092; m/z 178 (M', 24%) and 135 (100); dH(CDC13) 1.17 (t, J 7.27 (t, J 7.7, l'-H); GC-CDCl,) 12.5 (q), 26.5 (t), 50.1 (t), 50.4 C ~ H , O O ~ S ~ : M , 178.0122).
2-(2-Methoxypropylidene)- 1,3-dithiolane 1 -Oxide 19.-A methanolic solution of equivalent amounts of the dithiolane di- oxide 3h, acetic acid and MeMgCl(l.25 mol dm-3, diethyl ether) were stirred at room temperature for 1 h to give the title com- pound 19 ( 5 5 7 3 , containing four diastereoisomers (a, b, c and d, 33 : 33 : 17 : 17). Isomers a and b had 2-configuration whereas
c and d had E-configuration by analysis of the 'H NMR spectrum. Oil, TLC (MeOH-EtOAc, 1 : 9) R, 0.5; v,,,(neat)/ cm-' 2973, 1594, 1444, 1084 and 1042; m/z 192 (M', 7%) and 175 (86); dH(CDC1,) 1.30 (d, J 6.5, E-isomer)/l.315 (d, J 6.3, Z-isomer)/l.32 (d, J6.6, E-isomer)/l.38 (d, J6.2,2-isomer), 2.75 (1 H, m), 3.28 (s, OMe Z-isomer)/3.31 (s, E-isomer)/3.36 (s, E-
isomer)/3.37 (s, Z-isomer), 3.49-3.86 (2 H, m), 3.90-4.13 (m, Z- isomer)/4.44-4.57 (m, E-isomer) and 5.94 (d, J 8.7, 1'-H, Z - isomer)/5.99 (d, J 8.7, Z-isomer)/6.40 (d, J 5.0, E-isomer)/6.44 (d, J 5.0, E-isomer) (Found: M + , 192.0271. Calc. for C,H120,S,: M , 192.0278).
1 -Allyl-2-methylpent-4-enylidene- 1,3-dithiolane 20.-Under an atmosphere of argon, trifluoroacetic anhydride (0.15 cm3, 1 mmol) was added dropwise to a solution of the dithiolane dioxide 3h (89 mg, 0.5 mmol) in CH,Cl, (2 cm3) at 0 "C. The reddish mixture was stirred for 15 min and then allyltrimethyl- silane (0.16 cm', 1 mmol) was added to it and the mixture stirred at room temperature for 12 h. After dilution with water (2 cm3) the mixture was concentrated then extracted with EtOAc (2 x 10 cm3). The combined extracts were washed with brine, dried (Na,SO,) and filtered. The filtrate was concentrated and the residue chromatographed on silica gel by elution with EtOAc-hexane (2 : 98) to give the title compound 20 (60 mg, 53%). Oil, TLC (EtOAc-hexane, 2 : 98) R, 0.35; v,,,(neat)/ cm-' 1673; m/z 226 ( M + , 11%) and 185 (100); dH(CDC13) 1.03 (3 H, d, J6.9), 2.02-2.27 (2 H, m), 2.61-2.78 (1 H, m), 2.86-2.91 (2 H, m), 2.32 (4 H, s, dithiolane ring H), 4.91-5.14 (4 H, m) and 5.63-5.86 (2 H, m); Gc(CDCl,) 18.25 (4, Me), 36.8 (t), 37.4 (t, dithiolane ring C), 39.45 (t), 42.3 (d), 115.6 (t, 2 C), 128.5 (s), 131.0 (s), 135.5 (d) and 137.25 (d) (Found: M + , 226.0853. Calc. for C,,H,,S,: M , 226.0850).
Acknowledgements
We are grateful to the National Science Council for financial support (Grant NSC82-0208-M002-3 1).
References
1 T. Durst, in Comprehensive Organic Chemistry, eds. D. Barton and W. D. Ollis, Pergamon Press, 1979, vol. 3, pp. 121-170; eds. S. Patai, Z. Rappoport and C. Sterling, The Chemistry of Sulphones and
Sulphoxides, Wiley, 1988.
2 G. Solladie, in Asymmetric Synthesis, ed. J. D. Morrison, Academic Press, 1983, vol. 2, p. 157; G. H. Posner, Asymmetric Synthesis, ed. J. D. Morrison, Academic Press, 1983, vol. 2, p, 225.
3 P. C. B. Page and E. S. Namwindwa, Synlett., 1991, 80 and references cited therein; V. K. Aggarwal, M. Lightowler and S. D. Lindell, Synlett., 1992,730 and references cited therein; N, Khiar, I. Fernandez and F. Alcuda, Tetrahedron Lett., 1993,34, 123. 4 V. K. Aggarwal, I. W. Davies, J. Maddock, M. F. Mahon and K. C.
Molloy, J. Chem. SOC., Perkin Trans. I , 1992,662.
5 F. A. Carey, 0. D. Dailey Jr. and T. E. Fromuth, Phosphorus
Sulfur, 1981,10, 163.
6 0. Samuel, B. Ronan and H. B. Kagan, J. Organomet. Chem., 1989, 370,43.
7 J.-M. Fang, W.-C. Chou, G.-H. Lee and S.-M. Peng, J . Org. Chem., 1990,55, 5515 and the references cited therein.
8 0. Bortolini, F. D. Furia, G. Licini and M. Rossi, Tetrahedron Lett., 1986,27,6257.
9 F. A. Carey, 0. D. Dailey Jr. and W. C. Hutton, J . Org. Chem., 1978, 43,96.
10 C. H. Chen, Tetrahedron Lett., 1976, 25; N. Ueda, H. Shimizu, T. Kataoka and M. Hori, Tetrahedron Lett., 1984, 25, 757; W. S. Lee, K. Lee, K. D. Nam and Y. J. Kim, Tetrahedron, 1991, 47, 8091.
1 1 D. R. Hogg, in Comprehensive Organic Chemistry, eds. D. Barton and W. D. Ollis, Pergamon Press, 1979, vol. 3, pp. 264265.
12 G. H. Posner and P. W. Tang, J. Org. Chem., 1978,43,413 1. 13 S. Oae and Y. Uchida, Acc. Chem. Res., 1991,24202.
14 D. A. Evans and G. C. Andrews, Acc. Chem. Rex, 1974,7,147; F. A. Carey, P. M. Smith, R. J. Maher and R. F. Bryan, J. Org. Chem., 1977, 42,961.
15 B. C. Ranu, S. Bhar and D. C. Sarkar, Tetrahedron Lett., 1991,32, 2811.
16 F. A. Carey and 0. D. Dailey Jr., Phosphorus Sulfur, 1981, 10,
169; E. Schaumann, in Perspectives in Organosulfur Chemistry, eds. B. Zwanenburg and A. Klunder, Elsevier, 1986, p. 251.
17 D. Pandy-Szekeres, G. Deleris, J.-P. Picard, J.-P. Pillot and R. Calas, Tetrahedron Lett., 1980, 21, 4267; R. Hunter and C. D. Simon, Tetrahedron Lett., 1986,27,1385; I. Mori, P. A. Bartlett and C. H. Heathcock, J. Org. Chem., 1990,55, 5966; W.-L. Cheng and T.-Y. Luh, J. Org. Chem., 1992,57,3516; W. H. Chan, A. W. M. Lee and E. T. T. Chan, J. Chem. SOC., Perkin Trans, 1, 1992,945. 18 C.-C. Yang and J.-M. Fang, J. Chem. SOC., Perkin Trans. I , 1992,
3085.
19 A. Hoppmann, P. Weyerstahl and P. Zummack, Justus Liebigs Ann.
Chem., 1977, 1547; J.-M. Fang, L.-F. Liao and B.-C. Hong, J. Org.
Chem., 1986,51,2828.
20 E. J. Corey and A. P. Kozikowski, Tetrahedron Lett., 1975, 11,925.
Paper 3/04409B Received 26th July 1993 Accepted 6th October 1993