Brain Research, 508 (1990) 225-233 225 Elsevier
BRES 15170
Involvement of amygdala pathways in the influence of post-training
intra-amygdala norepinephrine and peripheral epinephrine on
memory storage
K.C.
L i a n g 1, J a m e s L. M c G a u g h 2 and
H.-Y.
Yao 1
1Department of Psychology, National Taiwan University, Taipei (Taiwan) and 2Department of Psychobiology and Center for the Neurobiology of Learning and Memory, University of California, lrvine, CA 92 717 (U.S.A. )
(Accepted 5 July 1989)
Key words: Amygdala; Adrenaline; Inhibitory avoidance; Memory; Noradrenaline; Retention; Stria terminalis; Ventral amygdalofugal pathway
These experiments examined the role of two major amygdala afferent-efferent pathways - - the stria terminalis (ST) and the ventral amygdalofugal pathway (VAF) - - in mediating the effects, on memory storage, of post-training intra-amygdala injections of norepinephrine (NE) and subcutaneous (s.c.) injections of epinephrine (E). Rats with either ST lesions or VAF transections and sham-operated rats were trained on a one-trial step-through inhibitory avoidance task and immediately after training received intra-amygdala injections of NE or a buffer solution. Other groups of VAF-transected animals received post-training s.c. injections of E or saline. ST lesions blocked the memory-enhancing effect of intra-amygdala injections of a low dose of NE (0.2 ~g) as well as the amnestic effect of a high dose of NE (5.0 ~g). In contrast, VAF transections did not block the memory-enhancing effect of NE (0.2 ,ug). ttowever, VAF transections attenuated the memory-enhancing effect of s.c. injections of E: the effective dose of E was shifted from 0.1 to 0.5 mg/kg. These findings, considered together with previous evidence that ST lesions block the memory-enhancing effect of peripheral E injections, suggest that the VAF is involved in mediating the central influences of peripheral E on amygdala functioning, while the ST is involved in mediating amygdala influences on memory storage elsewhere in the brain.
INTRODUCTION
E x t e n s i v e e v i d e n c e suggests that the a m y g d a l a is involved in the m o d u l a t i o n of m e m o r y s t o r a g e processes. Lesions o r electrical s t i m u l a t i o n of the a m y g d a l a i m p a i r r e t e n t i o n of a variety o f recently l e a r n e d r e s p o n s e s (for review, see refs. 33, 34). In view of the findings that d a m a g e of the a m y g d a l a in rats o r h u m a n patients results in r e t r o g r a d e a m n e s i a which is t e m p o r a l l y limited 23'35, it s e e m s u n l i k e l y that the a m y g d a l a is a site of p e r m a n e n t m e m o r y storage. Results f r o m a n u m b e r of studies suggest that a m y g d a l a influences on m e m o r y s t o r a g e m a y involve its two m a j o r i n p u t - o u t p u t p a t h w a y s - - the stria t e r m i n a l i s (ST) and the ventral a m y g d a l o f u g a l p a t h w a y
(VAF) 3"13'16'17'20"21"27'28"32.
The findings that ST lesions significantly a t t e n u a t e the amnestic effect of p o s t - t r a i n i n g electrical s t i m u l a t i o n o f the a m y g d a l a argue that the a m y g d a l a m a y be involved in m o d u l a t i n g m e m o r y storage processing t h r o u g h its influences on n e u r a l processes in o t h e r b r a i n regions 2°.T h e a m y g d a l a receives n o r a d r e n e r g i c p r o j e c t i o n s from the locus c o e r u l e u s as well as o t h e r b r a i n s t e m nuclei 8'~'~5.
T h e r e is extensive e v i d e n c e indicating that t r e a t m e n t s influencing n o r a d r e n e r g i c activity in the a m y g d a l a influ- ence m e m o r y f o r m a t i o n : in an inhibitory a v o i d a n c e task, post-trial i n t r a - a m y g d a l a infusions of f l - a d r e n e r g i c antag- onists i m p a i r r e t e n t i o n 1°'26, and r e t e n t i o n is e n h a n c e d by low doses of n o r e p i n e p h r i n e ( N E ) 19 and i m p a i r e d by higher doses 6. In a d d i t i o n , n a l o x o n e , which e n h a n c e s N E release by blocking o p i o i d r e c e p t o r s , p r o d u c e s a p r o p r a - n o l o l - r e v e r s i b l e m e m o r y e n h a n c e m e n t when adminis- t e r e d directly into the a m y g d a l a 14. I n t r a - a m y g d a l a injec- tions of N E have also b e e n shown to affect taste aversion learning 2. T h e s e findings suggest that the a m y g d a l a N E system m a y be involved in the e n d o g e n o u s m o d u l a t i o n of m e m o r y storage.
It is well e s t a b l i s h e d that p o s t - t r a i n i n g systemic injec- tions of e p i n e p h r i n e ( E ) , which d o e s not r e a d i l y e n t e r the brain 31"38, i m p r o v e r e t e n t i o n in b o t h a p p e t i t i v e and a v o i d a n c e learning tasks j~'25'~6. Several r e c e n t findings from our l a b o r a t o r i e s indicate that the m e m o r y - e n h a n - cing effect of p e r i p h e r a l E involves the a m y g d a l a N E system: p o s t - t r a i n i n g i n t r a - a m y g d a l a injections of pro- p r a n o l o l a t t e n u a t e the m e m o r y - e n h a n c i n g effects of
Correspondence: K.C. Liang, Department of Psychology, National Taiwan University, Taipei, Taiwan 10764, R.O.C.
peripherally a d m i n i s t e r e d E and post-training intra- amygdala injections of N E a t t e n u a t e the learning deficit p r o d u c e d by adrenal d e m e d u l l a t i o n 19. We have also f o u n d that the m e m o r y - e n h a n c i n g effect of peripherally a d m i n i s t e r e d E is a t t e n u a t e d in animals given intra-
amygdala injections of the N E n e u r o t o x i n DSP-4
TM.
C o n s i d e r e d together, these findings suggest that periph- eral E may activate N E projections from the brainstem to various brain regions, including the amygdala, and that N E released in the amygdala may, in turn, activate pathways which serve to m o d u l a t e m e m o r y processing elsewhere in the brain. This view is consistent with the view that the locus coeruleus m a y play a critical role in i n t e g r a t i n g i n t e r n a l or external stimuli from the periphery to e m o t i o n a l or other behavior 37 as well as the finding that N E released in the central amygdala nucleus is t e m p o r a l l y correlated with plasma N E level in rats 4.
A c c o r d i n g to our i n t e r p r e t a t i o n of these findings, severance of the afferents conveying the influences of peripheral E to the amygdala should a t t e n u a t e the m e m o r y - m o d u l a t i n g effect of peripheral E injections but should n o t block those p r o d u c e d by intra-amygdala N E injections. In contrast, severance of the pathway trans- mitting the m e m o r y m o d u l a t o r y influences from the amygdala to other brain regions should a t t e n u a t e the effects of both peripherally a d m i n i s t e r e d E and intra- amygdally injected NE. We have previously shown that ST lesions a t t e n u a t e the m e m o r y e n h a n c i n g effect of peripherally a d m i n i s t e r e d E 21. To investigate these im- plications further, the present e x p e r i m e n t s e x a m i n e d the
effects, on m e m o r y storage, of: (1) peripherally admin- istered E, in rats with V A F transections: and (2) intra-amygdally injected N E , in rats with lesions of either the ST or VAF.
MATERIALS AND METHODS
Subjects
Male Sprague-Dawley rats, 50-60 days old, were obtained from the breeding center of National Yang-Ming Medical College (Expts. I and II) and Charles River Labs (Expt. III). They were individually housed upon arrival and maintained on a 12-h light-dark cycle (lights on at 07.00 h) with food and water ad libitum.
Surgery
Approximately 3 weeks after arrival, the rats were subjected to stereotaxic surgery under sodium pentobarbital anesthesia (40 mg/kg). Atropine sulfate (0.3 mg/kg) was given to prevent conges- tion. Rats in Expts. I and II received bilateral amygdala cannula implantation plus bilateral ST lesions or VAF transections or sham operations on either pathway. Rats in Expt. III received only VAF transections or sham operation.
S T lesions. To produce bilateral ST lesions, a Radionic TCZ electrode was placed into the brain with the tip at the fimbra-fornix level (AP -1.0 mm from bregma, ML +2.5 mm, DV -4.0 mm from dura) 3°. Radio frequency currents (2.5-3.0 mA) generated by a DKI RFG-4A lesion maker were passed through the electrode for 30 s. The electrode was then withdrawn from the brain and the burr hole was sealed with Gelfoam and bone wax. The sham operation followed the same procedure except that no current was delivered.
VAF transection. The VAF transecting procedure was similar to that described previously 2°. A wire knife composed of a 30-gauge cannula containing a stainless-steel wire (0.13 mm in diameter) was lowered into the brain (AP 0.0 mm, ML +2.5 mm, DV -5.5 mm). The wire was then forced out from the slightly curved opening of the cannula tip so that the wire-blade would protrude 2.0 mm from the tip in a position perpendicular to the cannula and suspend an angle
of 15 ° laterally to the parasagittal plane of the cannula shaft. A cut was made between the lateral hypothalamus and the amygdala by moving the entire knife down and up once between -5.5 mm and the floor o f the brain. The knife was then retracted into the cannula and the cannula was withdrawn from the brain. Since simultaneous bilateral V A F transections are known to produce severe aphagia as well as adipsia resulting in high death rates, two unilateral transections were p e r f o r m e d , with a 2-week interval between the surgeries. In the sham V A F operation, the cannula containing the knife was lowered to -5.5 m m below the dura and then withdrawn (without extending the wire). The two unilateral sham operations were also separated by an interval of 2 weeks.
Amygdala cannula implantation. The amygdala cannula implan- tation surgery was performed following the ST or V A F surgery. Stainless-steel cannulae (23 gauge) were implanted bilaterally into the center of the amygdala ( A P - 2 . 0 ram, ML +4.5 mm, D V - 6 . 0 mm). Two small stainless-steel screws were implanted over the right frontal and left posterior cortices serving as anchors. The entire complex was affixed on the skull with dental cement. A stylet was inserted into each cannula to maintain patency. For animals receiving V A F transections or sham operations, both cannulae were implanted at the second V A F surgery.
Inhibitory avoidance task
Two weeks after the final surgery the rats were trained on a one-trial step-through inhibitory avoidance task. The apparatus consisted of a trough-shaped alley divided by a sliding door into an illuminated safe c o m p a r t m e n t and a dark shock c o m p a r t m e n t 19, The rat was placed in the safe c o m p a r t m e n t facing away from the door. As the rat turned around, the door was o p e n e d and the rat had free access to the dark side. A f t e r the rat entered the dark compartment, the door was closed and a footshock was administered through a Lafayette constant-current stimulator (Model 82400SS, Lafayette).
2 2 7
The shock intensity was calculated as the root mean square of 60 Hz sinusoidal A C currents. The rat was removed from the alley about 5 s after receiving the shock and administered the appropriate post-training treatment, and returned to its home cage. O n the retention test given 24 h later, the rat was again placed in the illuminated c o m p a r t m e n t and the latency to step into the dark c o m p a r t m e n t was recorded as a measure of retention performance. Rats which did not enter the dark c o m p a r t m e n t within 600 s were removed from the alley and assigned a ceiling score of 600.
Drug administration
In Expts. I and II, rats received bilateral intra-amygdala injections (vehicle or NE) immediately following training. The animal was gently restrained by the experimenter when receiving injections. The injection was administered through a 30-gauge injection needle connected to a 10 ~1 Hamilton microsyringe by 0.5 m PE-20 polyethylene tubing. The injection needle was bent at a length such that, when inserted into the cannula, the needle tip would protrude approximately 1.5 mm beyond the tip of cannula. Drug solutions were introduced into the P E tubing and the microsyringe, and were delivered into the amygdala bilaterally by a Sage microinjection pump (model 355) at a rate of 1 ~ul/min. (-)-Norepinephrine hydrochloride (Sigma, A-7131) was dissolved into a buffered vehicle, which in 100 ml, contained 0.9 g of NaC1, 4.05 ml of 0.2 M Na2HPO 4 and 0.95 ml of 0.2 M NaH~POa.2H20. All concentrations were calculated as the salt weight. O n e microliter of solution was injected bilaterally. The injection needle was kept in the cannula for 1 min after completing the injection to allow for diffusion.
In Expt. IlI, the rats received s.c. injections of saline or E immediately following training. E was obtained from Elkins-Sinn. Inc. as a 1 mg/ml solution (containing 0.1% sodium bisulfite) and was diluted with saline to appropriate concentrations. The injection volume was 1 ml/kg b.wt.
/ • 1.80 \ -2.12 -2.30 -2.56 Fig. 3. Distribution of cannulae tips in the amygdala. - ~.80 • 3 .I4 .30 -60
Histology
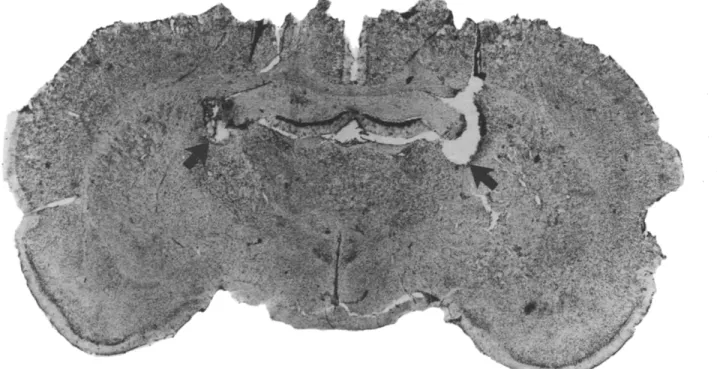
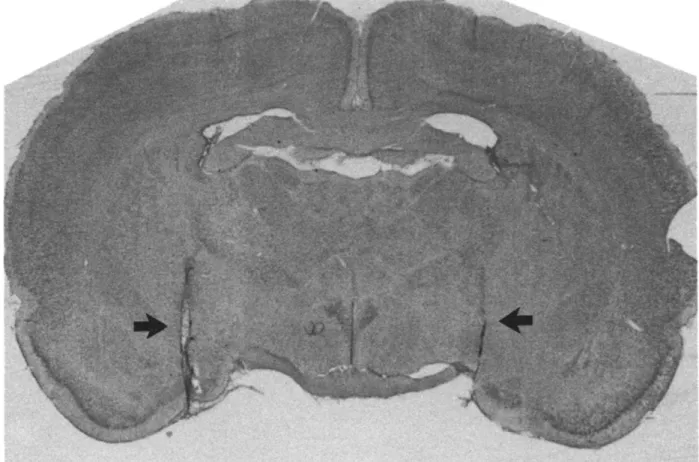
The rats were sacrificed with an overdose of sodium pentobarbital and perfused intracardiacally with 0.9% saline followed by 10% formalin. The brain was removed and stored in formalin for at least 48 h and then sectioned into 40-~tm slices. Slices through the ST lesions, VAF transections and the cannula tract were taken and processed through standard Cresyl violet staining. Animals with cannulae not in the amygdala, or with misplaced lesions or transections were not included in data analyses. The 35 rats discarded for this reason were evenly distributed among various groups. Typical ST lesions, VAF transections and the distribution of amygdala cannula tips within the amygdala are shown in Figs. 1-3.
RESULTS
Experiment I
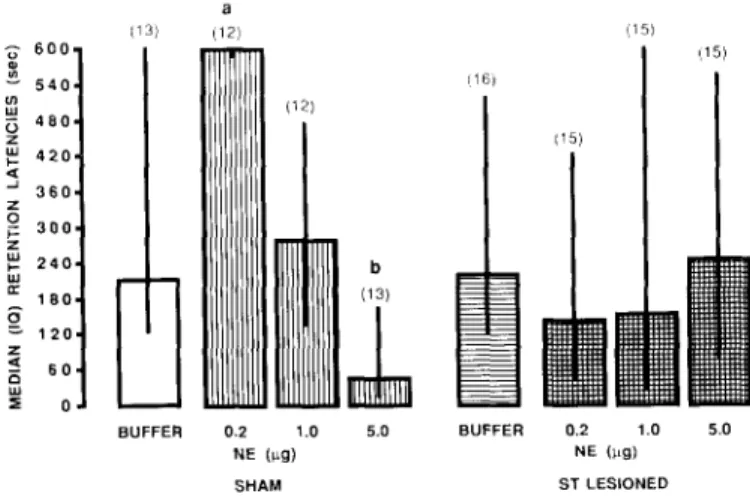
T h e first e x p e r i m e n t e x a m i n e d the i n v o l v e m e n t of the ST a n d the V A F in m e d i a t i n g the m e m o r y facilitation i n d u c e d by post-training i n t r a - a m y g d a l a injections of 0.2 ktg NE. In a previous e x p e r i m e n t t9 we found that this dose reliably e n h a n c e d r e t e n t i o n of the inhibitory avoid- ance response. I m p l a n t e d rats with ST lesions (ST-L), ST sham o p e r a t i o n (ST-S), V A F transections ( V A F - T ) or V A F sham o p e r a t i o n (VAF-S) were trained on the inhibitory avoidance task with a 0.9 mA/0.9 s footshock. T h e y received i n t r a - a m y g d a l a injections of buffered vehicle (Buf) or 0.2 ktg N E i m m e d i a t e l y after training. T h e 24 h r e t e n t i o n p e r f o r m a n c e is shown in Fig. 4. The d i s t r i b u t i o n of the r e t e n t i o n scores was t r u n c a t e d at 600. C o n s e q u e n t l y , m e d i a n and interquartile ranges were used to express the central t e n d e n c y a n d the dispersion, respectively, of the data, a n d n o n - p a r a m e t r i c statistics were used for data anlyses.
In both groups of s h a m - o p e r a t e d rats (ST-S and V A F - S ) , post-trial i n t r a - a m y g d a l a injections of 0.2 ~tg N E
229 improved r e t e n t i o n : the r e t e n t i o n latencies of the ST- S/NE group were significantly higher than those of the ST-S/Buf group ( M a n n - W h i t n e y two-tailed U-test, U = 29.5, P < 0.02), and the latencies of the V A F - S / N E group were significantly higher than those of the VAF-S/Buf group ( U = 20, P < 0.01). Lesions of the ST blocked the m e m o r y e n h a n c e m e n t p r o d u c e d by i n t r a - a m y g d a l a injec- tions of 0.2 /~g NE: the r e t e n t i o n latencies of the ST-L/NE group did not differ significantly from those of the ST-L/Buf group. F u r t h e r , the r e t e n t i o n latencies of the ST-L/NE group were significantly lower than those of the ST-S/NE group (U = 19, P < 0.02). Transections of the V A F did not block the m e m o r y facilitation caused by NE: the r e t e n t i o n scores of the V A F - T / N E group were significantly higher than those of the V A F - T / B u f group (U = 47, P < 0.05) and were not significantly different from those of the V A F - S / N E group.
Experiment II
The findings of Expt. 1 indicate that the m e m o r y - e n h a n c i n g effect of i n t r a - a m y g d a l a injections of 0,2 l~g N E is readily blocked by ST lesions but is unaffected by V A F transections. Expt. I1 was designed to e x a m i n e the possibility that the lack of effectiveness of intra-amygdala N E on r e t e n t i o n in ST-L rats might be due to shifts in the d o s e - r e s p o n s e curve. Expt. II also e x a m i n e d the possi- bility that the lack of N E facilitation in the ST-L rats might be due to a p e r f o r m a n c e - i m p a i r i n g effect of the lesion which was not revealed u n d e r the low-footshock training conditions used in Expt. 1,
I m p l a n t e d rats with ST-lesions, V A F - t r a n s e c t i o n s or sham operations were t r a i n e d on the inhibitory avoidance task with a 1.2 mA/1.2 s footshock. I m m e d i a t e l y after
~" 6 0 0 -
=
5 4 0 -4301
4 2 0 , 3 6 0 - F- 3 0 0 . ~ 240. # 1 8 0 , A 0 1 2 0 , -- 6 0 , ~ O , a a (13) (13) (12) (~5} b "~" 6 0 0 1 (15) {11) 5 4 0 (16) OZ 4 8 0 115) 3 6 0 ~13) ~1
1 2 0 60BUFFER NE BUFFER NE BUFFER NE BUFFER NE
SHAM LESIONED SHAM LESIONED
S T V A F
Fig. 4. Retention performance (median +_ interquartile range) of ST-lesioned or VAF-transected rats receiving intra-amygdala injec- tions of 0.2 l~g NE immediately after training, a, different from ST Sham/Buf and ST lesioned/NE, P < 0.02; b, different from VAF Sham/Buf, P < 0.01; c, different from VAF Transected/Buf, P < 0.05. Number of subjects in parentheses.
BUFFER 0.2 1.0 5.0 BUFFER 0.2 1.0 5.0
NE (~g) NE (~g)
SHAM ST LESIONED
Fig. 5. Retention performance (median _+ mtcrquartile range) of ST-lesioned or sham-operated rats receiving intra-amygdala injec- tions of NE in the doses indicated, a, different from Sham/Bur, P < 0.01 : different from Lesioned/0.2 ug NE, P < 0.001 ; b, different from Sham/Buf, P < 0.05; different from Lesioned/5.0 t~g NE, P < 0.05. Number of subjects in parentheses.
training, the rats received intra-amygdala injections of Buf or one of 3 doses of NE: 0.2, 1.0 or 5.0/ag.
The 24 h retention performance of the ST-operated groups is shown in Fig. 5. In ST-S rats, intra-amygdala injections of NE significantly affected retention perfor- mance: a Kruskal-Wallis one-way A N O V A revealed significant differences among various groups (H = 22.60, P < 0.001). Paired comparisons by Mann-Whitney two-tailed U-tests indicated that 0.2 /~g NE facilitated retention (ST-S/0.2/~g vs ST-S/Buf, U = 30, P < 0.01), while 5.0 /~g NE impaired retention (ST-S/5.0 ktg vs ST-S/Buf, U = 42, P < 0.05).
of the ST-L/Buf rats was not that of the ST-S/Buf rats.
The retention performance significantly different from However, in ST-L rats, post-training intra-amygdala injections of NE did not affect retention performance: none of the 3 ST-L groups treated with NE had retention scores which were signifi- cantly different from those of the ST-L/Buf group. Comparisons between the lesioned and the sham-oper- ated groups receiving the same dose of NE indicated that the retention of the ST-S/0.2 ¢tg group was significantly better than that of the ST-L/0.2/~g group (U -- 9.5, P < 0.001), and the retention of the ST-S/5.0/ag group was significantly poorer than that of the ST-L/5.0/tg group (U = 49.5, P < 0.05).
The retention performance of the VAF-operated groups is shown in Fig. 6. In VAF sham-operated animals, post-trial intra-amygdala injections of NE pro- duced biphasic effects on retention. A Kruskal-Wallis one-way A N O V A revealed significant differences among various groups (H = 25.98, P < 0.001). Paired compar- isons indicated that 0.2 ~g NE facilitated retention (VAF-S/0.2 /~g vs VAF-S/Buf, U = 22.5, P < 0.02), whereas 5.0/ag NE impaired retention (VAF-S/5.0/~g vs
VAF-S/Buf, U = 2, P < 0.001). "Iransecting the VAF tended to impair retention: the VAF-T/Buf group had lower retention scores than the VAF-S/Buf group, how- ever, the difference only approached statistical signifi- cance (U = 49.5, 0.05 < P < 0.06). A Kruskal-Wallis one-way A N O V A showed that the differences among various VAF transected groups were significant (H = 13.99, P < 0.01). Paired comparisons indicated that 0.2 #g NE produced significant retention enhancement in the VAF transected rats (VAF-T/0.2 ~g vs VAF-T/Buf, U = 43.5, P < 0.005). While retention scores of the VAF- T/0.2/~g group appeared to be lower than those of the VAF-S/0.2 #g group, the difference failed to reach statistical significance (U = 50, P = (i.10). The retention performance of the VAF-T/5.0/~g group did not differ significantly from that of either the VAF-T/Buf group, or the VAF-S/5.0/~g group.
Experiment III
Previous findings have indicated that noradrenergic innervation of the amygdala is mediated, in part, by the VAF pathway 7. Thus, if the effects of peripheral E on memory involve NE innervation of the amygdala, tran- section of the VAF should be expected to attenuate, but not completely block, the effects of E. Expt. III exam- ined this implication.
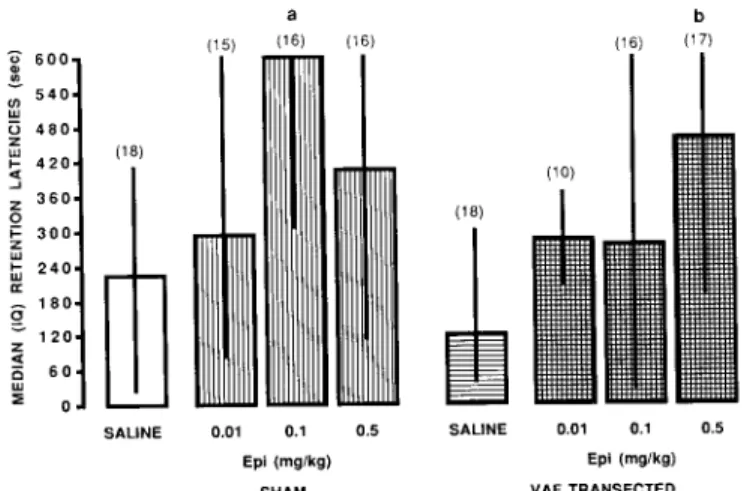
VAF-T and VAF-S rats were trained on the inhibitory avoidance task with a 0.7 mA/1 s footshock, They received s.c. injections of saline (Sal) or 0.01, 0.1, 0.5 mg/kg E immediately after training. The findings of retention performance tested 24 h later are shown in Fig. 7. In VAF-S rats, a Kruskal-Wallis one-way A N O V A revealed statistically significant differences among the
~
6 0 0 , u) 5 4 0 u.I O z 4 8 0 uJ ~ 4 2 0 Z 3 6 0 '~
3oo' 2 4 0 , 1 8 0 ' 1 2 0 . 6 0 . ,,s O, (12) BUFFER a (11) C (15) 0.2 1.0 5.0 BUFFER 0.2 1.0 5.0 NE (pg) NE (Isg)SHAM VAF TRANSECTED
Fig. 6. Retention performance (median + interquartile range) of VAF-transected or sham-operated rats receiving intra-amygdala injections of NE at various doses, a, different from Sham/Buf, P < 0.01; b, different from Sham/Bur, P < 0.001; c, different from Transected/Buf, P < 0.05. Number of subjects in parentheses.
" •
6 0 0 , 5 4 0 , (n tU 4 8 0 , ~ 4 2 0 , .J ~. 3 6 0 , 0 ..~ 3 0 0 , uJ 2 4 0 1 0 0 "~ 1 2 0 z ~ 0o ~ 0 o a (15) (16) (16) (18) SALINE 0.01 0.1 0.5 Epl (mg/kg) SHAM b {16) (17) SALINE 0.01 0,1 0.5 Epi (mg/kg) VAF TRANSECTEDFig. 7. Retention performance (median + interquartile range) of VAF-transected rats receiving subcutaneous injections of E. a, different from Sham/Sat, P < 0.01; b, different from Transected/Sal, P < 0.02. Number of subjects in parentheses.
groups ( H = 8.150, P < 0.05). Paired comparisons
indicated that the retention of the VAF-S/0.1 mg group
was significantly better than that of the VAF-S/Sal group
(U = 63.5, P < 0.01). The retention scores of the VAF-T
rats given Sal tended to be lower than those of the VAF-S
rats given Sal, although the difference was not statisti-
cally significant. A Kruskal-Wallis one-way A N O V A
revealed that the differences among VAF-transected
groups only approached statistical significance ( H = 6.38,
0.05 < P < 0.10). However, the scores of various groups
distributed differently, according to a previously set
criterion 23, among low (below 60), medium (60-400) and
high (over 400) latencies (X 2 = 13.60, P < 0.05). This
difference was thus pursued further. Paired comparisons
indicated that the retention latencies of the VAF-T/0.5
mg group were significantly greater than those of the
VAF-T/Sal group (U = 88, P < 0.05). In contrast, 0.1
mg/kg dose of E, which was most effective in enhancing
m e m o r y of the VAF sham-operated rats, did not signifi-
cantly influence the retention performance of the VAF-T
group.
DISCUSSION
Three major findings emerged from the present study.
First, ST lesions blocked the memory-modulating effects
of NE administered intra-amygdally immediately post-
training. Second, transections of the VAF did not block
the m e m o r y enhancing effect of intra-amygdala injections
of NE. Third, transections of the VAF attenuated, but
did not block the memory-enhancing effect of peripher-
ally administered E: the effective enhancing dose of E
was shifted from 0.1 mg/kg to 0.5 mg/kg.
In the present study, ST lesions did not affect retention
performance of otherwise untreated animals, but blocked
both the enhancing and impairing effects of intra-
amygdala injections of NE at, respectively, low and high
doses. These findings, which are consistent with those of
previous studies 13'14'2°-22'27, indicate that the blocking of
the modulating effect of NE on retention is not due to a
general influence of the lesions on retention perfor-
mance.
Our findings, considered together with those of pre-
vious evidence 2~, clearly indicate that an intact ST is
essential for the memory-modulating effects of intra-
amygdala NE, as well as peripheral E. Thus, the findings
are consistent with the view that amygdala efferents
within the ST mediate the memory-modulating influences
initiated by activation of NE receptors within the amyg-
daloid complex. Alternatively, it might be that the
memory modulatory effects of amygdala NE involve
efferent influences through other pathways but require
integrated ST-mediated input to the amygdala. While the
231
present findings are consistent with both possibilities, our
previous findings that the amnestic effect of electrical
amygdala stimulation can be blocked by either ST lesions
or naloxone, an opiate antagonist, injected into the bed
nucleus of the
ST 2°'24 argue that the ST mediates
amygdala outputs involved in modulating memory stor-
age processes.
While the radio frequency used to lesion the ST may
have also damaged structures immediately surrounding
the ST, such damage does not appear to contribute
significantly to the effect of the lesion in blocking the
memory modulatory influences. In a previous study we
found no correlation between the extent and location of
extra-ST damage and the loss of E-induced memory
enhancement in ST-lesioned rats 2~. The effect of the ST
lesions reported here is also unlikely to be due to a
transient effect of the lesions. We previously found that
ST lesions made 2 - 4 weeks prior to training blocked the
memory modulatory effect of post-training electrical
stimulation of the amygdala without affecting retention
performance of otherwise untreated animals
TM.
VAF transections, which were produced in sequential
unilateral surgeries, failed to block the memory enhan-
cing effect of intra-amygdala NE injections. Such findings
are congruent with our previous finding that single-stage
bilateral VAF transections failed to attenuate the mem-
ory enhancing effect of posttraining electrical stimulation
of the amygdala 2°. In sham-transected (VAF-S) rats,
retention was impaired by the high dose of NE (5.0 ktg).
In transected (VAF-T) rats, the effect of the high dose of
NE was less clear. The retention performance of the
VAF-T/5.0 ~g group was not significantly poorer than
that of the VAF-T/Buf controls but was not significantly
better than that of the VAF-S/5.0 ~tg group either. And,
as was noted above, the retention performance of the
latter group was significantly impaired in comparison
with that of the VAF-S/Buf controls. Therefore, the
question of whether VAF transections attenuate the
memory impairing effect of NE at high doses remains
inconclusive and requires further elucidation.
It has been reported that VAF transections, produced
by surgical procedures comparable to those used in the
present study, result in a 70% reduction of NE within the
amygdala 7. Thus, on the assumption that the VAF
mediates E effects on NE release within the amygdala,
VAF transections should be expected to shift the d o s e -
response effects of E on retention. Our findings are
consistent with this assumption: in the VAF-transected
rats, the dose of E required for enhancement of retention
was greater than that required for the VAF sham-
operated rats. While the present results cannot rule out
possible involvement of non-VAF fibers damaged by the
transection in the effect, our interpretation of the
findings are consistent with evidence indicating that partial depletion of amygdala N E (produced by the n e u r o t o x i n DSP-4) a t t e n u a t e s the effects of peripherally a d m i n i s t e r e d E on r e t e n t i o n
TM,
as well as other evidence indicating that the effect of peripherally a d m i n i s t e r e d E on m e m o r y d e p e n d s u p o n the integrity of amygdala f u n c t i o n i n g 19-2I .C o n s i d e r e d together, the findings of the present e x p e r i m e n t s are consistent with the view that the V A F mediates, at least in part, peripheral E effects on amygdala N E which influences m e m o r y storage through effects involving the ST pathway. This general hypothesis is also consistent with extensive evidence indicating that central n o r a d r e n e r g i c n e u r o n s are responsive to periph- eral visceral challenge, including systemic injections of E 5'12'29'37. Peripheral E might m o d u l a t e amygdala func- t i o n i n g by activating visceral pathways influencing brain- stem N E n e u r o n s . A l t e r n a t i v e l y , E might activate other
n o n - N E pathways in the V A F that m o d u l a t e N E release within the amygdala.
As yet, little is k n o w n a b o u t brain systems influenced by activation of the ST in the m o d u l a t i o n of m e m o r y storage. T h e ST provides reciprocal i n t e r c o n n e c t i o n b e t w e e n the central nucleus of the amygdala and the bed nucleus of the ST, which project to m a n y brain areas, including the basal forebrain m a g n o c e l l u l a r cholinergic n e u r o n s 1. Thus, it would be of considerable interest to d e t e r m i n e whether e n d o g e n o u s m o d u l a t o r y influences on m e m o r y storage is based, at least in part, on interactions of the amygdala with basal forebrain cholinergic systems.
Acknowledgements. We thank Ines Introini-Collison and Nancy
Collett for assistance in preparation of this manuscript. This research was supported by Grant NSC-76-0301-H002-02 from the National Science Council of the Republic of China (to K.C.L.) and Office of Naval Research Contract N00014-87-0518 and USPHS Grant MH12526 from the National Institute of Mental Health and the National Institute of Drug Abuse (to J.L.McG).
REFERENCES
1 Alheid, G.E and Heimer, L., New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal com- ponents of substantia innominata, Neuroscience, 27 (1988) 1-39. 2 Borsini, F. and Rolls, E.T., Role of noradrenaline and serotonin in the basolateral region of the amygdala in food preferences and learned taste aversion in the rat, Physiol. Behav., 33 (1984) 37-43.
3 Davis, M., Hitchcock, J.M. and Rosen, J.B., Anxiety and amygdala: pharmacological and anatomical analysis of the fear-potentiated startle paradigm. In G.H. Bower (Ed.), The
Psychology of Learning and Motivation, Vol. 21, Academic,
London, 1987, pp. 263-305.
4 Dietl, H., Temporal relationship between noradrenaline release in the central amygdala and plasma noradrenaline secretion in rats and tree shrews, Neurosci. Lea., 55 (1985) 41-46. 5 Elam, M., Thoren, P. and Svensson, T.H., Locus coeruleus
neurons and sympathetic nerves: activation by visceral afferents,
Brain Research, 375 (1986) 117-125.
6 Ellis, M.E. and Kesner, R.P., The noradrenergic system of the amygdala and aversive memory processing, Behav. Neurosci., 97 (1983) 399-415.
7 Emson, P.C., Bj6rklund, A., Lindvall, O. and Paxinos, G., Contribution of different afferent pathways to catecholamine and 5-hydroxytryptamine innervation of the amygdala: a neu- rochemical and histochemical study, Neuroscience, 4 (1979) 1347-1357.
8 Fallon, J.H., Koziell, D.A. and Moore, R.A., Catecholamine innervation of the basal forebrain. II. Amygdalal suprarhinal cortex and entorhinai cortex, J. Comp. Neurol., 180 (1978) 509-532.
9 Fallon, J.H., Histochemical characterization of dopaminergic, noradrenergic and serotonergic projections to the amygdala. In Y. Ben-Ari (Ed.), The Amygdala Complex, Elsevier, Amster- dam, 1981, pp. 175-184.
10 Gallagher, M., Kapp, B.S., Pascoe, J.P. and Rapp, P.R., A neuropharmacology of amygdala systems which contribute to learning and memory. In Y. Ben-Ari (Ed.), The Amygdaloid
Complex, Elsevier, Amsterdam, 1981, pp. 343-354.
11 Gold, P.E. and van Buskirk, R., Effects of post-trial hormone injections on memory process, Horm. Behav., 7 (1976) 509-517. 12 Holdefer, R.N. and Jensen, R.A., The effects of peripheral
D-amphetamine, 4-OH amphetamine, and epinephrine on main- tained discharge in the locus coeruleus with reference to the modulation of learning and memory by these substances, Brain
Research, 417 (1987) 108-117.
13 Introini-Collison, I.B., Arai, Y. and McGaugh, J.L., Stria terminalis lesions attenuate the effects of posttraining atropine and oxotrcmorine of retention, submitted.
14 Introini-Collison, I.B., Nagahara, A. and McGaugh, J.L., Memory-enhancement with intra-amygdala posttraining nalox- one is blocked by concurrent administration of propranolol,
Brain Research, 476 (1989) 94-101.
15 Jones, B.E. and Moore, R.Y., Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study, Brain
Research, 127 (1977) 23-53.
16 Kapp, B.S., Frysinger, R.C., Gallagher, M. and Bretschneider, A.J., Effects of amygdala and stria terminalis lesions on aversive conditioning in the rabbit, Soc. Neurosci. Abstr., 3 (1973) 236. 17 LeDoux, J.E., Iwata, J. and Reis, D.J., Different projections of the central amygdaloid nucleus mediate autonomic and behav- ioral correlates of conditioned fear, J. Neurosci., 8 (1988) 2517-2529.
18 Liang, K.C. and Chen, L., Intra-amygdala injections of DSP-4 attenuate the memory enhancing effect of peripheral epineph- rine, Soc. Neurosci. Abstr., 12 (1986) 702.
19 Liang, K.C., Juler, R.G. and McGaugh, J.L., Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system, Brain Research, 368 (1986) 125-133.
20 Liang, K.C. and McGaugh, J.L., Lesions of the stria terminalis attenuate the amnestic effect of amygdaloid stimulation on avoidance responses, Brain Research, 274 (1983) 309-31& 21 Liang, K.C. and McGaugh, J.L., Lesions of the stria terminalis
attenuate the enhancing effect of posttraining epinephrine on retention of an inhibitory avoidance response, Behav. Brain
Res., 9 (1983) 49-58.
22 Liang, K.C. and McGaugh, J.L., Effects of adrenal demedul- lation and stria terminalis lesions on retention of inhibitory avoidance response, Psychobiology, 15 (1987) 154-160. 23 Liang, K.C., McGaugh, J.L., Martinez, Jr., J.L., Jensen, R.A.,
Vasquez, B.J. and Messing, R.B., Posttraining amygdaloid lesions impair retention of an inhibitory avoidance response,
Behav. Brain Res., 4 (1982) 237-249.
24 Liang, K.C., Messing, R.B. and McGaugh, J.L., Naloxone attenuates amnesia caused by amygdaloid stimulation: the
involvement of a central opioid system, Brain Research, 271
(1983) 41-49.
25 McGaugh, J.L., Hormonal influences on memory, Ann. Rev.
Psychol., 27 (1983) 297-323.
26 McGaugh, J.L., lntroini-Collison, I. and Nagahara, A., Mem- ory-enhancing effects of posttraining naloxone: involvement of
fl-noradrenergic influences in the amygdaloid complex, Brain
Research, 446 (1988) 37-49.
27 McGaugh, J.L., Introini-Collison, I., Juler, R.G. and lzquierdo, I., Stria terminalis lesions attenuate the effects of posttraining
naloxone and fl-endorphin on retention, Behav. Neurosci., 100
(1986) 839-844.
28 Mishkin, M., Malamut, B. and Bachevalier, J., Memories and habits: two neural systems. In G. Lynch, J.L. McGaugh and
N.M. Weinberger (Eds.), Neurobiology of Learning and Mem-
ory, Guilford, New York, 1984, pp. 65-77.
29 Morilak, D.A., Fornal, C.A. and Jacobs, B., Effects of physiological manipulations on locus coeruleus neuronal activity
in freely moving cats. II. Cardiovascular challenge, Brain
Research, 422 (1988) 24-31.
30 Paxinos, G. and Watson, C., The Rat Brain in Stereotaxic
Coordinates, Academic, New York, 1986.
31 Peskind, E.R., Raskind, M.A., Wilkinson, C.W., Flatness, D.E. and Halter, J.B., Peripheral sympathectomy and adrenal me-
233
dullectomy do not alter cerebrospinal fluid norepinephrine,
Brain Research, 367 (1986) 258-264.
32 Ross, J.F. and Grossman, S.P., Transections of stria medullaris or stria terminalis in the rat: effects of aversively controlled
behavior, J. Comp. Physiol. Psychol., 70 (1977) 907-915.
33 Sarter, M. and Markowitsch, H.J., Involvement of the amygdala in learning and memory: a critical review, with emphasis on
anatomical relationship, Behav. Neurosci., 99 (1985) 342-380.
34 Squire, L., Mechanisms of memory, Science, 232 (1986) 1612-
1619.
35 Squire, L.R, and Cohen, N.J., Human memory and amnesia. In
G. Lynch, J.L. McGaugh and N.M. Weinberger (Eds.), Neu-
robiology of Learning and Memory, Guilford, New York, 1984,
pp. 3-64.
36 Sternberg, D.B., Isaacs, K.R., Gold, P.E. and McGaugh, J.L., Epinephrine facilitation of appetitive learning: attenuation with
adrenergic receptor antagonists, Behav. Neural Biol., 44 (1985)
447-453.
37 Svensson, T.H., Peripheral, autonomic regulation of locus coeruleus noradrenergic neurons in brain: putative implications
for psychiatry and psychopharmacology, Psychopharmacology,
92 (1987) 1-7.
38 Weil-Malherbe, H., Axelrod, H. and Tomchick, R., Blood-