Title: Performance of classification criteria for gout in early and late disease
Authors: William J. Taylor1, Jaap Fransen2, Nicola Dalbeth3, Tuhina Neogi4, H. Ralph Schumacher5, Melanie Brown1, Worawit Louthrenoo6, Janitzia Vazquez-Mellado7, Maxim Eliseev8, Geraldine McCarthy9 10, Lisa K. Stamp11, Fernando Perez-Ruiz12, Francisca
Sivera13,Hang-Korng Ea14 15, Martijn Gerritsen16, Carlo Scire17, Lorenzo Cavagna17, Chingtsai Lin18, Yin-Yi Chou19, Anne-Kathrin Tausche20, Geraldo da Rocha Castelar-Pinheiro21, Matthijs
Janssen22, Jiunn-Horng Chen23 24, Ole Slot25, Marco Cimmino26, Till Uhlig27, Tim L. Jansen2
Institutions: 1University of Otago Wellington, New Zealand. 2Radboud University, Nijmegen, Netherlands. 3University of Auckland, New Zealand.
4Boston University School of Medicine, Boston MA. 5University of Pennsylvania, Philadelphia PA.
6Division of Rheumatology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
7Servicio de Reumatología, Hospital General de México, México City, México.
8Nasonova Research Institute of Rheumatology of Russia, Moscow, Russia.
9School of Medicine and Medical Science, University College Dublin, Ireland
10Mater Misericordiae University Hospital, Eccles St, Dublin, Ireland
11Department of Medicine, University of Otago Christchurch, PO Box 4345, Christchurch 8140, New Zealand
12Rheumatology Division, Hospital Universitario Cruces & BioCruces Health Institute, Vizcaya, Spain.
13Department Reumatologia, Hospital General Universitario de Elda, Alicante, Spain.
14Univ. Paris Diderot, Sorbonne Paris Cité, UFR de Médecine, F-75205 Paris, France ; INSERM, UMR 1132, Hôpital Lariboisière, F-75475 Paris, France
15Assistance Publique-Hôpitaux de Paris (AP-HP), Hôpital Lariboisière, Service de Rhumatologie, Centre Viggo Petersen, Pôle Appareil Locomoteur, 2, Rue Ambroise Paré, F-75010 Paris, France
16Amsterdam Rheumatology Immunology Center (ARC), Department of Rheumatology, Westfries Gasthuis, Hoorn, The Netherlands.
17University and IRCCS Policlinico S. Matteo Foundation, Viale Golgi 3, 27100, Pavia, Italy.
18Division of Rheumatology and Immunology, Taichung Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taiwan
19Taichung Veterans' General Hospital, 160 Taichung Road Section 3, Taichung, Taiwan 40705, ROC.
20Division of Rheumatology, Department of Internal Medicine III, University Hospital Carl Gustav Carus, Fetscherstrasse 74, D-01307 Dresden, Germany.
21Faculdade de Ciências Médicas da Universidade do Estado do Rio de Janeiro (FCM-UERJ), Brasil.
22Rijnstate Hospital, Arnhem, The Netherlands.
23School of Medicine, China Medical University, Taichung, Taiwan, ROC
24Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan, ROC
24Dipartimento di Medicina Interna e Specialità Mediche, Università di Genova, Italy
25Department of Rheumatology. Copenhagen University Hospital Glostrup DK-2600, Denmark.
26Research Laboratory and Academic Division of Clinical Rheumatology, Department of Internal Medicine, University of Genova, Italy.
27National Advisory Unit on Rehabilitation in Rheumatology, Department of Rheumatology, Diakonhjemmet Hospital, Box 23 Vinderen, 0319 Oslo, Norway.
Corresponding author: Associate Professor William Taylor, Department of Medicine, University of Otago Wellington, PO Box 7343, Wellington, New Zealand
Phone +64 4 806 1801 Fax +64 4 389 5427
Email will.taylor@otago.ac.nz
Keywords: Gout, classification criteria
Acknowledgements: This study was supported by the American College of
Rheumatology, European League against Rheumatism, Arthritis New Zealand, Association Rhumatisme et Travail, and Asociación de Reumatólogos del Hospital de Cruces.
We gratefully acknowledge the help of Ana Beatriz-Vargas (Rio de Janeiro, Brazil), Fatima Kudaeva (Moscow, Russia), Angelo Gaffo (Birmingham AL), Douglas White (Hamilton, New Zealand), and Juris Lazovskis (Sydney, Canada) with data collection.
We particularly acknowledge Victoria Barskova (Moscow, Russia) who died during the course of this study and wish to dedicate this manuscript to her memory.
Abstract:
Objectives: To compare the sensitivity and specificity of different classification criteria for gout in early and late disease.
Methods: This was a case-control study of rheumatology clinic patients in which gout was defined by presence or absence of monosodium urate (MSU) crystals as observed by a certified examiner. Early disease was defined as patient-reported onset of symptoms of 2 years or less. Results: Data from 983 patients (509 cases, 474 controls) were collected. Early disease was present in 144 cases and 228 controls. Sensitivity across criteria was better in late disease (95.3% vs 84.1%, p<0.001) and specificity was better in early disease (79.9% vs 52.5%, p<0.001). The overall best performing criteria were the New York criteria with sensitivity/specificity in early and late disease of 100/87.7 and 100/70.1. No criteria that did not require synovial fluid analysis had sensitivity and specificity of more than 80% in both early and late disease.
Conclusions: Existing classification criteria for gout have sensitivity of over 80% in early and late disease but currently available criteria that do not require synovial fluid analysis have inadequate specificity especially in later disease. Classification criteria for gout with better specificity are required.
Gout is the most common inflammatory arthritis in men and is increasing in prevalence and incidence (1). Most gout is managed in primary care where the diagnosis seldom relies upon identification of MSU crystals. Therefore, classification criteria that do not require MSU crystal identification would be useful for clinical research conducted in primary care settings. Six classification criteria for gout have been developed but the most widely used is the 1977 American Rheumatism Association criteria (2).
Current classification criteria have been tested in patient populations with average disease duration of 7 to 10 years (2, 3) or where disease duration was not reported (4-7). However, identification of patients with early disease is likely to be important to test important questions related to early treatment of gout or in order to study the natural history of gout in inception cohorts.
The Study for Updated Gout ClAssification CRiteria (SUGAR) was undertaken as part of an American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) project to update gout classification criteria (8) and allows a direct comparison of existing criteria in early disease compared to later disease duration.
Methods
Consecutive patients attending a rheumatology clinic with joint swelling or a subcutaneous nodule within the previous 2 weeks, either of which was judged by a clinical investigator to be conceivably due to gout were enrolled into this case-control study. A clinical diagnosis was recorded by the rheumatologist prior to synovial fluid/tissue microscopy. Clinical data including all items within published classification criteria were collected at the index visit. Each centre received Ethics Committee Approval or Institutional Review Board approval according to local requirements.
Gold-standard for gout (case definition)
All patients underwent arthrocentesis or tissue aspiration for polarizing microscopy to identify MSU crystals. All microscopy was undertaken by observers who had passed a 2-stage MSU-identification certification procedure, which consisted of a web-based crystal recognition test followed by examination of 5 to 8 vials of synovial fluid (SF) from the laboratories of Eliseo Pascual (European centres) or H. Ralph Schumacher (rest of the world). The web-based test was strict and had a high non-pass rate (9). Each SF sample in the second stage needed to be correctly identified as demonstrating MSU crystals or not to achieve certification.
Cases (gout) were defined as patients with MSU crystals identified by a certified observer. Controls (non-gout) were defined as patients without MSU crystals, irrespective of the clinical diagnosis. Synovial fluid/tissue microscopy by a certified observer was performed within 1 month of the index visit and was blinded to the collection of potential classification items.
Disease duration
Disease duration was defined by patient self-report of the time since onset of first symptoms. Early disease was defined as symptom onset of no more than 2 years; late disease was defined as symptom duration of more than 2 years.
Statistical analysis
The comparison criteria sets from published studies were the 1977 ARA preliminary criteria (both survey format and complete format) (2), an abbreviated form of the ARA criteria (Mexico) (10), a criteria set developed in primary care (Netherlands) (4), the Rome and New York (11) criteria and modified versions of the Mexico, Rome and New York criteria that excluded SF or tissue microscopy. The details of these criteria are shown in Supplementary Table 1. The
sensitivity and specificity of each criteria set was calculated in early and late disease separately. In addition, a sensitivity analysis that excluded the control patients who had a clinical diagnosis of gout but were MSU crystal negative was performed to check that specificity estimates were not unduly under-estimated by contamination of the control sample with gout cases. It should be noted that specificity is likely to be over-estimated in this analysis.
Statistical comparison of differences in sensitivity or specificity was done using logistic
regression in cases (for sensitivity) and controls (for specificity). The ARA (survey) criteria were the reference category for criteria so that the quoted odds ratio are relative to the
sensitivity/specificity of the ARA (survey) criteria. Separate regression models were used for early and late disease to compare sensitivity/specificity by disease duration and a full regression model that included disease duration (early/late) as a categorical covariate and as an interaction term was also calculated to assess the overall effect of disease duration on sensitivity and specificity across all criteria sets. For zero cells, 0.5 was added to permit estimation.
Receiver operating characteristic curves were plotted. These are the false positive (proportion of controls classified as cases) and true positive (proportion of cases classified as cases) rates plotted against each other. Plots for each criteria-set were constructed separately for early and late disease.
Results
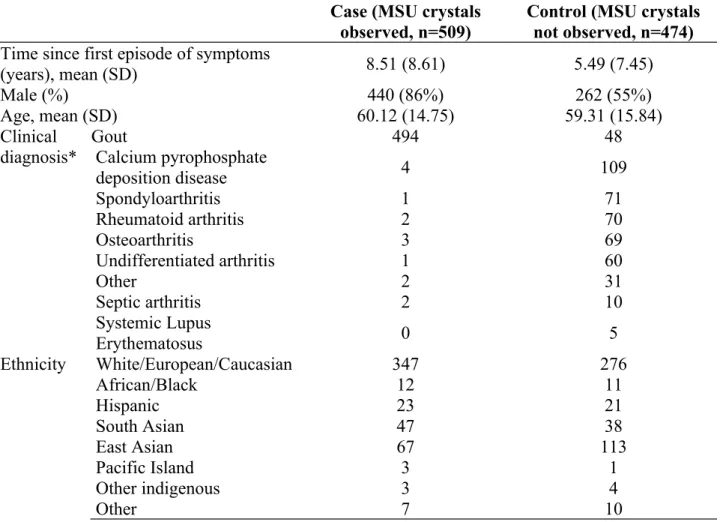
Twenty-five centres from 16 countries were involved with the data collection. Data from 983 patients (509 cases, 474 controls) were collected, of whom 702 (71.4%) were male (Table 1).
Early disease (2 years or less) was observed in fewer gout cases (144, 28.5%) than non-gout controls (228, 48.5%). Controls had various clinical diagnoses shown in Table 1.
Across all criteria-sets, later disease was associated with better sensitivity (95.3%) than early disease (84.1%), (OR 4.4, 95%CI 2.5 to 7.8, p<0.001). Conversely, early disease was associated with better specificity (79.9% versus 52.5%), (OR 4.7, 95%CI 2.8 to 7.7, p<0.001). There was no significant interaction between disease duration and particular criteria in respect of sensitivity or specificity.
The sensitivity (Table 2) and specificity (Table 3) for each classification criteria by disease duration are shown. The point estimates for sensitivity and specificity are also shown plotted on a receiver operating characteristic curve (Supplementary Figure 1) and performance of each criteria in the combined data is shown in Supplementary Table 2). Note that criteria which include MSU crystals in synovial fluid or tissue alone as sufficient for classification will show 100% sensitivity by definition, since case-ness in the SUGAR dataset only required
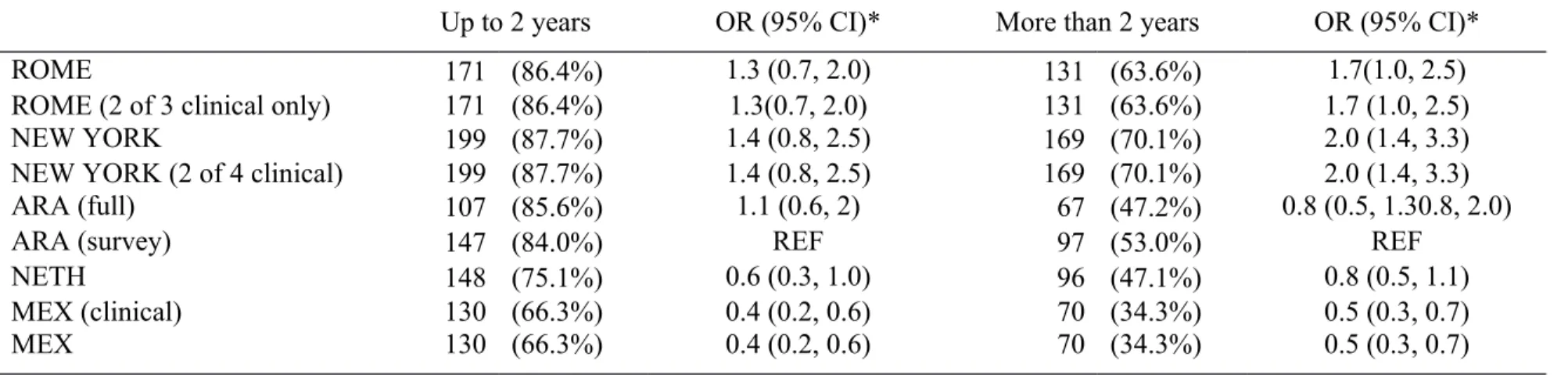
demonstration of MSU crystals. Exclusion of MSU crystal examination as a criterion for classification generally led to a marked reduction in sensitivity. Nevertheless, all criteria had adequate sensitivity in later disease. The Netherlands criteria and the version of the Mexico criteria that did not rely upon MSU crystals demonstrated adequate sensitivity even in early disease. Conversely, specificity was generally less satisfactory and worse in later disease, particularly for the Mexico and Netherlands criteria.
Excluding controls with a clinical diagnosis of gout (but who were negative for MSU crystals) specificity estimates improved somewhat to 69.3% to 90.4% (early disease) and 39.3% to 77.1% (later disease) (Supplementary Table 2). The criteria with the best specificity were the New York criteria (90.4% in early disease and 77.1% in later disease).
Discussion
Early disease is more challenging to accurately classify since not all characteristic features will present early. This analysis of the SUGAR dataset has shown that older gout classification criteria that include MSU examination perform well in late disease and that newer clinical-only classification criteria (Mexico and Netherlands) have fairly good sensitivity even for early disease. Specificity in early disease for all criteria is better than in late disease, but specificity is problematic for well-established disease.
The context of study is likely to be important when deciding on the optimal sensitivity and specificity of classification criteria. For early phase studies of new treatments of unknown toxicity, criteria with very high specificity is likely to be necessary, whereas epidemiological or outcomes researchers may wish to be more inclusive and value sensitivity over specificity. For studies in early disease, the preferred trade-offs in misclassification also depend on the purpose of the study. Probably intervention studies that aim to test the effectiveness of treatment in early disease would also require highly specific classification criteria.
A previous study showed the specificity of the clinical versions of the Rome, New York and ARA criteria to be 78.8% to 88.5%, which is somewhat greater than we observed (6). The difference may be due to the selection of patients in that study, who were recruited because they had undergone synovial fluid aspiration at any time (6), leading to the possibility of diagnostic confounding. In another study of the ARA criteria in general practice patients presenting with symptoms possibly due to gout, the specificity was only 64% (5), which is closer to what we observed. It is likely that the large number of controls with CPPD (23% of control group) and inclusion of patients with a clinical diagnosis of gout but negative for MSU crystals as control patients (10%) contributed to the lower specificity estimates observed in our study.
The strengths of this study include the rigorous gold-standard diagnostic test being available in all cases and controls, the large numbers of participants from multiple geographic sites, pertinent comparator diseases, and the comprehensive data collection that allowed classification by
multiple criteria sets. In addition, we confirmed specificity estimates in controls without clinical diagnoses of gout. The main limitation is the recruitment of patients from specialist
rheumatology centres, which confers unavoidable spectrum bias (likelihood of more severe disease than is seen in primary care).
Nonetheless, the results of this study suggest that the major problem for existing classification criteria is not so much inadequate sensitivity in early disease but rather low specificity in early and (even more so) in later disease, particularly for criteria that do not require MSU crystal examination. Criteria with better specificity are required, especially for well-established disease.
Table 1. Participant characteristics
Case (MSU crystals
observed, n=509) Control (MSU crystalsnot observed, n=474) Time since first episode of symptoms
(years), mean (SD) 8.51 (8.61) 5.49 (7.45)
Male (%) 440 (86%) 262 (55%)
Age, mean (SD) 60.12 (14.75) 59.31 (15.84)
Clinical
diagnosis* GoutCalcium pyrophosphate 494 48
deposition disease 4 109 Spondyloarthritis 1 71 Rheumatoid arthritis 2 70 Osteoarthritis 3 69 Undifferentiated arthritis 1 60 Other 2 31 Septic arthritis 2 10 Systemic Lupus Erythematosus 0 5 Ethnicity White/European/Caucasian 347 276 African/Black 12 11 Hispanic 23 21 South Asian 47 38 East Asian 67 113 Pacific Island 3 1 Other indigenous 3 4 Other 7 10
Table 2. Sensitivity of criteria sets, by duration of symptoms (n, %)
Up to 2 years OR (95% CI)* More than 2 years OR (95% CI)*
ROME 131 (92.9%) 5.4 (2.5,11.4) 350 (99.4%) 16.1 (3.8, 68.6)
ROME (2 of 3 clinical) 85 (60.3%) 0.62 (0.37, 1.04) 297 (84.4%) 0.5 (0.3, 0.8)
NEW YORK† 144 (100.0%) 117.8 (7.1, 1944.4) 362 (100.0%) 66.8 (4.0, 1100.6)
NEW YORK (2 of 4 clinical) 83 (57.6%) 0.56 (0.3, 0.9) 315 (87.5%) 0.6 (0.4, 1.07)
ARA (full)† 144 (100.0%) 117.8 (7.1, 1944.4) 362 (100.0%) 66.8 (4.0, 1100.6)
ARA (survey) 88 (71.0%) REF 282 (91.6%) REF
NETH 124 (87.9%) 3.0 (1.6, 5.6) 333 (96.0%) 2.2 (1.1, 4.3)
MEX (clinical) 123 (87.2%) 2.8 (1.5, 5.2) 342 (98.6%) 6.3 (2.4, 16.6)
MEX (full)† 144 (100.0%) 117.8 (7.1, 1944.4) 362 (100.0%) 66.8 (4.0, 1100.6)
* OR represent the odds of each criteria being positive vs being negative in cases compared to the ARA (survey) criteria. Higher OR means that the criteria are more sensitive than the ARA (survey) criteria.
†These criteria can be fulfilled with presence of MSU crystals alone
Table 3. Specificity of each criteria, by duration of symptoms (n, %)
Up to 2 years OR (95% CI)* More than 2 years OR (95% CI)*
ROME 171 (86.4%) 1.3 (0.7, 2.0) 131 (63.6%) 1.7(1.0, 2.5)
ROME (2 of 3 clinical only) 171 (86.4%) 1.3(0.7, 2.0) 131 (63.6%) 1.7 (1.0, 2.5)
NEW YORK 199 (87.7%) 1.4 (0.8, 2.5) 169 (70.1%) 2.0 (1.4, 3.3)
NEW YORK (2 of 4 clinical) 199 (87.7%) 1.4 (0.8, 2.5) 169 (70.1%) 2.0 (1.4, 3.3)
ARA (full) 107 (85.6%) 1.1 (0.6, 2) 67 (47.2%) 0.8 (0.5, 1.30.8, 2.0)
ARA (survey) 147 (84.0%) REF 97 (53.0%) REF
NETH 148 (75.1%) 0.6 (0.3, 1.0) 96 (47.1%) 0.8 (0.5, 1.1)
MEX (clinical) 130 (66.3%) 0.4 (0.2, 0.6) 70 (34.3%) 0.5 (0.3, 0.7)
MEX 130 (66.3%) 0.4 (0.2, 0.6) 70 (34.3%) 0.5 (0.3, 0.7)
* OR represent the odds of each criteria being negative vs being positive in controls compared to the ARA (survey) criteria. Higher OR means that the criteria are more specific than the ARA (survey) criteria.
Supplementary Table 1. Existing classification criteria for gout Rome (1963)
1. Serum uric acid >7mg/dl (male) or >6mg/dl (female) 2. Presence of tophi
3. MSU crystals in synovial fluid or tissue
4. History of attacks of painful joint swelling with abrupt onset and resolution within two weeks
TWO OR MORE criteria required
New York (1968)
1. At least two attacks of painful joint swelling with complete resolution with two weeks 2. A history or observation of podagra
3. Presence of tophi
4. Rapid response to colchicine treatment, defined as a major reduction in the objective signs of inflammation within 48 hours
TWO OR MORE criteria required
OR MSU crystals in synovial fluid or tissue
ARA (full) (1977)
1. More than one attack of acute arthritis
2. Maximum inflammation developed within one day 3. Monoarthritis attack
4. Redness observed over joints
5. First metatarsophalangeal joint painful or swollen 6. Unilateral first metatarsophalangeal joint attack 7. Unilateral tarsal joint attack
8. Tophus (suspected or proven) 9. Hyperuricemia
10. Asymmetric swelling within a joint on x-ray 11. Subcortical cysts without erosions on xray 12. Joint fluid negative for organisms during attack
SIX OR MORE criteria required
OR MSU crystals in joint fluid or tophus
ARA (survey) (1977)
1. More than one attack of acute arthritis
2. Maximum inflammation developed within one day 3. Oligoarthritis attack
4. Redness observed over joints
5. First metatarsophalangeal joint painful or swollen 6. Unilateral first metatarsophalangeal joint attack 7. Unilateral tarsal joint attack
8. Tophus (suspected or proven) 9. Hyperuricemia
10. Asymmetric swelling within a joint on x-ray 11. Complete termination of an attack
SIX OR MORE criteria required
Mexico (2011)
1. More than 1 attack of arthritis
3. Mono and/or oligo-articular attacks 4. Podagra
5. Joint erythema
6. Unilateral tarsal joint attack 7. Tophus (suspected or proven)
8. Hyperuricemia (more than 2 SD greater than the normal population average)
FOUR OR MORE criteria required OR MSU crystal identification
Netherlands (2010) 2 Male sex
2 Previous patient reported arthritis attack 0.5 Onset within one day
1 Joint redness
2.5 MTP1 involvement
1.5 Hypertension or more than 1 cardiovascular disease 3.5 Serum uric acid level > 5.88 mg/dL
13 Presence of a tophus
Each item contributes its weighted score as shown. A summed score of 4 or less excludes gout; 8 or more suggests gout; between 4 and 8 suggests need for synovial fluid analysis.
Supplementary Table 2. Overall performance of each classification criteria set (combined early and late disease)
Sensitivity (%) Specificity (%) AUC (95%CI)* Not able to be classified (%) ROME 97.6 74.5 0.87 (0.84, 0.90) 8.1 ROME (2 of 3 clinical only) 77.6 74.5 0.77 (0.73, 0.81) 8.1 NEW YORK 100.0 78.4 0.89 (0.86, 0.92) 0.2 NEW YORK (2 of 4 clinical) 78.9 78.4 0.79 (0.76, 0.83) 0.5 ARA (full) 100.0 64.8 0.83 (0.79, 0.86) 20.8 ARA 85.7 68.1 0.79 (0.75, 0.83) 19.2 MEX (Clinical) 95.3 49.5 0.74 (0.70, 0.78) 9.1 MEX 95.3 49.5 0.76 (0.72, 0.80) 7.1 NETH 93.7 60.7 0.78 (0.74, 0.82) 9.0
* Area under the curve of a receiver operating characteristic curve. Values of 1 indicate that the criteria perfectly discriminate between cases and controls and values of 0.5 indicate that the discrimination is no better than chance.
Supplementary Table 3. Specificity of each criteria, having excluded controls with a clinical diagnosis of gout
Up to 2 years (n, %) More than 2 years (n, %)
ROME 162 (90.0%) 126 (70.0%)
ROME (2 of 3 clinical only) 162 (90.0%) 126 (70.0%)
NEW YORK 188 (90.4%) 162 (77.1%)
NEW YORK (2 of 4 clinical) 188 (90.4%) 162 (77.1%)
ARA (full) 102 (86.4%) 66 (51.6%)
ARA (survey) 141 (87.6%) 96 (58.2%)
MEX (clinical) 124 (69.3%) 70 (39.3%)
MEX 124 (69.3%) 70 (39.3%)
Supplementary Figure 1. Performance of each criteria-set is shown on a receiver operating characteristic curve. [A] Disease duration less than or equal to 2 years; [B] disease duration greater than 2 years.
0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 0.93 0.6 0.58 0.71 0.88 0.87
False positive rate (1-specificity)
Tr u e p os iti ve r a te ( se n si ti vi ty ) 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 0.99 0.84 1 0.88 1 0.92 0.96 0.99
False positive rate (1-specificity)
Tr u e n e ga ti ve r a te ( se ns iti vi ty )
[A]
[B]
References
1. Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364(5):443-52.
2. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895-900.
3. Rigby AS, Wood PHN. Serum uric acid levels and gout: what does this herald for the population? Clin Exp Rheumatol. 1994;12:395-400.
4. Janssens HJ, Fransen J, van de Lisdonk EH, van Riel PL, van Weel C, Janssen M. A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis. Arch Intern Med.
2010;170(13):1120-6.
5. Janssens HJEM, Janssen M, van de Lisdonk EH, Fransen J, van Riel PLCM, van Weel C. Limited validity of the American College of Rheumatology criteria for classifying patients with gout in primary care. Ann Rheum Dis. 2009:-.
6. Malik A, Schumacher HR, Dinnella JE, Clayburne GM. Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis. JCR: Journal of Clinical Rheumatology. 2009;15(1):22-4.
7. Vazquez-Mellado J, Hernandez-Cuevas CB, Alvarez Hernandez E, Ventura-Rios L,
Pelaez-Ballestas I, Casasola, et al. The diagnostic value of the proposal for clinical gout diagnosis (CGD). Clin Rheumatol. 2011;DOI 10.1007/s10067-011-1873-4.
8. Dalbeth N, Fransen J, Jansen TL, Neogi T, Schumacher HR, Taylor WJ. New classification criteria for gout: a framework for progress. Rheumatology (Oxford). 2013;52(10):1748-53.
9. Berendsen D, Jansen TL, Taylor W, Neogi T, Fransen J, Pascual E, et al. A critical appraisal of the competence of crystal identification by rheumatologists (abstract). Ann Rheum Dis. 2013;72(Suppl 3):981.
10. Pelaez-Ballestas I, Hernandez-Cuevas C, Burgos-Vargas R, Hernandez-Roque L, et al. Diagnosis of chronic gout: evaluating the american college of rheumatology proposal, European league against rheumatism recommendations, and clinical judgment. J Rheumatol. 2010;37(8):1743-8.
11. Decker JL. Report from the subcommittee on diagnostic criteria for gout. In: Bennett PH, Wood PHN, editors. Population studies of the rheumatic diseases - Proceedings of the third International Symposium New York, June 5-10, 1966. Amsterdam: Excerpta Medica Foundation; 1968. p. 385-7.