ORIGINAL ARTICLE
Clinical and pathophysiological correlates of the symptom
severity of stress urinary incontinence
Jenn-Ming Yang&Shwu-Huey Yang&Shu-Yu Yang&
Evelyn Yang&Wen-Chen Huang&Chii-Ruey Tzeng
Received: 3 October 2009 / Accepted: 29 December 2009 / Published online: 5 February 2010 # The International Urogynecological Association 2010
Abstract
Introduction and hypothesis The pathophysiology of stress urinary incontinence (SUI) is multifactorial. The aim of this study was to explore the factor determining the symptom severity of SUI.
Methods One hundred twenty-four women with SUI were retrospectively investigated. Clinical data for analyses included demographics, pelvic organ prolapse quantifica-tion, SUI severity using a 4-point Likert scale, ultrasound, 1-h pad tests, and urodynamic studies. Data were analyzed using the Spearman's rho test and Kruskal–Wallis test. Results The symptom severity was not correlated with risk factors of SUI or the morphologic manifestations represent-ing urethral support defect, but was significantly correlated with urine loss on 1-h pad test, Valsalva leak point pressure (VLPP) grading, and maximum urethral closure pressure (MUCP). Women with higher SUI severity had greater urine loss on 1-h pad tests, worse VLPP grading, and lower MUCP.
Conclusions Urethral sphincter function appears to be an important determinant for the symptom severity of SUI. Keywords Stress urinary incontinence . Symptom severity . Urethral sphincter function . Urethral support .
Urodynamic stress incontinence Abbreviations
ICS International Continence Society MUCP Maximum urethral closure pressure
POP-Q Pelvic Organ Prolapse Quantification System SUI Stress urinary incontinence
UPP Urethral pressure profilometry USI Urodynamic stress incontinence VLPP Valsalva leak point pressure rUD Urethral distance at rest rUA Urethral angle at rest sUD Urethral distance with strain sUA Urethral angle with strain J.-M. Yang
:
W.-C. Huang:
C.-R. TzengDepartment of Obstetrics and Gynecology, Taipei Medical University,
Taipei, Taiwan, Republic of China J.-M. Yang
Department of Obstetrics and Gynecology, Taipei Medical University-Shuang Ho Hospital, Taipei, Taiwan, Republic of China
S.-H. Yang
School of Nutrition and Health Sciences, Taipei Medical University,
Taipei, Taiwan, Republic of China S.-Y. Yang
:
W.-C. HuangSchool of Medicine, Fu Jen Catholic University, Taipei, Taiwan, Republic of China
E. Yang
Department of Bioengineering, UCSD, Jacobs School of Engineering, La Jolla, CA, USA
W.-C. Huang (*)
Department of Obstetrics and Gynecology, Cathay General Hospital,
280, Ren-Ai Road, Section 4, Taipei 106 Taiwan, Republic of China e-mail: huangwc0413@hotmail.com
C.-R. Tzeng
Department of Obstetrics and Gynecology, Taipei Medical University Hospital, Taipei, Taiwan, Republic of China DOI 10.1007/s00192-009-1094-4
Introduction
Urodynamic stress incontinence (USI) is defined by the International Continence Society (ICS) as a urodynamic observation of involuntary urine leakage with increased abdominal pressure and with an absence of detrusor contraction [1]. The pathophysiology of USI is multifacto-rial [2,3]. Among the pathophysiological factors, urethral support defect (urethral hypermobility) and urethral sphinc-ter dysfunction (intrinsic sphincsphinc-ter deficiency) can coexist with varying degrees and are commonly regarded as the two major components accounting for the pathogenesis of USI [4, 5]. In addition, the Integral Theory introduced by Petros and Ulmsten [6] has become widely accepted and has highlighted the importance of musculoelastic closure mechanisms in maintaining normal pelvic floor structures and function.
As patient-assessed subjective health measures have been becoming the mainstream in clinical and academic practices for women with stress urinary incontinence (SUI), disease severity stratified by symptom bother-someness has been increasing in popularity. Neverthe-less, the agreement of symptom severity assessed between the patients and physicians is only slight, with up to 53% of assessments being different [7]. Moreover, the existing severity measures for urinary incontinence are varied, not interchangeable, and they appear to measure different aspects of the incontinence condition [8]. Previous researches attempting to determine factors associated with symptom severity of urinary incontinence did not yield definite conclusions [5, 9–11]. Studies addressing how the pathophysiological factors, urethral hypermobility, or urethral sphincter dysfunction, contrib-ute to symptom severity of SUI were limited [5]. Women who suffered from higher incontinence symptom index scores had poorer urethrovesical support [5].
Being non-invasive and reproducible, ultrasound has replaced radiology in the diagnosis of urethral hypermo-bility [12]. Ultrasound is useful in identifying patients who are at higher risk of pelvic organ prolapse or treatment failure [13], and serving as a biofeedback and quantification tool for pelvic floor muscle contraction [14]. By determining morphological changes in the geometry of pelvic floor structures, ultrasound appears to be a promising tool for providing reliably quantitative and qualitative analyses [14].
Given that a better understanding of the pathogenesis of USI is imperative to providing women with better cure rates and less risk of complications [3, 11], this study was conducted based on the hypothesis that both pathophysio-logical factors including urethral hypermobility and urethral sphincter dysfunction were involved in the symptom severity of SUI [5]. We aimed to investigate the correlations
of symptom severity of SUI with clinical and pathophys-iological factors.
Materials and methods
We retrospectively reviewed a urodynamic database compiled from July 2006 to June 2008 to identify women with objective evidence of SUI. Records of women with incomplete grading of SUI severity, >stage 2 prolapse on ICS Pelvic Organ Prolapse Quantification System (POP-Q), cerebrovascular disease, dementia, overt neurological disease, diabetes mellitus, previous pelvic surgeries, or coexisting urodynamically demon-strated detrusor overactivity were excluded, leaving 124 records for analyses. Clinical data recorded in the database at the time of evaluation included demographic information, symptom severity of SUI, POP-Q, introital ultrasound, 1-h pad test, and urodynamic studies. Approval to carry out this study was obtained from the local ethics committees. Informed consents were obtained from all of the participants. Methods, definitions, and units conform to the standards recommended by the ICS except where specifically noted [1].
Symptom severity of SUI was assessed after a question-directed interview which addressed the storage and voiding functions of the lower urinary tract and which encompassed urinary frequency, nocturia, urgency, urge incontinence, SUI, and voiding difficulty. The question for SUI was,“In the past 3 months, have you ever leaked urine while laughing, coughing, sneezing, during physical exercise, or while bending over, moving or heavy lifting?” If the woman answered “Yes”, symptom severity of SUI was further ranked by asking the woman how bothersome the symptom of SUI was on a 4-point Likert scale, ranging from 0 (not bothered at all) to 1 (mildly bothered), 2 (moderately bothered), and 3 (severely bothered) [15].
Pelvic support was assessed using a split speculum while the patients were maximally straining in the dorsal lithotomy position. Site-specific analysis of pelvic organ prolapse was defined using the ICS POP-Q system [16].
The urethral support was assessed by introital ultrasound using a Toshiba SSA-260A (Toshiba Medical Systems, Tokyo, Japan) or Voluson 730 (GE Medical Systems, Zipf, Austria) scanner and a 5.0- to 9.0-MHz endovaginal probe with an estimated bladder volume of 200 mL to 300 mL while the patient was lying supine [12–14]. The urethral support was represented by morphological characteristics of the lower urinary tract, which were evaluated at rest, during a maximal Valsalva maneuver, and during squeezing pelvic floor muscles (PFM). This included measurements of the urethral position and observations of the opening (funneling) of the proximal urethra and/or bladder neck on
distance between the bladder neck and the inferior border of the symphysis pubis and the angle between the bladder neck-symphyseal line and the midline of the symphysis pubis. The rotational angle of the urethra was defined as the difference between the angles at resting and straining [12]. The vector of the urethral motion on Valsalva was calculated from the resting and straining urethral positions in polar coordinates by the following mathematic formula: √[rUD2
+sUD2–(2×rUD×sUD×cos(sUA–rUA))] [17], with rUD standing for urethral distance at rest, sUD for urethral distance with strain, sUA for urethral angle with strain, and rUA for urethral angle at rest.
The 1-h pad test was performed according to the recommendations of the ICS [1] before free uroflowmetry and served as an objective standard of SUI severity. One hour after drinking approximately 500 mL fluid, an absorbent pad was weighed and then placed inside the patients underpants. The patient was instructed to walk and climb stairs for 30 min, followed by the following activities: ten repetitions of sitting down and standing up, ten coughs, 1 min of running in place, picking up objects from the floor, and finally washing their hands under running water for 1 min. At the end of the test, the absorbent pad was removed and weighed. The absolute weight gain was recorded.
The full urodynamic studies included free uroflowmetry, filling phase cystometry including Valsalva leak point pressure (VLPP), pressure-flow study, and urethral pressure profilometry (UPP). At the maximum cystometric capacity, a standing stress test was performed with the feet apart parallel to the breadth of the shoulders. For a positive standing stress test, VLPP was measured by asking the patient to strain and the intravesical pressure was recorded at the point of visible urine loss. The lowest pressure obtained on two attempts was documented. VLPP values were classified as follows: low VLPP, less than 60 cm H2O;
intermediate VLPP, between 60 and 90 cm H2O; high
VLPP, greater than 90 cm H2O; and negative, no urinary
leakage [18]. After bladder emptying, the bladder was refilled with 200 mL of 0.9% saline solution. Resting and stress UPP were then performed with the patients sitting at 45° utilizing a trans-urethral microtransducer catheter (Gaeltec, Dunvegan, Scotland) which 4-638.1dts
functions assessed by peak flow rate, voided volume, and residual urine on free uroflowmetry, as well as bladder sensation represented by first desire to void and maximum cystometric capacity on filling-phase cystometry (Table3).
Discussion
In this study, using a 4-point Likert scale to measure SUI severity, we demonstrated that urethral sphincter function is the most important factor correlated with symptom severity of SUI. Symptom severity of SUI was not correlated with any risk factors of SUI or urethral support manifested on clinical and ultrasound examina-tions. Instead, a severe SUI symptom was in significant correlation with lower MUCP and VLPP. This study
further demonstrated that women with higher symptom severity of SUI had poorer urethral sphincter function or a possibility of weak urethral resistance. The significant correlations with both MUCP and VLPP illustrated in this study imply that urethral sphincter function plays an important role in SUI severity.
Our findings are consistent with others’ in the associa-tion of incontinence severity with urethral sphincter function measured by MUCP and/or VLPP [5, 11, 18– 23]. Notably, in the published literatures, the relationships between incontinence severity and either MUCP or VLPP are inconclusive, as the measurements of incontinence severity, MUCP, or VLPP were not uniform [11, 18–23]. In a case-control study exploring the pathophysiology of SUI, women with mild, moderate, and severe symptoms based on Incontinence Symptom Index have different Mild (N=56) Moderate (N=61) Severe (N=7) P value
Age 50 (43; 55) 50 (45; 58) 51 (45; 63) 0.450
Menopause 23 (41.1 %) 26 (42.6 %) 3 (42.9 %) 0.919
Body mass index (kg/m2) 23.6 (21.8; 25.6) 23.9 (21.9; 25.9) 25.4 (22.7; 28.1) 0.309
Parity 2 (2; 3) 2 (2; 4) 3 (2; 4) 0.781 C/S 5 (8.9 %) 6 (9.8 %) 1 (14.3 %) 0.903 POP-Q Aa -1 (-1.5; 1) -1.5 (-2; 0) -2 (-2.5; -1.0) 0.131 Ba -2 (-2.5; -1) -2.5 (-2.5; -1.5) -2.5 (-2.5; -2) 0.126 C −5 (−6; −4) −5 (−7; −4.5) −6 (−7.5; −4.5) 0.208 gh 2.5 (2; 3) 2.5 (2; 3) 2 (1.5; 3) 0.846 pb 3 (3; 3.5) 3 (3; 3.5) 3.5 (2.5; 4) 0.860 tvl 8 (7; 9) 8 (7; 9) 7.5 (6; 9.5) 0.684 Ap −2.5 (−2.5; −2) −2.5 (−2.5; −2) −2.5 (−3; −2) 0.524 Bp −2.5 (−2.5; −2) −2.5 (−2.5; −2) −2.5 (−3; −2) 0.427 D −6 (−7; −6) −7 (−7.5; −6) −7 (−8; −6) 0.175
Table 1 Differences in clinical characteristics among women with different symptom severity of SUI
Data is presented as median and (interquartile range) or n and (%) POP-Q pelvic organ prolapse quantification system
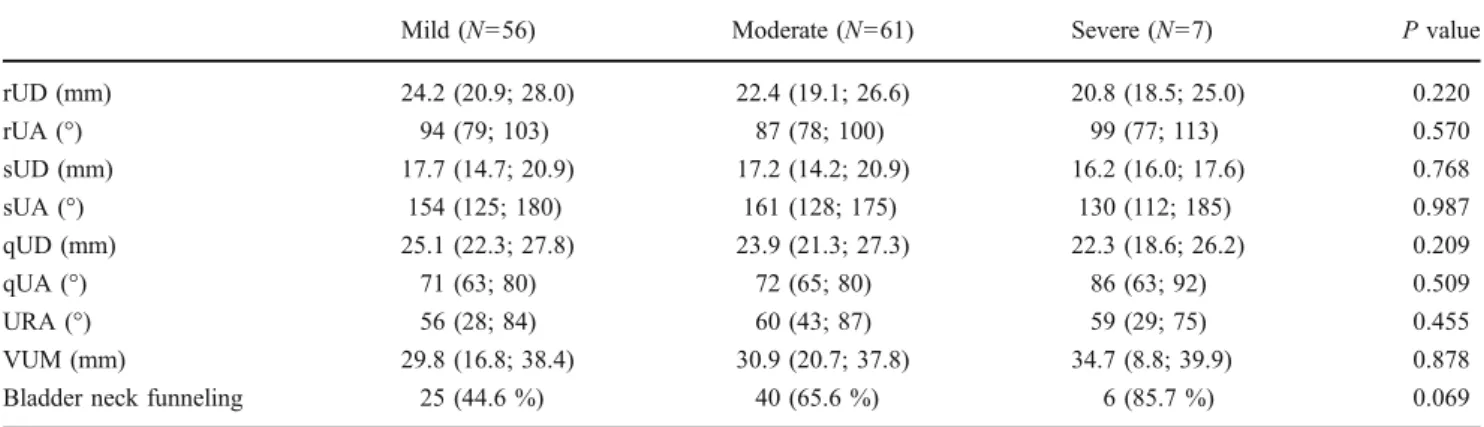
Table 2 Differences in morphological manifestations among women with different symptom severity of SUI
Mild (N=56) Moderate (N=61) Severe (N=7) P value
rUD (mm) 24.2 (20.9; 28.0) 22.4 (19.1; 26.6) 20.8 (18.5; 25.0) 0.220 rUA (°) 94 (79; 103) 87 (78; 100) 99 (77; 113) 0.570 sUD (mm) 17.7 (14.7; 20.9) 17.2 (14.2; 20.9) 16.2 (16.0; 17.6) 0.768 sUA (°) 154 (125; 180) 161 (128; 175) 130 (112; 185) 0.987 qUD (mm) 25.1 (22.3; 27.8) 23.9 (21.3; 27.3) 22.3 (18.6; 26.2) 0.209 qUA (°) 71 (63; 80) 72 (65; 80) 86 (63; 92) 0.509 URA (°) 56 (28; 84) 60 (43; 87) 59 (29; 75) 0.455 VUM (mm) 29.8 (16.8; 38.4) 30.9 (20.7; 37.8) 34.7 (8.8; 39.9) 0.878
Bladder neck funneling 25 (44.6 %) 40 (65.6 %) 6 (85.7 %) 0.069
Data is presented as median and (interquartile range) or n and (%)
rUD urethral distance at rest, rUA urethral angle at rest, sUD urethral distance with strain, sUA urethral angle with strain, qUD urethral distance at squeeze, qUA urethral angle at squeeze, URA urethral rotational angle, VUM vector of urethral motion on Valsalva
urethral sphincter function with respects to cough leak point pressure, VLPP, and MUCP [5]. In a prospective, case-control cohort study matched for age, race, parity and hysterectomy status, MUCP instead of urethral support was the predominant factor associated with SUI. Improving urethral function or preventing urethral damage rather than focusing solely on urethral support for future treatment paradigms was recommended for management of urinary incontinence [11]. Another study found that lower VLPP was independently relevant to the incontinence severity assessed by an Ingelman–Sunberg scale [21].
The MUCP measures static urethral resistance in response to a specific bladder volume, representing the passive urethral tone to maintain urinary continence at rest [19,20]. Factors affecting the urethral closure pressure that keeps the urethra closed at rest are a healthy and functioning striated pelvic musculature including the sphincter, a well vascularized urethral mucosa and submu-cosa, a properly aligned and functioning intrinsic urethral smooth muscle, an intact urethra and vaginal wall support, and an adequate reinforcement response to abdominal strain [19, 24]. The VLPP evaluates the urethral response to increased intra-abdominal pressure [20], representing active urethral resistance to the increased abdominal strain. During straining urinary continence is maintained by the combina-tion of intrinsic urethral closure pressure and the compen-satory occluding forces, such as abdominal pressure transmission to the urethra and reflex striated sphincter contraction [19].
The significant correlations with both MUCP and VLPP illustrated in this study imply that urethral sphincter function plays an important role in SUI severity. Our findings illustrating the significant correlations of inconti-nence severity with both passive and active urethral
sphincter function rather than urethral support may have significant implications. A complex combination of the pathophysiological factors may determine the severity of urinary incontinence, with urethral sphincter function appearing the most crucial component.
Our findings also can be explained by the Integral Theory [6, 25–28]. In integral theory, two distinct but related, involuntary closure mechanisms were involved in urinary continence. Both mechanisms narrow the urethra and close it along its length at rest and under stress via slow- and fast-twitch muscle fibers, respectively [26]. Stretching increases the pressure exerted by the urethral walls according to Laplace’s law. Narrowing the urethra increases the urethral resistance by the 4th power of the change in the radius (Hagen-Poiseuille’s law) [25, 27]. Thus, opening (or funneling) of the bladder neck on ultrasound demonstrable in 35% of women with primary SUI [12] implies a defect of musculoelastic mechanism for urethral closure [25, 27, 28] and decreased urethral resistance. The presence of bladder neck funneling has been reported to be associated with lower MUCP, higher incidence of low VLPP, more urine loss on a pad test, and higher peak flow rate on a pressure-flow study [12]. Thus, lower MUCP and VLPP in women with higher SUI severity indicate a decreased urethral resistance at rest and under stress, respectively, according to the Integral Theory. For women with a well-supported urethra, urinary leakage may still occur in the event of an ineffective responsive closure or fatigability of the urethral sphincter [29]. Therefore, it is likely that surgical procedures aiming at supporting the urethra instead of ameliorating urethral sphincter dysfunction may leave the incontinent symptoms unresolved, whereas those improving or compensating the urethral sphincter function (or urethral resistance) without Mild (N=56) Moderate (N=61) Severe (N=7) P value 1-h PT (g) 12.6 (2.4; 40.4) 28.0 (13.1; 56.4) 59.3 (35.5; 66.1) 0.008 Free uroflowmetry
Peak flow rate (mL/s) 21.5 (14.0; 26.0) 21.0 (15.5; 28.0) 24.0 (19.0; 27.0) 0.648 Voided volume (mL) 269 (216; 329) 266 (224; 326) 264 (225; 393) 0.864 Residual urine (mL) 5 (1; 24) 6 (1; 21) 1 (1; 12) 0.434 Filling-phase cystometry FDV (mL) 191 (151; 228) 190 (157; 227) 192 (164; 225) 0.900 MCC (mL) 388 (308; 459) 349 (308; 444) 385 (294; 500) 0.800 VLPP grading 3 (3; 3) 3 (2; 3) 2 (1; 2) 0.000 <60 cmH2O 4 (7.1 %) 9 (14.8 %) 3 (42.9 %) 60–90 cmH2O 5 (8.9 %) 10 (16.4 %) 4 (57.1 %) >90 cmH2O 39 (69.9 %) 37 (60.7 %) 0 (0 %)
Urethral pressure profile
MUCP (cmH2O) 58 (41; 71) 47 (33; 64) 33 (23; 48) 0.003
FUL (mm) 25.0 (22.0; 29.8) 25.0 (22.0; 28.0) 20.0 (13.0; 26.0) 0.054 Table 3 Differences in 1-h pad
test and urodynamic findings among women with different symptom severity of USI
Data is presented as median and (interquartile range) or n and (%) 1-h PT 1-h pad test, FDV first desire to void, MCC maximum cystometric capacity, VLPP Valsalva leak point pressure, MUCP maximum urethral closure pressure, FUL functional urethral length
correcting urethral support defect can still cure the symptom of SUI. This may explain why the mid-urethral tape procedures can cure or ameliorate SUI without correcting the supporting defect of the urethra [17].
One limitation of this study is that a 4-point Likert scale seems too simple to assess the symptom severity of SUI. Likert scales are commonly used to quantify attitudes, behaviors, and domains of health-related quality of life. It is possible that different results could be yielded by using other dimensions of severity measures. Never-theless, a single question that asks the patient to rate the severity of her incontinence on a 5-point scale correlates well with a validated severity index developed by Sandvik et al. and may provide a reasonable avenue for assessing severity and identifying patients in need of more timely evaluation and treatment [15]. Furthermore, our study demonstrated a significant correlation of the symptom severity of SUI with urine loss on the 1-h pad test, an ICS-recommended measure [1]. This justifies our use of this measure in assessing incontinent symptom severity. A further limitation is the retrospective design of this study, which may contain inherent bias. Finally, one may argue that the distribution of symptom severity in our sample population was not homogeneous, with 5.6 % having severe SUI. Interestingly, our observation is in concert with other’s findings showing the majority of women reporting mild SUI symptoms (74.4%), followed by moderate (20.2%) and severe symptoms (5.4%) [10]. Despite the above limitations, it appears that symptom severity of SUI was determined primarily by urethral sphincter function rather than by urethral support.
In conclusion, our findings demonstrate that urethral sphincter function appears a significant and important determinant for the symptom severity of SUI.
Acknowledgements This study was supported by grants from the National Science Council (NSC 97-2314-B-195-012-MY3 and NSC 97-2314-B-281-003-MY2).
Conflicts of interest None.
References
1. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U et al (2004) The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61:37–49
2. Whiteside JL, Walters MD (2007) Pathophysiology of stress urinary incontinence. In: Walters MD, Karram MM (eds) Urogynecology and reconstructive pelvic surgery. Mosby, Philadelphia, pp 157–164
3. Daneshgari F, Moore C (2006) Advancing the understanding of pathophysiological rationale for the treatment of stress urinary incontinence in women: the “trampoline theory”. BJU Int 98:8–14
4. McGuire EJ, Lytton B, Pepe V, Kohorn EI (1976) Stress urinary incontinence. Obstet Gynecol 47:255–264
5. Lewicky-Gaupp C, Wei JT, DeLancey JOL, Fenner DE, McGuire EJ, Morgan DM (2008) The association of Incontinence Symptom Index scores with urethral function and support. Am J Obstet Gynecol 199:680e1–680e5
6. Petros PE, Ulmsten UI (1993) An integral theory and its method for the diagnosis and management of female urinary incontinence. Scand J Urol Nephrol Suppl 153:3–93
7. Yalcin I, Viktrup L (2007) Comparison of physician and patient assessments of incontinence severity and improvement. Int Urogynecol J Pelvic Floor Dysfunct 18:1291–1295
8. Albo M, Wruck L, Baker J, Brubaker L, Chai T, Dandreo KJ, for the Urinary Incontinence Treatment Network et al (2007) The relationships among measures of incontinence severity in women undergoing surgery for stress urinary incontinence. J Urol 177:1810–1814
9. Richter HE, Burgio KL, Brubaker L, Moalli PA, Markland AD, Mallet V et al (2005) Urinary Incontinence Treatment Network. Factors associated with incontinence frequency in a surgical cohort of stress incontinent women. Am J Obstet Gynecol 193:2088–2093
10. Gasquet I, Tcherny-Lessenot S, Gaudebout P, Bosio Le Goux B, Klein P, Haab F (2006) Influence of the severity of stress urinary incontinence on quality of life, health care seeking, and treatment: a national cross-sectional survey. Eur Urol 50:818–825
11. DeLancey JO, Trowbridge ER, Miller JM, Morgan DM, Guire K, Fenner DE et al (2008) Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol 179:2286–2290
12. Huang WC, Yang JM (2003) Bladder neck funneling on ultrasound cystourethrography in primary stress incontinence: a sign associated with urethral hypermobility and intrinsic sphincter deficiency. Urology 61:936–941
13. Huang WC, Yang SH, Yang JM (2006) Anatomical and functional significance of urogenital hiatus in primary urodynamic stress incontinence. Ultrasound Obstet Gynecol 27:71–77
14. Yang SH, Huang WC, Yang SY, Yang E, Yang JM (2009) Validating new ultrasound parameters for quantifying pelvic floor muscle contraction. Ultrasound Obstet Gynecol 33:465–471 15. Melville JL, Miller EA, Fialkow MF, Lentz GM, Miller JL,
Fenner DE (2003) Relationship between patient report and physician assessment of urinary incontinence severity. Am J Obstet Gynecol 189:76–80
16. Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P et al (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175:10–17
17. Yang JM, Yang SH, Huang WC (2008) Dynamic interaction involved in the tension-free vaginal tape obturator procedure. J Urol 180:2081–2087
18. Nitti VW, Combs AJ (1996) Correlation of Valsalva leak point pressure with subjective degree of stress urinary incontinence in women. J Urol 55:281–285
19. Almeida FG, Bruschini H, Srougi M (2005) Correlation between urethral sphincter activity and Valsalva leak point pressure at different bladder distentions: revisiting the urethral pressure profile. J Urol 174:1312–1315
20. Martan A, Mašata J, Petri E, Švabík K, Drahorádová P, Voigt R et al (2007) Weak VLPP and MUCP correlation and their relationship with objective and subjective measures of severity of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 18:267–271
21. Ku JH, Shin JW, Oh SJ, Kim SW, Paick JS (2006) Clinical and urodynamic features according to subjective symptom severity in female urinary incontinence. Neurourol Urodyn 25:215–220
22. Theofrastous JP, Bump RC, Elser DM, Wyman JF, McClish DK (1995) Correlation of urodynamic measures of urethral resistance with clinical measures of incontinence severity in women with pure genuine stress incontinence. The Continence Program for Women Research Group. Am J Obstet Gynecol 173:407–414
23. Chen CCG, Rooney CM, Paraiso MF, Kleeman SD, Walters MD, Karram MM et al (2008) Leak point pressure does not correlate with incontinence severity or bother in women undergoing surgery for urodynamic stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct 19:1193–1198
24. Rud T (1980) Urethral pressure profile in continent women from childhood to old age. Acta Obstet Gynecol Scand 59:331–335
25. Petros PE (2003) Changes in bladder neck geometry and closure pressure after midurethral anchoring suggest a musculoelastic mechanism activates closure. Neururol Urodyn 22:191–197 26. Petros PE, Woodman PJ (2008) The Integral theory of continence.
Int Urogynecol J 19:35–40
27. Petros PE, Ulmsten U (1997) Role of the pelvic floor in bladder neck opening and closure: I muscle forces, II vagina. Int J Urogynecol Pelvic Floor 8:69–80
28. Petros PE, Von Konsky B (1999) Anchoring the midurethra restores bladder neck anatomy and continence. Lancet 354:997– 998
29. Fritel X, Fauconnier A, Pigné A (2008) Circumstances of leakage related to low urethral closure pressure. J Urol 180:223–226