Anti-inflammatory properties of yellow and orange pigments from Monascus purpureus NTU 568
Li-Chuan Hsu, †§Yu-Han Liang, †§Ya-Wen Hsu, †§ Yao-Haur Kuo*, §‡ and Tzu-Ming Pan*,†
Department of Biochemical Science & Technology, College of Life Science, National Taiwan University, Taipei 106, Taiwan, R.O.C.
National Research Institute of Chinese Medicine, Taipei 112, Taiwan, R.O.C.
Graduate Institute of Integrated Medicine, China Medical University, Taichung 404, Taiwan, R.O.C.
* Corresponding Author: Tzu-Ming Pan, Professor; Tel: +886-2-3366-4519 ext 10; Fax: +886-2-3366-3838; E-mail: tmpan@ntu.edu.tw; Yao-Haur Kuo, Professor; Tel: +886-2-2820-1999 ext. 7061; Fax: +886-2-2823-6150; E-mail: kuoyh@nricm.edu.tw; †Department of Biochemical Science and Technology, National Taiwan University, Taipei 10617, Taiwan
§National Research Institute of Chinese Medicine
‡Graduate Institute of Integrated Medicine, China Medical University 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
ABSTRACT
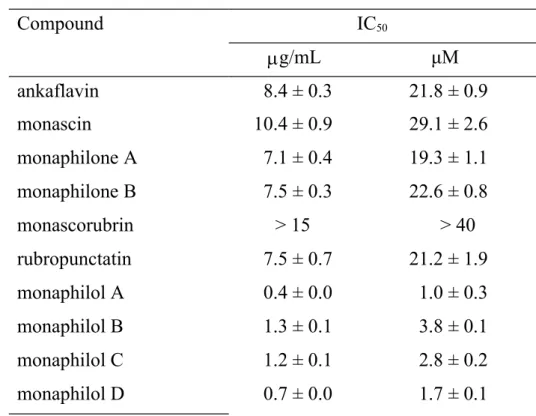
The Monascus species has been used on foods for thousands of years in China. In this study, ten azaphilone pigments, including four yellow and six orange pigments, were isolated from the fermented rice and dioscorea of Monascus purpureus NTU 568. By employing lipopolysaccharide (LPS)-stimulated murine macrophage RAW 264.7 cells, we determined the inhibitory activities of these pigments on the nitric oxide (NO) production. As a result, four orange pigments, monaphilols A–D, showed the highest activities (IC50 = 1.0–3.8 M), compared with the other two orange pigments, monascorubrin (IC50 > 40 M) and rubropunctatin (IC50 = 21.2 M), and the four yellow pigments ankaflavin (IC50 = 21.8 M), monascin (IC50 = 29.1 M), monaphilone A (IC50 = 19.3 M), and monaphilone B (IC50 = 22.6 M). Using Western blot and ELISA kits, we found that treatments with 30 M of the yellow pigments and 5 M of the orange pigments could down-regulate the protein expression of inducible nitric oxide synthase (iNOS) and suppress the production of tumor necrosis factor-(TNF-), interleukin-1(IL-1), and interleukin-6 (IL-6). We also used two animal experiments to evaluate the anti-inflammatory effects of these pigments. In a 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ear edema model, eight kinds of these pigments (0.5 mg/ear) could prevent the ear edema against TPA administrations on the ears of BALB/c mice. In an LPS-injection mice model, several kinds of these pigments (10 mg/kg) could inhibit the NO, TNF-, IL-1 and IL-6 levels in the plasma of BALB/c mice. As concluded from the in vitro and in vivo studies, six kinds of azaphilonoid pigments, namely ankaflavin, monaphilone A, and monaphilols A–D, showed high potential to be developed into chemopreventive foods or drugs against inflammation-associated diseases.
Keywords: Monascus purpureus, azaphilonoid pigment, anti-inflammation, 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43
lipopolysaccharide, 12-O-tetradecanoylphorbol-13-acetate
44 45
INTRODUCTION
Monascus species have been used as food additives in Asian countries for thousands of years. Recently, Monascus-fermented products, such as red mold rice (RMR) and red mold dioscorea (RMD), were reported to block or retard the development of some diseases. For example, they could decrease the cholesterol level and thus lower cardiovascular diseases,1 reduce injuries from diabetes,2,3 inhibit carcinogenesis4 or tumor progression,5 and improve the learning ability in patients with Alzheimer’s disease.6,7 Similarly, many evidences have also revealed that chronic inflammations are the key events for cardiovascular diseases,8 diabetes,9,10 cancers,11,12 and Alzheimer’s disease.13,14 These coincidences highlight that the chemoprotective functions of RMR and RMD possibly result from their anti-inflammatory properties.
The anti-inflammatory functions of Monascus sp. are contributed by their azaphilonoid pigments. In 1994, azaphilonoid pigments from M. anka were demonstrated to inhibit 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ear edema in mice.15 In 2005, Monascus pigments from M. pilosus were also tested on a TPA-induced ear edema model.16 Compared with non-azaphilonoid pigments, the inhibitory effects of azaphilonoid pigments on TPA-induced ear edema were much stronger. In total, five azaphilonoid pigments, including two yellow, two orange, and one red pigment, were proven to exhibit anti-inflammatory activities. Recently, a 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64
variety of new azaphilonoid pigments, named monasnicotinates A–D, were isolated from M. pilosus BCRC 38093 and showed inhibitory effects against nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated Raw 264.7 cells.17
In our research group, we found that ethanol extracts of rice or dioscorea fermented from M. purpureus NTU 568 could down-regulate the productions of NO, PEG2, and pro-inflammatory cytokines in a 7',12'-dimethylbenz[a]anthracene (DMBA)-stimulation hamsters model.18,19 From the fermented products of M. purpureus NTU
568, we have identified a series of new azaphilonoid pigments with inhibitory effects against nitric oxide production in LPS-stimulated RAW 264.7 cells.20-23 Here, we
report the structures and anti-inflammatory activities of ten azaphilonoid pigments isolated from M. purpureus NTU 568, including four yellow and six orange pigments. For these purposes, we obtained these pigments on a large scale and evaluated their anti-inflammatory properties for in vitro and in vivo experiments.
MATERIALS AND METHODS
General Experimental Procedures. Nuclear magnetic resonance (NMR) spectra were generated on a Brucker NMR unit (Unity Plus 400 MHz; Brucker BioSpin, Rheinstetten, Germany) using d4-methanol as the solvent. High-performance liquid chromatography (HPLC) separations were performed on a Shimadzu LC-6AD series 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83
preparative Cosmosil AR-II column (Nacalai Tesque, Inc., Kyoto, Japan).
Materials. Methanol and acetonitrile (HPLC grade), and acetone and methanol (analytical grade) were purchased from ECHO (Miaoli, Taiwan). Fetal bovine serum (FBS), Dulbecco’s minimum essential medium (DMEM), and phosphate-buffered saline (PBS) were purchased from Biological Industries (Kibbutz Beit-Haemek, North District, Israel). Other chemicals such as LPS (from Escherichia coli O55:B5), TPA, and dimethyl sulfoxide were obtained from Sigma (St. Louis, MO, USA). The powders of RMR and RMD were generously provided by SunWay Biotech Co., Ltd. (Taipei, Taiwan). The QC quality of commercial RMR and RMD provided by SunWay Biotech Co. was described as: monascin, > 3 mg/g; ankaflavin, > 1.1 mg/g; citrinin, < 2 ppm; total plate count, < 5 × 104 CFU/g; coliform, < 103 CFU/g; E. coli,
negative; mold and yeast, < 100 CFU/g; Staphylococcus, negative; Salmonella, negative; moisture, < 10%.
Extraction of Monascus Pigments. The procedures for the extraction and isolation of Monascus pigments of RMR and RMD were adapted and modified from the methods published by our group.20,23 Briefly described, RMR (5 kg) and RMD (30 kg)
were extracted respectively with methanol (50 L, 50°C) and acetone (150 L, 40°C), for three times each. The methanol extracts of RMR and the acetone extracts of RMD were further chromatographed on several types of columns, including silica gel 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103
(Kieselgel 60), Sephadex LH-20, and HPLC. All of the pure substances were purified twice by HPLC at the final step, and the purities of compounds (> 98%) were checked by photo diode array detector. Four yellow pigments, namely ankaflavin (AK), monascin (MS), and monaphilones A and B (MA and MB), were harvested from RMR; and six orange pigments, namely rubropunctatin (RPT), monascorubrin (MCB), and monaphilols A–D (MPA–MPD), were harvested from RMD. The structures of these ten azaphilonoid pigments are shown in Figure 1. The structural elucidations of these compounds were determined by NMR, mass, infrared, and ultraviolet spectroscopies.
Cell Lines and Culture. The murine macrophage cell line RAW 264.7 was obtained from Bioresource Collection and Research Center (Hsinchu, Taiwan). The cells were cultured in DMEM (5% FBS) in a humidified incubator with 5% CO2 at 37°C. DMSO was used to prepare stock solutions (20000 g/mL) of the pigments and then diluted to appropriate concentrations in cell model.
Assay of Nitrite Production. RAW 264.7 cells (2 × 105 per well) were seeded and maintained with 500 L of DMEM in 24-well plates at 37°C. After 24 h, the cells were co-treated with LPS (1 g/mL) and test agents (0.625, 2.5, 10, 20, and 40 M) dissolved in DMEM. After 24 h of incubation, determination of the nitrite levels in the supernatants was performed using a Griess reagent kit (Promega, Madison, WI, 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122
USA), as adapted from reported methods.24
Western Blot Analysis. The procedures were modified from published literature.25
Cells (about 8 × 106 RAW 264.7) were seeded with 10 mL of medium in a 75 cm2 flask. After 12 h, the medium was replaced with 10 mL of test agents (yellow
pigments, 30 M; orange pigments, 5 M) dissolved in fresh medium. After 24 h of
incubation, the cells were harvested and extracted with RIPA lysis buffer (Millipore, Bellerica, MA, USA) containing 1% protease inhibitor (Sigma, St. Louis, MO, USA). The cell lysates were further separated by polyacrylamide gel electrophoresis (10% sodium dodecyl sulfate) and analyzed with primary antibodies, including -actin murine monoclonal antibody (Epitomics, Burlingame, CA, USA), and inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) murine polyclonal antibody (Cayman Chemical, Ann Arbor, MI, USA). A goat anti-mouse secondary IgG (Jackson ImmunoResearch, West Grove, PA, USA) was further added. Finally, the detection was performed using the Western Lightning chemiluminescence reagent (PerkinElmer Life Sciences, Waltham, MA, USA).
Cytokine Assay. RAW 264.7 cells (2 × 105 per well) were seeded and maintained with 500 L of DMEM in 24-well plates at 37°C. After 12 h, cells were treated with LPS (1 g/mL) and test agents (yellow pigments, 30 M; orange pigments, 5 M)
dissolved in 10 mL of DMEM. After 24 h of incubation, the cells were harvested and
123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141
tested by using a colorimetric assay kit (eBioscience, San Diego, CA, USA). Cytokines, including interleukin-1 (IL-1, interleukin-6 (IL-6), and tumor necrosis factor- (TNF- were determined according to the manufacturer’s protocols.
Animal Experiments. The procedures were modified from published literature.16,
26 Male Balb/cByJNarl mice (8 weeks old, n = 3) were purchased from the National
Animal Institute. The animals were stimulated with inflammatory agents by two methods: LPS injection (2 mg/kg, intraperitoneal injection) or TPA induction (1 g/ear, scribbled on the both side of ear). Mice were grouped randomly for a) vehicle control (PBS or acetone); b) inflammatory agents (LPS or TPA); c) sample (inflammatory agents combined with test agents); and d) positive control (inflammatory agents combined with quercetin or indomethacin). Sample administration was performed at 0.5 h before LPS injection or TPA induction. (In TPA-induction model, we chose acetone as vehicle for drug administrations on the ears of mice because it was a fast evaporating and not a toxic solvent.) In the LPS-injection model, mice were sacrificed at 12 h after LPS LPS-injection and further evaluated for the alteration of serum NO levels and pro-inflammatory cytokines. In the TPA-induced ear edema model, mice were sacrificed at 4 h after TPA administrations and further evaluated for the changes of ear biopsies weight.
Statistical Analysis. Data are presented as the mean ± standard deviation (n = 3). 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160
The statistical comparisons were performed by one-way analysis of variance (ANOVA) with Duncan’s test. The significant differences (p < 0.05) were presented as different letters for each group.
RESULTS
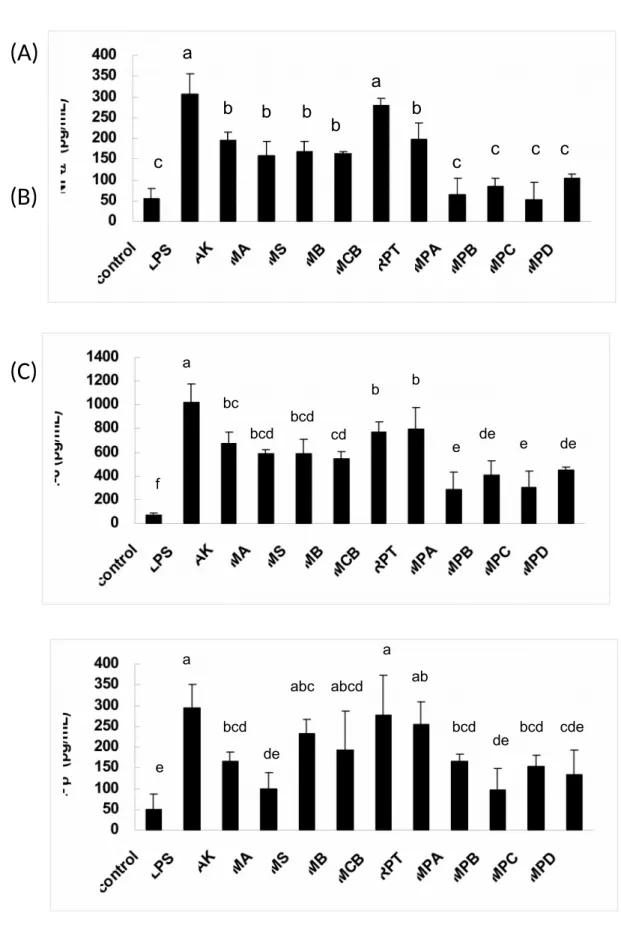
Anti-NO Activities of Azaphilonoid Pigments from RMR and RMD. In this study, we performed large-scale preparations of four yellow pigments (from the fermented rice) and six orange pigments (from the fermented dioscorea) of M. purpureus NTU 568. The ten pigments were structural analogs and all belonged to the azaphilone family. In Table 1, we show the anti-NO activities of these pigments. The four yellow pigments, AK (IC50 = 21.8 M), MS (IC50 = 29.1 M), MA (IC50 = 19.3 M), and MB (IC50 = 22.6 M), and one orange pigment, RPT (IC50 = 21.2 M), showed moderate anti-NO activities. Four orange pigments, MPA, MPB, MPC, and MPD, showed high anti-NO activities (IC50 = 1.0–3.8 M). However, MCB showed low anti-NO activity (IC50 > 40 M). To a certain extent, the anti-NO activities of the orange pigments were stronger than those of the yellow pigments. Thus, we decided to use different concentrations (30 M for yellow pigments; 5 M for orange pigments) to explore the correlations between the NO activities and other anti-inflammatory parameters. In Figure 2, the anti-NO activities of the yellow and orange 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179
pigments had been screened in separate groups. Comparing among the yellow pigments, AK, MA, MS and MB possessed significantly (p < 0.05) higher anti-NO activities than LPS group. As for the orange pigments, MPA, MPB, MPC, MPD, MCB and RPT possessed significantly (p < 0.05) higher anti-NO activities than LPS group.
Inhibition of iNOS/COX-2 Expression by Azaphilonoid Pigments. Using Western blotting, the anti-inflammatory effects of the azaphilonoid pigments were evaluated on the protein level; namely, the expressions of iNOS and COX-2. As shown in Figure 3, all of the yellow pigments could suppress the LPS-induced iNOS expressions significantly (p < 0.05), but only three of them (MS, MA, and MB) could down-regulate the LPS-induced COX-2 expressions significantly (p < 0.05). Comparing among the yellow pigments, MA and MB suppressed more iNOS expressions (significant difference, p < 0.05) than AK and MS.As shown in Figure 4, all of the orange pigments could suppress the LPS-induced iNOS expressions significantly (p < 0.05), but none of them could down-regulate the LPS-induced
COX-2 expressions. Comparing among the orange pigments, MPD suppressed more
iNOS expressions (significant difference, p < 0.05) than MPA, MPB, MPC, MCB and RPT. The results suggested that the azaphilonoid pigments worked like iNOS inhibitors, but not COX-2 inhibitors. Our data also revealed the correlations between 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196 197 198
the inhibitions on iNOS expression and NO production.
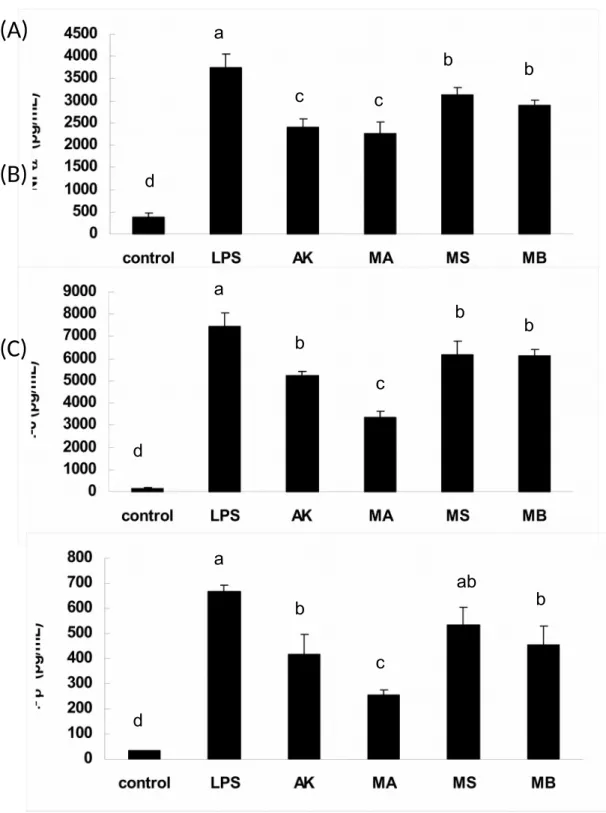
Inhibition of Inflammatory Cytokines Production by Azaphilonoid Pigments. Using ELISA kits, the anti-inflammatory properties of the azaphilonoid pigments were explored on the cytokines TNF-, IL-6, and IL-1. As evident in Figure 5, most of the yellow pigments could suppress cytokines production significantly (p < 0.05) than LPS group, but MS could not down-regulate the IL-1 production significantly. Comparing among the yellow pigments, AK and MA suppressed more TNF- productions (significant difference, p < 0.05) than MSand MB. In the case of IL-6 productions, MA suppressed more productions (significant difference, p < 0.05) than AK, MS and MB. With regard to IL-1productions, MA suppressed more productions (significant difference, p < 0.05) than AK and MB. In Figure 6, most of the orange pigments are shown to suppress cytokines production significantly (p < 0.05) than LPS group, but MCB and RPT could not down-regulate the IL-1
production significantly. Comparing among the orange pigments, MPA, MPB, MPC
and MPD suppressed more TNF- productions than MCB and RPT. In the case of IL-6 productions, MPA, MPB, MPC and MPD suppressed more productions than MCB and RPT. With regard to IL-1productions, MPD suppressed more productions than
MPA, MPB and MPC. The findings implied that the yellow and orange pigments
were suitable agents to reduce the production of inflammatory cytokines on LPS-199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217
stimulated RAW 264.7 cells. Therefore, our aims turned to animal experiments in the subsequent steps.
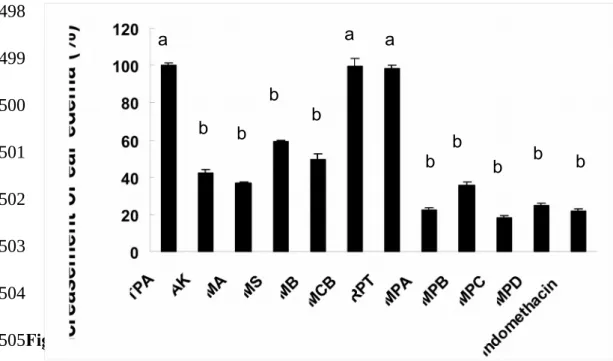
Inhibition of TPA-induced Ear Edema by Azaphilonoid Pigments. Using a TPA-induced ear edema model, the anti-inflammatory effects of the azaphilonoid pigments were evaluated on mice. At the dosage of 0.5 mg/ear, AK, MA, MS, MB, MPA, MPB, MPC, MPD and positive control indomethacin could inhibit ear edema significantly (p < 0.05) than TPA group, but MCB and RPT could not (Figure 7).The results indicated that all four yellow pigments and four orange pigments (MPA, MPB, MPC, and MPD) were excellent chemoprotective agents against inflammatory responses that occurred on the body surface.
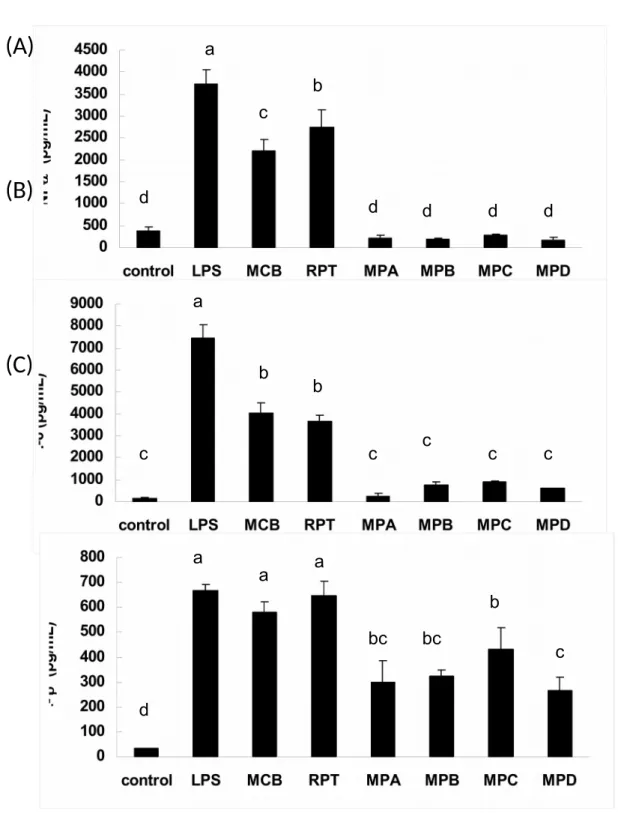
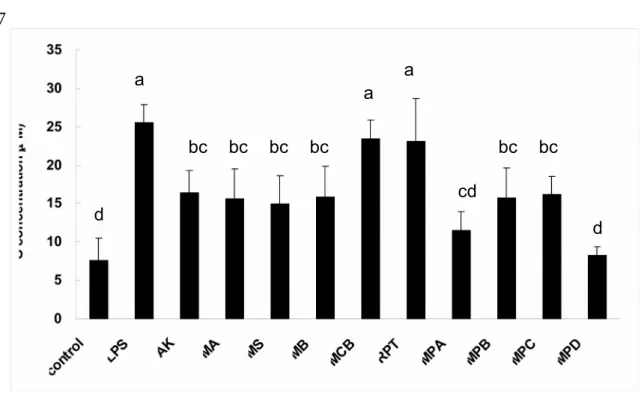
Inhibition of LPS-induced Inflammations by Azaphilonoid Pigments. Using LPS-injected mice, the anti-inflammatory potentials of azaphilonoid pigments were evaluated according to the NO and cytokines (TNF-, IL-6, and IL-1 found in the blood. At the dosage of 10 mg/kg, AK, MA, MS, MB, MPA, MPB, MPC and MPD could inhibit serum NO levels significantly (p < 0.05) than LPS group, but MCB and
RPT could not (Figure 8). However, the inhibitions of cytokines by the azaphilonoid
pigments were more complicated (Figure 9). All four yellow pigments and five orange pigments (RPT, MPA, MPB, MPC, and MPD) could reduce TNF- production significantly (p< 0.05), but MCB could not. In the case of IL-6, all the 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234 235 236
azaphilonoid pigments showed significant inhibitory abilities (p < 0.05). With regard to IL-1, only six of the azaphilonoid pigments (AK, MA, MPA, MPB, MPC and MPD) showed inhibitory abilities (significant difference, p < 0.05). Generally speaking, two yellow pigments (AK and MA) and four orange pigments (MPA, MPB, MPC, and MPD) were the most effective chemoprotective agents against the inflammatory responses in the blood.
DISCUSSION
RMR and RMD fermented by M. purpureus NTU 568 are recognized to contain various azaphilonoid pigments, which are associated with antitumor or anti-inflammatory properties.20-23 In this study, we prepared ten azaphilonoid pigments
from RMR and RMD. With the help of in vitro and in vivo experiments, we further investigated the structure–activity relationship (SAR) for the anti-inflammatory effects by these azaphilonoid pigments. In the case of the yellow pigments, alterations of the alkyl side chain and the γ-lactone ring could affect the anti-inflammatory activities slightly. For example, AK and MA (R = C7H15) possessed higher anti-inflammatory activities than MS and MB (R = C5H11). Moreover, MA (lacking the γ-lactone ring) possessed higher anti-inflammatory activity than AK. However, the alterations that occurred on C-8 affected the anti-inflammatory activities more 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253 254 255
obviously in the case of the orange pigments. For example, MPA, MPB, MPC, and MPD (a hydroxyl group on C-8) possessed much higher anti-inflammatory activities than MCB and RPT (a ketone group on C-8). From the SAR studies, we successfully identified key structures that could enhance the anti-inflammatory activities of azaphilonoid pigments.
In our in vitro designs, we selected LPS as a chemical challenge to stimulate the
RAW 264.7 cells. Upon analyzing the iNOS/COX-2 expressions, we found that two yellow pigments (AK and MA) and four orange pigments (MPA, MPB, MPC, and MPD) were effective chemoprotective agents against the LPS-induced inflammation.
According to the findings, we suggested that the down-regulation of excess iNOS protein expression was likely to be a common event exerted by azaphilonoid pigments. The findings were also similar to our published literature, which claim that AK and MA could inhibit the expression of iNOS, but not COX-2.22
In our in vivo designs, two yellow pigments (AK and MA) and four orange pigments (MPA, MPB, MPC, and MPD) showed good anti-inflammatory effects on TPA- or LPS-stimulated mice. The six azaphilonoid pigments were all effective inhibitors against ear edema, serum NO, and inflammatory cytokines. According to the findings, we suggest that these pigments are capable of preventing anti-inflammatory responses that occur outside or inside the body. The findings were also 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274
consistent with our published literatures, which claim that RMR and RMD can reduce inflammatory cytokines on DMBA/TPA-stimulated mice18,19 or STZ-induced diabetic
rats.2,3
In this study, four orange pigments (MPA–MPD) were found to possess the highest anti-inflammatory activities on mice. In our previous studies,20,23 however, the optimal
yields of yellow pigments (from RMR) were almost 6-fold higher than those of orange pigments (from RMD). Briefly described, the RMR was found to contain 15.2 mg/kg of AK, 19.9 mg/kg of MS, 2.0 mg/kg of MA, and 3.0 mg/g of MB.20 As for the
RMD, it contained only 2.0–3.0 mg/kg of MCB or RPT, and even a lesser amount of
MPA–D (0.3–1.1 mg/kg).23 We hope that the yields of MPA–D can be enhanced by
some adjustments of the fermentation process. Otherwise, for the concerns of industrial yields from M. purpureus NTU 568, AK and MA are better choices for development into anti-inflammatory drugs or foods than any of the other orange pigments.
ACKNOWLEDGMENT
The research grant was provided by the National Science Council (NSC), National Taiwan University (NTU), and National Research Institute of Chinese Medicine (NRICM). The RMR and RMD powders were generously donated by Sun Way Co., 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293
LITERATURE CITED
(1) Lee, C. L.; Tsai, T. Y.; Wang, J. J.; Pan, T. M. In vivo hypolipidemic effects and safety of low dosage Monascus powder in a hamster model of hyperlipidemia. Appl. Microbiol. Biotechnol. 2006, 70, 533–540.
(2) Shi, Y. C. and Pan, T. M. Anti-diabetic effects of Monascus purpureus NTU 568 fermented products on streptozotocin-induced diabetic rats. J. Agri. Food Chem. 2010, 58: 7634-7640.
(3) Shi, Y. C. and Pan, T. M. Antioxidant and pancreas-protective effect of red mold fermented products on streptozotocin-induced diabetic rats. J Sci. Food & Agri. 2010, 90: 2519-2525.
(4) Hsu, W. H.; Lee, B. H.; Pan, T. M. Red mold dioscorea-induced G2/M arrest and apoptosis in human oral cancer cells. J. Sci. Food. & Agri. 2010, 90, 2709–2715. (5) Ho, B. Y.; Wu, Y. M.; Hsu, Y. W.; Hsu, L. C.; Kuo, Y. H.; Chang, K. J.; Pan, T.
M. Effects of Monascus-fermented rice extract on malignant cell-associated neovascularization and intravasation determined using the chicken embryo chorioallantoic membrane model. Integr. Cancer Ther. 2010, 2, 204–212.
(6) Lee, C. L.; Kuo, T. F.; Wu, C. L.; Wang, J. J.; Pan, T. M. Red mold rice promotes neuroprotective sAPPalpha secretion instead of Alzheimer’s risk factors and amyloid beta expression in hyperlipidemic A40-infused rats. J. Agric. Food 295 296 297 298 299 300 301 302 303 304 305 306 307 308 309 310 311 312 313 314
Chem. 2010, 58, 2230–2238.
(7) Lee, C. L.; Kuo, T. F.; Wang, J. J.; Pan, T. M. Red mold rice ameliorates impairment of memory and learning ability in intracerebroventricular amyloid β-infused rat by repressing amyloid β accumulation. J. Neurosci. Res. 2007, 85, 3171–3182.
(8) Cottones, S., Lorito, M. C., Riccobene, R., Nardi, E., Mule, G., Buscemi, S., Geraci, C., Guarneri, M., Arsena, R. and Cerasola, G. Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J. Nephrol. 2008, 21: 175-179.
(9) Wellen, K. E. and Hotamisligil. G. S. Inflammation, stress, and diabetes. J. Clin. Invest. 2005, 115: 1111-1119.
(10) Heilbronn, L. K., and Campbell, L. V. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr. Pharm. Des. 2008, 14: 1225-1230.
(11) Wink, D. A., Vodovotz, Y., Laval, J., Laval, F., Dewhirst, M. W. and Mitchell. J. B. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998, 19: 711-721.
(12) Mantovani, A., Allavena, P., Sica, A. and Balkwill, F. Cancer-related inflammation. Nature. 2008, 454: 436-444.
(13) Kamer, A. R., Craig, R. G., Dasanayake, A. P., Brys, M., Glodzik-Sobanska, L. 315 316 317 318 319 320 321 322 323 324 325 326 327 328 329 330 331 332 333 334
and de Leon, M. J. Inflammation and Alzheimer's disease: possible role of periodontal diseases. Alzheimer’s Dement. 2008, 4: 242-250.
(14) Vasto, S., Candore, G., Listi, F., Balistreri, C. R., Colonna-Romano, G., Malavolta, M., Lio, D., Nuzzo, D., Mocchegiani, E., Di Bona, D. and Caruso, C. Inflammation, genes and zinc in Alzheimer's disease. Brain Res. Rev. 2008, 58: 96-105.
(15) Yasukawa, K., Takahashi, M., Natori, S., Kawai, K., Yamazaki, M., Takeuchi, M. and Takido, M. Azaphilones inhibit tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mice. Oncology. 1994, 51: 108-112.
(16) Akihisa, T., Tokuda, H., Yasukawa, H., Ukiya, M., Kiyota, A., Sakamoto, N., Suzuki, T., Tanabe, N. and Nishino, H. 2005. Azaphilones, furanoisophthalides, and amino acids from the extracts of Monascus pilosus-fermented rice (red-mold rice) and their chemopreventive effects. J. Agric. Food Chem. 53: 562-565.
(17) Wu, M.D.; Cheng, M.J., Yech, Y.J.; Chen, Y.L.; Chen, K.P.; Chen, I.H.; Yang, P.H.; Yuan, G.F. Monasnicotinates A–D, four new pyridine alkaloids from the fungal strain Monascus pilosus BCRC 38093. Molecules. 2011, 16, 4719–4727. (18) Tsai, R. L.; Ho, B. Y.; Pan, T. M. Red mold rice mitigates oral carcinogenesis in
7,12-dimethyl-1,2-benz[a]anthracene-induced oral carcinogenesis in hamster. Evid. Based Complement Alternat. Med. 2009, doi:10.1093/ecam/nep215.
335 336 337 338 339 340 341 342 343 344 345 346 347 348 349 350 351 352 353 354
(19) Hsu, W. H., Lee, B. H. and Pan, T. M. Protection of Monascus-fermented dioscorea against DMBA-induced oral injury in hamster by anti-inflammatory and antioxidative potentials. J. Agric. Food Chem. 2010, 58: 6715-6720.
(20) Hsu, Y.W.; Hsu, L.C.; Liang, Y.H.; Kuo, Y.H.; Pan, T.M. Monaphilones A-C, three new antiproliferative azaphilone derivatives from Monascus purpureus NTU 568. J. Agric. Food Chem. 2010, 58, 8211–8216.
(21) Hsu, Y.W.; Hsu, L.C.; Chang, C.L.; Liang, Y.H.; Kuo, Y.H.; Pan, T.M. New anti-inflammatory and anti-proliferative constituents from fermented red mold rice Monascus purpureus NTU 568. Molecules 2010, 15, 7815–7824.
(22) Hsu, L.C.; Hsu, Y.W.; Liang, Y.H.; Kuo, Y.H.; Pan, T.M. Anti-tumor and anti-inflammatory properties of ankaflavin and monaphilone A from Monascus purpureus NTU 568. J. Agric. Food Chem. 2011, 59, 1124–1130.
(23) Hsu, Y. W.; Hsu, L. C.; Liang, Y. H.; Kuo, Y. H.; Pan, T. M. New bioactive orange pigments with yellow fluorescence from Monascus-fermented dioscorea. J. Agric. Food Chem. 2011, 59: 4512-4518.
(24) Fiddler, R. M. Collaborative study of modified AOAC method of analysis for nitrite in meat and meat products. J. AOAC. 1977, 60, 594-599.
(25) Burnette, W. N. Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate—polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal.
355 356 357 358 359 360 361 362 363 364 365 366 367 368 369 370 371 372 373 374
Biochem. 1981, 60, 195-203.
(26) Barsoumian, H., El-Rami, F. and Abdelnoor, A. M. The effect of antibacterial
agents on the production of nitric oxide induced by lipopolysaccharide in mice. Adv. Biosci. Biotechnol. 2010, 1, 187-206.
375 376 377 378 379
Figure legends
Figure 1. Structures of azaphilonoid pigments.
Figure 2. Azaphilonoid pigments suppressed lipopolysaccharide (LPS)-induced NO production in RAW 264.7 cells. (A) Cells were treated with LPS (1 g/mL) or in combination with yellow pigments (30 M) for 24 h. (B) Cells were treated with LPS (1 g/mL) or in combination with tested agents (5 M) for 24 h. Different letters indicate statistically significant differences between the means (p < 0.05) for each group.
AK: ankaflavin; MA: monaphilone A; MS: monascin; MB: monaphilone B; MCB: monascorubrin; RPT: rubropunctatin; MPA: monaphilol A; MPB: monaphilol B; MPC: monaphilol C; MPD: monaphilol D.
Figure 3. (A) Yellow pigments suppressed LPS-induced inflammatory enzyme expressions in RAW 264.7 cells. Cells were treated with LPS (1 g/mL) or in combination with tested agents (30 M). After 24 h, cells were harvested and determined for iNOS and COX-2 expressions. (B) Quantification of iNOS and (C) quantification of COX-2. Different letters indicate statistically significant differences between the means (p < 0.05) for each group.
AK: ankaflavin; MA: monaphilone A; MS: monascin; MB: monaphilone B. 380 381 382 383 384 385 386 387 388 389 390 391 392 393 394 395 396 397 398 399 400
expressions in RAW 264.7 cells. Cells were treated with LPS (1 g/mL) or in combination with tested agents (5 M). After 24 h, cells were harvested and determined for iNOS and COX-2 expressions. Different letters indicate statistically significant differences between the means (p < 0.05) for each group.
MCB: monascorubrin; RPT: rubropunctatin; MPA: monaphilol A; MPB: monaphilol B; MPC: monaphilol C; MPD: monaphilol D.
Figure 5. Yellow pigments suppressed LPS-induced inflammatory cytokines in RAW 264.7 cells. Cells were treated with LPS (1 g/mL) or in combination with tested agents (30 M). After 24 h, cells were harvested and determined for (A) TNF- (B) IL-6and (C) IL-1. Different letters indicate statistically significant differences between the means (p < 0.05) for each group.
AK: ankaflavin; MA: monaphilone A; MS: monascin; MB: monaphilone B.
Figure 6. Orange pigments suppressed LPS-induced inflammatory cytokines in RAW 264.7 cells. Cells were treated with LPS (1 g/mL) or in combination with tested agents (5 M). After 24 h, cells were harvested and determined for (A) TNF- (B) IL-6and (C) IL-1. Different letters indicate statistically significant differences between the means (p < 0.05) for each group.
MCB: monascorubrin; RPT: rubropunctatin; MPA: monaphilol A; MPB: monaphilol B; MPC: monaphilol C; MPD: monaphilol D.
Figure 7. Inhibitory effect of Monascus-fermented pigments on 12-O-402 403 404 405 406 407 408 409 410 411 412 413 414 415 416 417 418 419 420 421 422 423 424
were topically applied about 30 min before the TPA treatment. Different letters indicate statistically significant differences between the means (p < 0.05) for each group.
AK: ankaflavin; MA: monaphilone A; MS: monascin; MB: monaphilone B; MCB: monascorubrin; RPT: rubropunctatin; MPA: monaphilol A; MPB: monaphilol B; MPC: monaphilol C; MPD: monaphilol D.
Figure 8. Monascus-fermented pigments suppressed plasma NO levels in LPS-injected mice. The compounds (10 mg/kg) were LPS-injected 30 min before the LPS (2 mg/kg) treatment. Different letters indicate statistically significant differences between the means (p < 0.05) for each group.
AK: ankaflavin; MA: monaphilone A; MS: monascin; MB: monaphilone B; MCB: monascorubrin; RPT: rubropunctatin; MPA: monaphilol A; MPB: monaphilol B; MPC: monaphilol C; MPD: monaphilol D.
Figure 9. Monascus-fermented pigments suppressed the plasma inflammatory cytokines level in LPS-injected mice. The compounds (10 mg/kg) were injected 30 min before the LPS (2 mg/kg) treatment. Different letters indicate statistically significant differences between the means (p < 0.05) for each group.
AK: ankaflavin; MA: monaphilone A; MS: monascin; MB: monaphilone B; MCB: monascorubrin; RPT: rubropunctatin; MPA: monaphilol A; MPB: monaphilol B; MPC: monaphilol C; MPD: monaphilol D. 426 427 428 429 430 431 432 433 434 435 436 437 438 439 440 441 442 443 444 445 446 447
Figure 1. 448
449 450
A. B. Figure 2. c d b de b a e a b c d e ef f 451 452 453 454 455 456 457 458 459 460 461 462 463
A. B. C. Figure 3. A.
COX-2 (72 kD)
Control LPS AK MA MS MB
iNOS (130 kD)
-actin (44 kD)
a b c d d a ab b b b 464 465 466 467 468 469 470 471 472 473 474 475 476 477 478 479B. C. Figure 4. a b b c cd cd d 480 481 482 483 484 485 486 487 488 489
Figure 5.
(A)
(B)
(C)
a b b c c d a b b b c d a ab b b c d 490 491 492 493Figure 6.
(A)
(B)
(C)
a b c d d d d d a b b c c c c c a a a bc bc b c d 494 495 496Figure 7. a a a b b b b b b b b b 497 498 499 500 501 502 503 504 505
Figure 8. a a a bc bc bc bc bc bc cd d d 506 507 508 509
Figure 9.
(A)
(B)
(C)
a a b b b b b c c c c c a b b bc bcd bcd cd de de e e f a a ab abc abcd bcd bcd bcd cde de de e 510 511 512Table 1. Anti-NO activity of azaphilonoid pigments on LPS-stimulated RAW 264.7 cells. Compound IC50 g/mL μM ankaflavin 8.4 ± 0.3 21.8 ± 0.9 monascin 10.4 ± 0.9 29.1 ± 2.6 monaphilone A 7.1 ± 0.4 19.3 ± 1.1 monaphilone B 7.5 ± 0.3 22.6 ± 0.8 monascorubrin > 15 > 40 rubropunctatin 7.5 ± 0.7 21.2 ± 1.9 monaphilol A 0.4 ± 0.0 1.0 ± 0.3 monaphilol B 1.3 ± 0.1 3.8 ± 0.1 monaphilol C 1.2 ± 0.1 2.8 ± 0.2 monaphilol D 0.7 ± 0.0 1.7 ± 0.1
Quercetin (IC50 = 13.2 ± 1.2 M) was used as the positive control. 513
514
TOC Graphic. Structures of azaphilonoid pigments. 516
517 518