脂肪激素在兒童過敏性鼻炎所扮演的角色; The Roles of Adipokines in Childhood Allergic Rhinitis
全文
(2) 中文摘要 背景:雖然已經有證據顯示,在成人及兒童血清中的瘦素(leptin)濃度 與氣喘有正向關聯性,但是血清中的瘦素(leptin)及脂締素(adiponectin) 在兒童過敏性鼻炎所扮演的角色仍不清楚。 目標:本實驗的目的是要來評估患有過敏性鼻炎的兒童在治療前血清中的 瘦素(leptin)及脂締素(adiponectin)的濃度,並且進一步的評估瘦素 (leptin)及脂締素(adiponectin)的濃度與過敏性發炎指標的關連性。 方法:我們收集 51 位過敏性鼻炎的兒童(18 位女童及 33 位男童;平均年紀 7.3 ± 2.08 歲)以及 47 位正常的兒童(15 位女童及 32 位男童;平均年紀 6.43 ± 2.59 歲)納入本實驗中,測量他們的身體質量指數(BMI)及血清中的瘦 素(leptin)及脂締素(adiponectin)的濃度,並且測量他們的血中 IgE 的濃度、塵蟎特異性 IgE 的濃度及嗜伊紅性球陽離子蛋白質的濃度。 結果:過敏性鼻炎的兒童與正常的兒童兩組血清中的瘦素(leptin)及脂 締素(adiponectin)的濃度有顯著的差異。瘦素(leptin)中位數的濃度 兩組分別是 4.60 (2.16-14.82) ng/ml 及 3.31 (1.08-7.10) ng/ml,脂締素 (adiponectin)中位數的濃度兩組分別是 30.36 (21.08-41.85) μg/ml 及 39.07 (30.83-45.46) μg/ml,進一步的分析發現這樣的差異在男性比女性來的 顯著。利用邏輯性回歸分析法分析發現只有瘦素(leptin)及脂締素 (adiponectin)是罹患過敏性鼻炎的預測因子,其勝算比分別為 27.06 (95% 信賴區間, 1.01-1209) 及 13.14 (95%信賴區間, 1.84-115.1)。利用多變相迴歸 1.
(3) 分析發現身體質量指數(BMI)以及過敏性鼻炎與血清中瘦素(leptin)濃 度有顯著相關,另ㄧ方面身體質量指數(BMI)、年紀和過敏性鼻炎與血清 中脂締素(adiponectin)濃度有顯著相關。過敏性鼻炎兒童的脂締素 (adiponectin)濃度與血清中嗜伊紅性球陽離子蛋白質的濃度有顯著的負 相關。這兩種脂肪激素與血清中 IgE 的濃度、塵蟎特異性 IgE 的濃度都沒 有相關性。 結論:過敏性鼻炎的兒童,具有較高的血清瘦素(leptin)濃度及較低的 血清脂締素(adiponectin)的濃度,這樣的結果暗示脂肪激素在過敏性鼻 炎的致病機轉扮演一定的角色,至於是否可以應用在治療上仍需進一步的 研究。. 2.
(4) 英文摘要 Background: Although there is evidence of a positive association between leptin and asthma in adults and children, very little is known about the role of adiponectin and leptin in children with allergic rhinitis (AR). Objectives: The aims of this study were to evaluate serum leptin and adiponectin levels in a group of children with allergic rhinitis before the initiation of therapy and to examine the relationship between leptin and adiponectin and allergic inflammatory markers in AR children. Methods: Body mass index (BMI) and serum leptin and adiponectin levels were measured in 51 (18 female, 33 male; mean age, 7.3 ± 2.08 years) allergic rhinitis children and 47 (15 female, 32 male; mean age, 6.43 ± 2.59 years) healthy children. Total serum IgE and mite-specific IgE and serum eosinophil cationic protein (ECP) levels were also measured. Results: A significant difference was observed in serum leptin and adiponectin levels between AR and healthy children. Median (interquartile range) levels of leptin were 4.60 (2.16-14.82) ng/ml and 3.31 (1.08-7.10) ng/ml, respectively (P = 0.041). Median (interquartile range) levels of adiponectin were 30.36 (21.08-41.85) μg/ml and 39.07 (30.83-45.46) μg/ml, respectively (P = 0.005). Further analysis revealed that these differences in leptin and adiponetin levels appeared to be far more significant in boys than girls. By logistic regression analysis, only leptin and adiponectin were predictive factors for having allergic rhinitis with their odds ratios being 27.06 (95% confidence interval (CI), 3.
(5) 1.01-1209) and 13.14 (95% CI, 1.84-115.1), respectively. In the multiple regression analysis, only BMI and AR were significantly associated with leptin levels and BMI, age and AR correlated with adiponectin levels. A significantly negative but weak correlation was observed between log adiponectin and log ECP levels among children with AR (r = -0.29; P = 0.036). There was no relation between adipokines levels and total IgE or mite-specific IgE levels. Conclusion: Patients with allergic rhinitis have a marked increase in serum levels of leptin but a marked decrease in adiponectin levels. These data confirm a relevant role for adipokines in the pathogenesis of allergic rhinitis and suggest important therapeutic implications that need further exploration.. 4.
(6) 序言 在繁忙的臨床工作之餘還要從事研究工作確實不容易,很感謝我的指導教 授蘇百弘主任給我的指導與協助,有了他的指導才令我引發動機去研究探 討脂肪激素在過敏領域所扮演的角色。經過此次的研究才發現脂肪激素在 過敏領域裡還有很多值得研究探討的地方,這也是我將來要繼續研究探討 的方向。 這個計畫的完成經歷了許多人的幫忙,包括研究助理黃美菁小姐幫忙 完成檢體的實驗室分析 以及我的姐夫周文才先生幫忙資料的統計分析。. 5.
(7) 目錄 內容 中文摘要 ---------------------------------------------------- 1. 英文摘要 -----------------------------------------------------3. 序言--------------------------------------------------------. 5.. 目錄 ---------------------------------------------------------6. 表目錄 ------------------------------------------------------ 8. 第一章. 前言. 第一節 研究背景---------------------------------------- 9. 第二節 研究目的----------------------------------------12. 第二章 研究方法 第一節 研究材料---------------------------------------- 13 第二節 研究設計-----------------------------------------15 第三節 統計方法---------------------------------------- 17 . 第三章 研究結果 第一節 描述性統計分析--------------------------------- 18 3-1-1 過敏性鼻炎與正常小朋友兩組基本資料比較--------3-1-2 過敏性鼻炎與正常小朋友血清中瘦素及脂締素差異性-. 18 18. 第二節 推論性統計分析--------------------------------- 19 3-2-1 影響血清中瘦素及脂締素濃度相關因子的探討------ 19 6.
(8) 3-2-2 發生過敏性鼻炎與瘦素及脂締素濃度的相關性探討--. 19. 3-2-3 血清中瘦素及脂締素濃度與過敏性發炎指標的關連性- 20 第四章 討論 第一節 結果討論--------------------------------------. 21. 4-1-1 過敏性鼻炎與正常小朋友兩組基本資料及血清中瘦素及脂締 素差異性---------------------------------------------------------- 21 4-1-2 影響血清中瘦素及脂締素濃度相關因子的探討------. 21. 4-1-3 過敏性鼻炎與瘦素及脂締素濃度以及過敏性發炎指標的相關 性以及免疫學相關機轉的探討-------------------- 23 第二節 其他相關性討論--------------------------------- 28 第五章 結論與建議-------------------------------------------- 30 參考文獻----------------------------------------------------- 34. 7.
(9) 表目錄 表 1、過敏性鼻炎與正常小朋友兩組基本資料、血清瘦素及脂締素濃度、血 清 IgE 的濃度、塵蟎特異性 IgE 的濃度及嗜伊紅性球陽離子蛋白質濃 度的比較 表 2、過敏性鼻炎與正常小朋友血清瘦素及脂締素濃度與人體測量指標及過 敏性發炎指標的相關性 表 3、血清瘦素濃度多變相迴歸分析 表 4、血清脂締素濃度多變相迴歸分析 表 5、發生過敏性鼻炎危險因子多變相迴歸分析. 8.
(10) 第一章 前言. 第一節 研究背景 Adipose tissue is no longer considered an inert tissue mainly devoted to energy storage. The current view of adipose tissue is that of an active secretory organ, sending out and responding to signals that modulate appetite, energy expenditure, insulin sensitivity, endocrine and reproductive systems, bone metabolism, and inflammation and immunity. Adipocytes produce and secret a number of hormones, collectively called adipokines, of importance in modulating immunity and inflammation. The most important two adipokines are leptin and adiponectin. Leptin is a 16-kd protein encoded by the ob gene (1). Adipocytes are the most important source of leptin, and circulating leptin levels directly correlate with adipose tissue mass (2). It acts in the hypothalamus to induce satiety and increase metabolism (3). In addition to its effects on the regulation of body weight, leptin is also proinflammatory (4). Hematopoietic cells express leptin receptors, and bone marrow cells cultured in the presence of leptin demonstrate growth of granulocyte-macrophage colonies (5, 6). CD4+ T cells, monocytes, and macrophages can each respond to leptin with increased proliferative responses, increased production of some cytokines, or both (5, 7-9). Leptin also causes activation of the transcription factors activating protein 1 and nuclear factor-κB in endothelial cells (10). Adiponectin is the adipokine that circulates at the highest levels. In human subjects and in animals, adiponectin 9.
(11) mRNA expression in adipocytes decreases in obesity and increases again with weight loss (11-13), and plasma adiponectin levels are inversely related to body mass index (14). Whereas this adipokine is best known for its role in the regulation of insulin sensitivity, adiponectin is also anti-inflammatory. Adiponectin inhibits inflammatory gene expression in a variety of cell types, inhibits or modulates nuclear factor κB (NF-κB) and extracellular signal–regulated kinase activation, and augments expression of anti-inflammatory genes, including the IL-1 receptor antagonist and IL-10 gene (15-18). Furthermore, adiponectin reduces induction of the endothelial adhesion molecules ICAM-1 and vascular cell adhesion molecule 1 by either TNF-α or resistin (19, 20). There is increasing evidence in the literature that adipokines may play a role in allergic inflammation. Several studies have shown an association between obesity and asthma (21-24), and much of the literature focusing on the relationship between obesity and airway inflammation and asthma has focused on the role of leptin and adiponectin. Shore and colleagues reported that exogenous leptin can augment allergic airway responses but exogenous adiponectin can attenuate allergic airway responses in ovalbumin (OVA)-sensitized and challenged mice, independent of obesity (25, 26). Clinical studies provide supporting evidence to the mouse-model observation that leptin may play a role in asthma that is to some extent independent of obesity. Guler and colleagues reported that a significant elevation in serum leptin was observed 10.
(12) in children with asthma when compared with healthy children despite similar mean body mass index (BMI), although this difference appeared to be far more significant in boys than girls (27). Sood and colleagues reported that high serum leptin concentrations in adults are associated with asthma and this association is stronger in women (28).. 11.
(13) 第一章. 前言. 第二節 研究目的 Allergic rhinitis (AR) is the most common atopic disorder in the United States, affecting between 9% and 24% of adults and up to 42% of children (29, 30). Higher frequencies of reported comorbidity, as well as functional, immunological and therapeutic considerations, had induced Passalacqua et al. to refer the nose-lung interaction in allergic rhinitis and asthma as united airways disease (UAD) (31). Not all patients with rhinitis present with asthma, however, and there are differences between rhinitis and asthma. The magnitude of inflammation may not be identical between nasal and bronchial mucosa. Despite the evidence of the role of adipokines in asthma, very little is known about the association between adipokines concentrations and AR. We hypothesized that altered systemic leptin and adiponectin levels in childhood would be associated with AR and both of these adipokines would be related to some allergic inflammatory markers. We tested this hypothesis in a population based, cross sectional study.. 12.
(14) 第二章 研究方法. 第一節 研究材料 Subjects Our study was performed at the outpatient allergy clinic of the Department of Pediatrics, China medical University Hospital in Taiwan. Fifty one prepubertal children (33 male, 18 female), aged 2–10 yr (mean age 7.3 ± 2.08 y/o) with newly diagnosed perennial allergic rhinitis were enrolled in the study group. Each patient demonstrated at least a 1-year history of moderate to severe persistent allergic rhinitis with a positive response to mite-specific IgE (>0.35 kU/l), which was determined using the Pharmacia CAP system (Pharmacia, Uppsala, Sweden).Children were excluded when any of the following criteria was present: (1) presence of past history of asthma, nasal abnormalities, concurrent purulent nasal infection, or any other significant medical conditions; (2) use of systemic or topical corticosteroids or sodium cromoglycate within the past 4 weeks; (3) use of histamine H1 antagonist within the past 7days; and (4) a history of any infectious states within the past 2 weeks. Forty seven prepubertal children (32 male, 15 female), aged 2–10 yr (mean age 6.43±2.59 y/o) without any documented diagnosis who had applied for a routine checkup and vaccination or elective preoperative evaluation for circumcision, and who were without a history of infection during the previous 2 weeks served as controls. Neither these children, their siblings, nor their parents had any atopic disease. 13.
(15) Serum IgE levels and allergen-specific IgE values (<0.35 kU/l) were all within normal ranges. Informed consent for participation in the study was obtained from the parents of all subjects. The protocols were approved by the Institutional Review Board of China Medical University Hospital.. 14.
(16) 第二章 研究方法 第二節 研究設計 Methods Body mass index (BMI) was calculated as body weight (kg)/height (m2). Blood samples were obtained between 6 AM and 9 AM after an overnight fast. After clotting at 4°C, the serum was separated by centrifugation and stored at -70°C until assay. Analyses of serum leptin and adiponectin The serum levels of leptin and adiponectin were measured using ELISA kits (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. The minimal detectable concentration of leptin is < 150 pg/ml (intra-assay and inter-assay coefficients of variation were 4.8 % and 7.7 %, respectively). The minimal detectable concentration of adiponectin is 1 ng/ml (intra-assay and inter-assay coefficients of variation were 4.8 % and 7.2 %, respectively). Serum eosinophil cationic protein (ECP) and Total IgE Serum total IgE (IU/ml) and ECP (ng/L) were determined by enzyme-linked immunosorbent assay method (Pharmacia CAP system). The detectable range of the assay for total IgE is 2-2000 IU/ml and for serum ECP is 2 to 100 ng/L. Allergen-specific IgE. 15.
(17) Specific IgE against Dermatophagoides pteronyssinus and Dermatophagoides farinae were measured using the Pharmacia CAP System (Pharmacia Diagnostics, Uppsala, Sweden), according to the instructions of the manufacturer. In the Pharmacia CAP System, results are reported in IU/ml, with a cutoff value of 0.35 IU/ml, and an upper limit of 100.00 IU/ml. The titers are classified as valence 0-6 according to the results: valence 0 (0-0.35 IU/ml), valence 1(0.36-0.70 IU/ml), valence 2 (0.71-3.50 IU/ml), valence 3 (3.51-17.50 IU/ml), valence 4 (17.51-50.00 IU/ml), valence 5 (50.01-100.00 IU/ml), valence 6 (>100.00 IU/ml).. 16.
(18) 第二章 研究方法 第三節 統計方法 Numerical variables were reported as means ± SDs. Numerical parameters with nonnormal distribution (serum leptin, adiponectin, IgE, allergen-specific IgE and ECP levels) were reported as medians and interquartile ranges and were log-transformed before analysis. Bivariate analyses were evaluated by using χ2 and Mann-Whitney U or Student t tests. Correlations between log leptin or log adiponectin and various other parameters were assessed by using the Pearson correlation coefficient. To test which variables independently contribute to the variation of leptin and adiponectin levels, multivariate linear regression analysis was performed. Adjusted R2 that corrected for the number of variables and the sample size was used to estimate the variance explained in each model. To investigate the relationships between sex (1, male; 0, female), age, BMI, leptin, adiponectin and the development of allergic rhinitis, a multivariate logistic regression analysis was done. Statistical analysis was performed with the SPSS software, version 7.5 for Windows (SPSS, Chicago, Ill). P < .05 was considered significant.. 17.
(19) 第三章 研究結果 第一節 描述性統計分析 3-1-1 過敏性鼻炎與正常小朋友兩組基本資料比較 Anthropometric parameters of the AR patients and the controls are shown in Table I. The mean values for age, weight, height, and BMI did not show any difference between the groups of allergic rhinitis and healthy children. There was no significant sex difference with respect to these parameters between and within the groups, either. 3-1-2 過敏性鼻炎與正常小朋友血清中瘦素及脂締素差異性 A significant elevation in serum leptin was observed in children with AR when compared with healthy children (p = 0.041) despite a similar BMI in both groups. There was a tendency toward higher leptin levels in AR boys compared with healthy boys with a borderline significance (P = 0.085) despite a similar BMI in both groups. In contrast, serum adiponectin levels in children with AR were significantly lower (p = 0.005) than those in healthy children in spite of a similar BMI in both groups. This significant difference was also apparent between the AR boys and healthy boys with similar BMI values (P = 0.009), whereas a similar difference was not observed between AR girls and healthy girls. No significant sex difference was detected in serum leptin and adiponectin levels among AR children, as well as among healthy children (Table I).. 18.
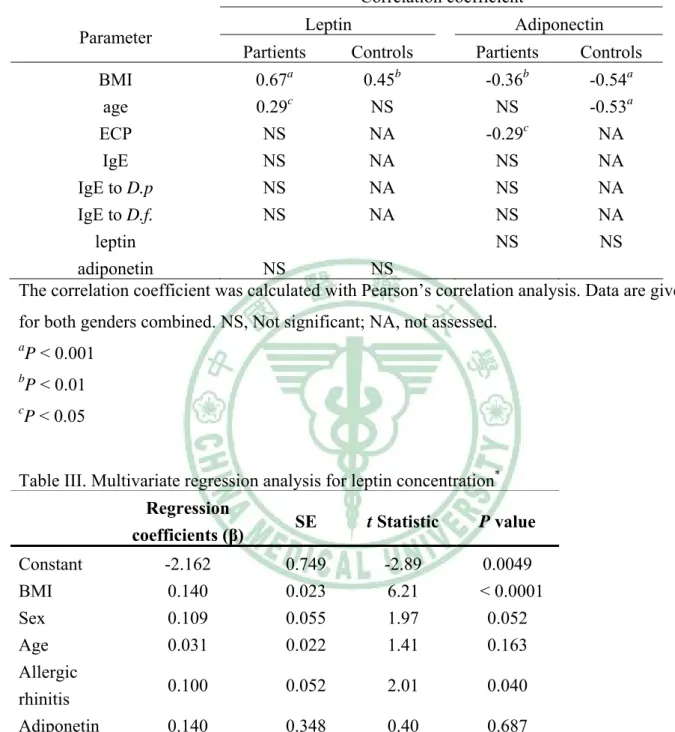
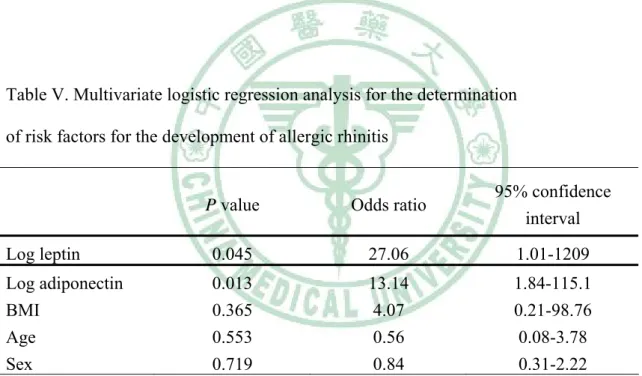
(20) 第三章 研究結果 第二節 推論性統計分析. 3-2-1 影響血清中瘦素及脂締素濃度相關因子的探討 Log leptin levels of both AR and healthy children showed positive correlations with BMI, but log adiponectin levels correlated negatively with BMI in both groups (Table II). A weak positive correlation existed between log leptin levels and age only in AR patients, whereas log adiponectin levels were significantly inversely related to age in healthy children (r = -0.53; P < 0.001) (Table II). In a multivariable regression model including BMI, sex, age, allergic rhinitis and log adiponectin with log leptin as the dependent variable, we identified BMI (p < 0.0001), and allergic rhinitis (P = 0.040) as significant independent determinants of leptin levels, explaining 35 % of the variance (adjusted R2= 0.35; p < 0.0001; Table III). In a multivariable regression model including BMI, sex, age, allergic rhinitis and log leptin with log adiponetin as the dependent variable, BMI (P = 0.020), age (P = 0.031), and allergic rhinitis (P = 0.011) were significant independent determinants of adiponetin levels, explaining 20 % of the variance (adjusted R2=0.20; p = 0.0001; Table IV). 3-2-2 發生過敏性鼻炎與瘦素及脂締素濃度的相關性探討 When sex, age, BMI, log leptin and log adiponetin were all included in the logistic regression model, using allergic rhinitis as the dependent variable, only log leptin and log adiponetin were independent risk factors for the development 19.
(21) of allergic rhinitis, with their odds ratios being 27.06 (95% confidence interval (CI), 1.01-1209) and 13.14 (95% CI,. 1.84-115.1), respectively (Table V).. 3-2-3 血清中瘦素及脂締素濃度與過敏性發炎指標的關連性 A significantly negative but weak correlation was observed between log adiponectin and log ECP levels among AR children (r = -0.29; P = 0.036) but a significant correlation was not found between log leptin and log ECP. Both leptin and adiponectin levels did not correlate with total IgE or mite-specific IgE levels among AR children. There was no statistical correlation between leptin and adiponectin levels in both groups (Table II) There was no statistically significant difference in the leptin and adiponetin levels between AR children with higher mite-specific IgE levels (valence 4-6) and those with lower mite-specific IgE levels (valence 1-3) (data not shown).. 20.
(22) 第四章 討論. 第一節 結果討論 4-1-1 過敏性鼻炎與正常小朋友兩組基本資料及血清中瘦素及脂締素差 異性 The aims of this study were to evaluate serum leptin and adiponectin levels in a group of children with AR before the initiation of therapy and to examine the relationship between leptin and adiponectin and allergic inflammatory markers in AR children. Our results show that a significant elevation in serum leptin and a significant reduction in serum adiponectin were observed in children with AR when compared with healthy children in spite of no difference in BMI levels, although these differences appeared to be far more significant in boys than girls. The lack of statistically significant differences in leptin and adiponectin levels between AR girls and healthy girls could be a result either of a small sample size to detect a difference or a true modifying effect of sex on leptin and adiponectin. Higher levels of leptin with a borderline significance and significantly lower levels of adiponectin in AR boys but not in girls may suggest that both of the adipokines might be involved in the pathophysiologic mechanisms of childhood AR, which is also seen more frequently in boys than in girls (32, 33).. 4-1-2 影響血清中瘦素及脂締素濃度相關因子的探討 Serum leptin levels show positive correlation and adiponectin levels. 21.
(23) correlated inversely with BMI in healthy prepubertal children, as has also been shown in our study in both the AR children and the healthy children (14, 34). Serum leptin levels are relatively low in prepubertal ages, showing a gradual rise in both sexes before the onset of puberty, followed by a significant increase during puberty in girls and a decrease in boys (34, 35). Our results showed that a weak correlation existed between leptin levels and age only in AR patients and in univariate linear regression, age was positively associated with leptin (P= 0.0006) which explained 10 % of the variation in leptin concentrations (data not shown). However, the effect of age on leptin levels was eliminated in a multiple regression model that included BMI, age, sex and AR. By contrast, as previous reports (37, 38), serum adiponectin levels were significantly inversely related to age during childhood and age was a significant independent determinant of adiponectin in a multiple regression model in our study. Leptin and adiponectin levels were found in similar concentrations in both sexes over the prepubertal years in some studies as has also been shown in children in our study (34, 38), whereas a significant sex difference in leptin levels was noted in other studies, with girls having higher leptin levels than boys despite the absence of difference in body weight (35, 36). Interestingly, in the multiple regression analysis, we found that sex correlated with plasma leptin with a borderline significance (p = 0.052) after the adjustment. The effect size of sex on leptin levels needs further study.. 22.
(24) 4-1-3 過敏性鼻炎與瘦素及脂締素濃度以及過敏性發炎指標的相關性以 及免疫學相關機轉的探討 Although the relationship among AR and adipokines cannot be adequately addressed in this study because of the small sample size, the results of multivariant analysis suggested that leptin and adiponectin may have some effects on the regulation of allergic inflammation in the allergic rhinitis. Whereas the primary physiologic function of leptin and adiponectin is in the regulation of metabolism, they also regulate immune function and are believed to be pro-inflammatory and anti-inflammatory, respectively. Both of them have been reported to be involved in a variety of inflammatory-autoimmune conditions, such as inflammatory bowel disease (38, 39), rheumatoid arthritis (40), multiple sclerosis (41), Kawasaki disease (42) and endometriosis (43). However, the pathogenic role played by adipokines in such disorders is far from understood. Few clinical studies have investigated the relationship between adipokines and atopic diseases, such as asthma and atopic dermatitis, with all of them focusing on leptin. In the study by Guler et al (27), serum leptin levels were found to be higher in children with atopic bronchial asthma when compared with nonatopic asthmatic subjects and healthy children with similar BMI. They found serum leptin to be an independent predictive factor for asthma in children, especially in boys. Gurkan et al, reported that, in a small cohort (n=23) of children with mild to moderate asthma, treatment with inhaled budesonide resulted in a significant reduction in serum leptin concentrations 23.
(25) after 4 weeks (44). In a smaller group (n= 50), reported by Kimata, patients with IgE-associated atopic eczema/dermatitis syndrome (AEDS) had significantly elevated serum leptin levels related to steatohepatitis and hyperlipidemia (45). To our knowledge, neither the association between adipokines and AR nor the role of adiponectin in atopic disorders has previously been studied clinically. Furthermore, although altered systemic adipokine levels in atopic disorders are noted, the exact mechanism involved in the alteration in adipokines expression and the pathogenic role played by adipokines in TH2-mediated inflammation has not been fully clarified. In two previous studies by Shore et al (25, 26), sensitization and repeated airway challenge with OVA in BALB/cJ mice increased serum leptin but caused a reduction in serum adiponectin and a corresponding decrease in adipose tissue adiponectin mRNA expression. Because adipocytes are the primary source of leptin and adiponectin, these data suggested that allergic responses in the airways are capable of altering adipocyte function. It has been well documented that proinflammatory cytokines including IL-1β, TNF-α and IL-6 are capable of inducing the release of leptin from adipocytes but inhibiting adiponectin expression in adipocytes (46-48). Furthermore they have been shown to contribute to allergic airway responses in mice (49-51) and serum levels of these cytokines are increased in asthma and allergic rhinitis (52). It is possible that proinflammatory cytokines might be involved in allergen-induced alterations in adipokines expression. However, there may be a further complexity in the regulation of adipokines expression in 24.
(26) allergic inflammation. As previous reports, the production of proinflammatory mediators is also markedly influenced by adipokines levels (7, 8, 15, 18). The interaction between proinflammatory cytokines, adipokines and other inflammatory cells and mediators may be complex and remains to be further elucidated. The findings in the studies by Shore et al also indicate that exogenous leptin augments OVA- induced airway hyperresponsiveness (AHR) to methacholine (MCh). The increase in OVA-induced airway responsiveness occurred in the absence of any effects of leptin on inflammatory cell influx or TH2 cytokine expression but was associated with an increase in OVA-induced IgE production in the leptin treated mice. In contrast, exogenous adiponectin can attenuate OVA-induced changes in inflammatory cell influx and TH2 cytokine expression in the lungs, as well as OVA-induced AHR. These investigators speculated that the ability of leptin to augment OVA-induced AHR may be related to the direct effects of leptin on B lymphocytes that contributed to the production of IgE and may be the result of increased activation of mast cells. Guler et al also reported a weak but significant correlation between serum leptin and serum IgE among the children with asthma (r = 0.231, p = 0.019) (27). However, the finding in the present study, indicating no significant correlation between serum leptin and serum IgE among children with AR, is in contrast to the findings by Shore et al and Guler et al. Indeed, discrepant results about the association between leptin and IgE existed. Kimata ever reported leptin can inhibit in vitro IgE and IgG4 production (45) and in a previous study of mice by 25.
(27) Hetland et al (53), leptin does not influence the IgE response to OVA in mice. Further studies regarding the effects of leptin on IgE production are necessary. The relationship between adiponectin and IgE is less studied. In the report by Shore et al, OVA challenge did not increase serum IgE levels in adiponectin-treated mice, although IgE levels were greater in adiponectinversus Tris-buffered saline (TBS)-treated mice, regardless of the PBS or OVA aerosol challenge. In the present study, there was no significant correlation between adiponectin and total IgE levels. Furthermore, we found both leptin and adiponectin levels did not correlate with mite allergen-specific IgE levels and there was no statistically significant difference in the leptin and adiponectin levels among AR children on the basis of allergen-specific IgE levels. In a recent study by Conus el al (54), human eosinophils were found to express leptin surface receptors under in vitro and in vivo conditions, and leptin directly activates eosinophils and delays spontaneous apoptosis of these cells. The authors speculated that leptin may contribute to eosinophil accumulation at inflammatory sites, and perhaps it might then also stimulate the release of proinflammatory mediators from these cells. In the present study, we did not find a significant correlation between serum leptin and serum ECP level, an activated eosinophil-derived inflammatory mediator and is significantly higher in AR patients than in controls (55). Interestingly, there was a significantly inverse but weak correlation between serum adiponectin and serum ECP level among the children with AR (r = -0.29; P = 0.036). According to the report by 26.
(28) Shore et al, this may be explained partly by an adiponectin-induced inhibition of TH2 cytokine expression and eosinophil influx in the inflammatory sites. Further work is required to understand whether adiponectin has direct negative effects on eosinophil function and activation.. 27.
(29) 第四章 討論 第二節 其他相關性討論 The overall pathogenic view of respiratory allergy has profoundly changed and evolved over the past 10 years. Particular attention has been paid to the relationship between rhinitis and asthma. Evidence for this strict link leads to the operative definition of allergic rhinobronchitis or united airways disease (UAD). In this regard, many researchers have focused their attention on the systemic aspects of allergy because of a series of important observations indicating that local allergic reactions lead to manifestations at distant sites via producing systemic inflammatory events. These systemic inflammatory events have two consequences: they feed back into the site of the original reaction and lead to pathologic responses in other parts of the body. The mechanisms that lead to the development of the systemic element, and particularly the mechanisms of distant manifestations after a local allergic reaction have not been fully elucidated but they probably include a constellation of various interactive or even independent pathways (56, 57). According to the above-mentioned clinical and animal studies indicating the role of leptin and adiponectin in asthma and our current preliminary study revealing the alterations in adipokines levels in AR patients, we speculate that adipokines might also be involved in the systemic inflammatory events of allergic disease, further supporting the evidence that white adipose tissue is an active player, and not simply a bystander, in the 28.
(30) modulation of inflammatory immune response.. 29.
(31) 第五章 結論與建議 In conclusion, patients with allergic rhinitis have a marked increase in serum levels of leptin but a decrease in adiponectin levels. These data confirm a relevant role for adipokines in the pathogenesis of allergic rhinitis and suggest important therapeutic implications that need further exploration.. 30.
(32) Table I: Anthropometric parameters and serum leptin, adiponectin, eosinophil cationic protein (ECP), total IgE and mite-specific IgE levels in children with allergic rhinitis and healthy controls* Patients. Controls. (n = 51)(female/. (n= 47)(female/. Male=18/33). Male=15/32). p. Total. 7.3±2.08. 6.43±2.59. .068. Girls. 7.26±2.43. 6.65±2.99. .522. Boys. 7.32±1.90. 6.32±2.42. .071. Total. 27.00±8.30. 23.92±8.41. .071. Girls. 26.64±8.25. 23.04±9.64. .611. Boys. 28.29±8.17. 24.33±7.90. .051. Total. 125.08±14.20. 119.57±16.83. .082. Girls. 124.06±15.61. 119.40±18.37. .437. Boys. 125.64±13.57. 119.66±16.37. .115. Total. 16.85±2.47. 16.30±2.46. .270. Girls. 15.59±2.25. 15.45±1.85. .846. Boys. 17.54±2.34. 16.69±2.63. .177. Total. 4.60 (2.16-14.82). 3.31 (1.08-7.10). .041. Girls. 4.76 (2.72- 9.69). 4.12 (1.51-5.32). .278. Boys. 4.44 (2.09-18.13). 3.26 (1.04-10.08). .085. Total. 30.36 (21.08-41.85). 39.07 (30.83-45.46). .005. Girls. 34.69 (23.11-44.19). 41.62 (34.18-49.27). .180. Boys. 26.97 (20.97-36.83). 37.27 (28.46-43.34). .009. ECP (ng/L). 21.40 (7.40-40.1). 6.86 (4.16-16.1). .018. Total Ig E (IU/ml) † IgE to D.p . (IU/ml) † IgE to D.f. (IU/ml). 356 (169.5-672.5). 32.6 (18.85-83.85). .000. 61.4 (12.15-100). < 0.35. .000. 48.0 (9.57-100). < 0.35. .000. Age(y). Weight(kg). Height(cm). BMI. Leptin(ng/ml). Adiponectin(μg/ml). *Age,. weight, height and BMI are given as arithmetic means ± SDs; The Student t test is used.. Leptin, adiponectin, ECP, IgE , IgE to D.p. and IgE to D.f. are given as median (interquartile range); the Mann-Whitney U test is used for comparison. †D.p.: Dermatophagoides pteronyssinus †D.f.: Dermatophagoides farinae 31.
(33) Table II Association of leptin and adiponectin levels with anthropometric and allergic inflammatory parameters in in children with allhinitiergic rs and healthy controls Correlation coefficient Leptin. Parameter. Partients. Adiponectin. Controls. a. Partients. b. Controls. b. BMI 0.67 0.45 -0.36 -0.54a age 0.29c NS NS -0.53a ECP NS NA -0.29c NA IgE NS NA NS NA IgE to D.p NS NA NS NA IgE to D.f. NS NA NS NA leptin NS NS adiponetin NS NS The correlation coefficient was calculated with Pearson’s correlation analysis. Data are given for both genders combined. NS, Not significant; NA, not assessed. a. P < 0.001. b. P < 0.01. c. P < 0.05. Table III. Multivariate regression analysis for leptin concentration*. Constant BMI Sex Age Allergic rhinitis Adiponetin *. Regression coefficients (β). SE. t Statistic. P value. -2.162 0.140 0.109 0.031. 0.749 0.023 0.055 0.022. -2.89 6.21 1.97 1.41. 0.0049 < 0.0001 0.052 0.163. 0.100. 0.052. 2.01. 0.040. 0.140. 0.348. 0.40. 0.687. The dependent variable was log leptin (adjusted R2= 0.35; F = 11.45; p < 0.0001). 32.
(34) Table IV. Multivariate regression analysis for adiponectin concentration* Regression coefficients (β). SE. t Statistic. P value. 1.916 -0.018 0.011 -0.014. 0.122 0.008 0.017 0.007. 15.65 -2.36 0.67 -2.19. < 0.0001 0.020 0.504 0.031. -0.040. 0.015. -2.58. 0.011. 0.013. 0.031. 0.40. Constant BMI Sex Age Allergic rhinitis Leptin. 0.688 2. The dependent variable was log adiponectin (adjusted R =0.20; F=5.611; p = 0.0001). Table V. Multivariate logistic regression analysis for the determination of risk factors for the development of allergic rhinitis. P value. Odds ratio. 95% confidence interval. Log leptin. 0.045. 27.06. 1.01-1209. Log adiponectin BMI Age Sex. 0.013 0.365 0.553 0.719. 13.14 4.07 0.56 0.84. 1.84-115.1 0.21-98.76 0.08-3.78 0.31-2.22. 33.
(35) 參考文獻 1. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM.. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425-32. 2. Maffei M, Fei H, Lee GH, Dani C, Leroy P, Zhang Y, et al. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci U S A 1995;92:6957-60. 3. Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol Behav 2001;74:703-8. 4. Huang L, Li C. Leptin: a multifunctional hormone. Cell Res 2000;10:81-92. 5. Gainsford T, Willson TA, Metcalf D, Handman E, McFarlane C, Ng A, et al. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci U S A 1996;93:14564-8. 6. Umemoto Y, Tsuji K, Yang FC, Ebihara Y, Kaneko A, Furukawa S, et al. Leptin stimulates the proliferation of murine myelocytic and primitive hematopoietic progenitor cells. Blood 1997;90:3438-43. 7. Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation induced immunosuppression. Nature 1998;394:897-901. 8. Sanchez-Margalet V, Martin-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood 34.
(36) mononuclear cells: mechanisms of action. Clin Exp Immunol 2003;133:11-9. 9. Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol 2000;199:15-24. 10. Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J 1999;13:1231-8. 11. Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 1996;271:10697-703. 12. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001;7:941-6. 13. Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes 2003;52:1779-85. 14. Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, et al. Association between adiponectin and mediators of inflammation in obese women. Diabetes 2003;52:942-7. 15. Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, et al. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology 2004;40:177-84. 16. Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, et al. Adiponectin specifically increased tissue inhibitor of metalloprotenase-1 35.
(37) through interleukin-10 expression in human macrophages.Circulation 2004;109:2046-9. 17. Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun 2004;323:630-5. 18. Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulate cytokines in porcine macrophages. Biochem Biophys Res Commun 2004;316:924-9. 19. Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 1999;100:2473-6. 20. Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, et al. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun 2004;314:415-9. 21. Chinn S. Obesity and asthma: evidence for and against a causal relation. J Asthma 2003;40:1–16. 22. Chinn S, Jarvis D, Burney P. Relation of bronchial responsiveness to body mass index in the ECRHS. European Community Respiratory Health Survey. Thorax 2002;57:1028–33. 23. Figueroa-Munoz JI, Chinn S, Rona RJ. Association between obesity and asthma in 4–11 year old children in the UK. Thorax 2001;56:133–7. 36.
(38) 24. Camargo CA Jr, Weiss ST, Zhang S, et al. Prospective study of body mass index, weight change, and risk of adult- onset asthma in women. Arch Intern Med 1999;159:2582–8 25. Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol 2005;115:103–109. 26. Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 2006;118:389-95. 27. Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol 2004;114:254–259. 28. Sood A, Ford ES, Camargo CA. Association between leptin and asthma in adults. Thorax 2006;61:300–305. 29. Nathan RA, Meltzer EO, Selner JC, Storms W. Prevalence of allergic rhinitis in the United States. J Allergy Clin Immunol 1997;99(suppl):S808-14. 30. Wright AL, Holberg CJ, Martinez FD, Halonen M, Morgan W, Taussig LM. Epidemiology of physician-diagnosed allergic rhinitis in childhood.Pediatrics 1994;94:895-901. 31. Passalacqua G, Ciprandi G, Canonica GW. The nose–lung interaction in allergic rhinitis and asthma: united airways disease. Curr Opin Allergy Clin Immunol 2001;1:7–13. 37.
(39) 32. Clough J. The effect of gender on the prevalence of atopy and asthma. Clin Exp Allergy 1993;23:883-5. 33. Bousquet J, van Cauwenberge P, Khaltaev N. ARIA workshop report allergic rhinitis and its impact on asthma. J Allergy Clin Immunol 2001;108:147s–334s 34. Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Müller J, et al. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage and testosterone. J Clin Endocrinol Metab 1997;82:2904-10. 35. Garcia-Mayor RV, Andrade MA, Rios M, Lage M, Dieguez C, Casanueva FF. Serum leptin levels in normal children: relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. J Clin Endocrinol Metab 1997;82:2849-55. 36. Hassink SG, Sheslow DV, Lancey E, Opentanova I, Considine RV, Caro JF. Serum leptin in children with obesity: relationship to gender and development. Pediatrics 1996;98:201-3. 37. Punthakee Z, Delvin EE, O’Loughlin J, Paradis G, Levy E, Platt RW, Lambert M Adiponectin, Adiposity, and Insulin Resistance in Children and Adolescents. J. Clin. Endocrinol. Metab., Jun 2006; 91: 2119 – 2125 38. Böttner A, Kratzsch J, Müller G, Kapellen TM, Blüher S, Keller E, Blüher M, Kiess W. Gender Differences of Adiponectin Levels Develop during the Progression of Puberty and Are Related to Serum Androgen Levels. J. Clin. 38.
(40) Endocrinol. Metab 2004; 89: 4053 - 4061 38. Tuzun A, Uygun A, Yesilova Z, Ozel AM, Erdil A, Yaman H, et al. Leptin levels in the acute stage of ulcerative colitis. J Gastroenterol Hepatol 2004;19:429-32. 39. Barbier M, Vidal H, Desreumaux P, Dubuquoy L, Bourreille A, Colombel JF, et al. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroenterol Clin Biol 2003;27:987-91. 40. Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gómez-Reino JJ, Gualillo O. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis 2006;65:1198–1201. 41. Batocchi AP, Rotondi M, Caggiula M, Frisullo G, Odoardi F, Nociti V, et al. Leptin as a marker of multiple sclerosis activity in patients treated with interferon-beta. J Neuroimmunol 2003;139:150-4. 42. Takeshita S, Takabayashi H, Yoshida N. Circulating adiponectin levels in Kawasaki disease. Acta Paediatr. 2006;95:1312-4. 43. Takemura Y, Osuga Y, Harada M, Hirata T, Koga K, Morimoto C, Hirota Y, Yoshino O, Yano T, Taketani Y. Serum adiponectin concentrations are decreased in women with endometriosis.Hum Reprod. 2005;20:3510-3 44. Gurkan F, Atamer Y, Ece A, Kocyigit Y, Tuzun H, Mete N. Serum leptin levels in asthmatic children treated with an inhaled corticosteroid. Ann Allergy Asthma Immunol 2004;93:277–280. 39.
(41) 45. Kimata H. Elevated serum leptin in AEDS [letter]. Allergy.2002;57:179. 46. Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, et al. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest 1996;97:2152-7. 47. Degawa-Yamauchi M, Moss KA, Bovenkerk JE, Shankar SS, Morrison CL, Lelliott CJ, et al. Regulation of adiponectin expression in human adipocytes: effects of adiposity, glucocorticoids, and tumor necrosis factor alpha. Obes Res 2005;13:662-9. 48. Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A & RichelsenB 2003 Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. American Journal of Physiology.Endocrinology and Metabolism 285 E527–E533. 49. Kanehiro A, Lahn M, Makela MJ, Dakhama A, Joetham A, Rha YH, et al. Requirement for the p75 TNF-alpha receptor 2 in the regulation of airway hyperresponsiveness by gamma delta T cells. J Immunol 2002;169:4190-7. 50. Iwasaki M, Saito K, Takemura M, Sekikawa K, Fujii H, Yamada Y, Wada H, Mizuta K, Seishima M, Ito Y. TNF-α contributes to the development of allergic rhinitis in mice J Allergy Clin Immunol 2003;112:134-40. 51. Nakae S, Komiyama Y, Yokoyama H, Nambu A, Umeda M, Iwase M, et al. IL-1 is required for allergen-specific Th2 cell activation and the development of airway hypersensitivity response. Int Immunol 2003;15:483-90. 40.
(42) 52. Amici MD, Puggioni F, Casali L, Alesina R. Variations in serum levels of interleukin (IL)-1b, IL-2, IL-6, and tumor necrosis factor-a during specific immunotherapy. Ann Allergy Asthma Immunol 2001;86:311–313. 53. Hetland G, Granum B, Groeng EC, Lovik M. Leptin does not influence the IgE response to ovalbumin in mice. Clin Immunol.2001;101:8–11. 54 Conus S, Bruno A, Simon HU. Leptin is an eosinophil survival factor. J Allergy Clin Immunol 2005;116:1228-34 55. Hsu PY, Yang YH, Lin YT, Chiang BL. Serum eosinophil cationic protein level and disease activity in childhood rhinitis. Asian Pac J Allergy Immunol 2004;22:19-24. 56. Togias A. Systemic effects of local allergic disease. J Allergy Clin Immunol 2004;113:S8-14. 57. Borish L. Allergic rhinitis: Systemic inflammation and implications for management. J Allergy Clin Immunol 2003;112:1021-31.. 41.
(43)
數據

相關文件
- Multi-layer perceptron with linear, logistic and softmax outputs and appropriate error functions. - Radial basis function (RBF) networks with both Gaussian and non-local
Curriculum planning - conduct holistic curriculum review and planning across year levels to ensure progressive development of students’ speaking skills in content, organisation
"Extensions to the k-Means Algorithm for Clustering Large Data Sets with Categorical Values," Data Mining and Knowledge Discovery, Vol. “Density-Based Clustering in
Therefore, it is our policy that no Managers/staff shall solicit or accept gifts, money or any other form of advantages in their course of duty respectively without the
Macro Evolution of core-collapse supernovae (giant P violation) Chiral kinetic theory. Son, Yamamoto (2012); Stephanov, Yin
5.1.1 This chapter presents the views of businesses collected from the business survey, 12 including on the number of staff currently recruited or relocated or planned to recruit
Teacher / HR Data Payroll School email system Exam papers Exam Grades /.
Classifying sensitive data (personal data, mailbox, exam papers etc.) Managing file storage, backup and cloud services, IT Assets (keys) Security in IT Procurement and