The effects of omega-3 fatty acids monotherapy in Alzheimer's disease and mild
cognitive impairment: A preliminary randomized double-blind
placebo-controlled study
Chih-Chiang Chiu
a,b,c, Kuan-Pin Su
d, Tsung-Chi Cheng
e, Hsing-Cheng Liu
a, Ching-Jui Chang
f,
Michael E. Dewey
c, Robert Stewart
c, Shih-Yi Huang
g,⁎
a
Department of Psychiatry, Taipei City Psychiatric Center, Taipei City Hospital, Taipei, Taiwan
b
Department of Psychiatry, Taipei Medical University, Taiwan
cKing’s College London (Institute of Psychiatry), London, United Kingdom dDepartment of General Psychiatry, China Medical University and Hospital, Taiwan e
Department of Statistics, National Chengchi University, Taiwan
f
Department of Psychiatry, Cathay General Hospital, Taipei, Taiwan
g
School of Nutrition and Health Sciences, Taipei Medical University, Taiwan
A B S T R A C T A R T I C L E I N F O
Article history:
Received 27 December 2007 Received in revised form 29 April 2008 Accepted 17 May 2008
Available online 25 May 2008 Keywords:
Alzheimer’s disease Cognition Fish oil
Mild cognitive impairment Polyunsaturated fatty acids
A 24-week, randomized, double-blind placebo-controlled study was carried out to test the feasibility of using omega-3 polyunsaturated fatty acids (PUFAs) monotherapy in people with cognitive impairment and to explore its effects on cognitive function and general clinical condition in these participants. Twenty three participants with mild or moderate Alzheimer’s disease and twenty three with mild cognitive impairment were randomized to receive omega-3 PUFAs 1.8 g/day or placebo (olive oil). The data of 35 (76%) participants with at least one post-treatment visit was analyzed. There were no severe adverse effects in either group and it suggests that omega-3 PUFAs were well tolerable in this population. The treatment group showed better improvement on the Clinician’s Interview-Based Impression of Change Scale (CIBIC-plus) than those in the placebo group over the 24 week follow-up (p = 0.008). There was no significant difference in the cognitive portion of the Alzheimer’s Disease Assessment Scale (ADAS-cog) change during follow-up in these two groups. However, the omega-3 fatty acids group showed significant improvement in ADAS-cog compared to the placebo group in participants with mild cognitive impairment (p = 0.03), which was not observed in those with Alzheimer’s disease. Higher proportions of eicosapentaenoic acid on RBC membranes were also associated with better cognitive outcome (p = 0.003). Further studies should be considered with a larger-sample size, diet registration, higher dosages, comparisons between different combinations of PUFAs, and greater homogeneity of participants, especially those with mild Alzheimer’s disease and mild cognitive impairment.
© 2008 Published by Elsevier Inc.
1. Introduction
Alzheimer’s disease (AD) is the most common age-related neurodegenerative disease and has become an urgent public health issue in most areas of the world. Mild cognitive impairment (MCI) has
been suggested to represent a prodromal stage of AD with up to 80% of patients receiving an AD diagnosis within 6 years (Cherrier et al., 2005). Recent evidence suggests that omega-3 polyunsaturated fatty acids (PUFAs) may play an important role in cognitive function in patients with AD (Mazza et al., 2007).
PUFAs are derived solely from dietary sources and mainly include n−6 (omega-6) fatty acids such as arachidonic acid (AA, C20:4n−3), synthesized in the body using linoleic acid (LA, 18:2n−6) as a precursor, and n−3 (omega-3) fatty acids such as eicosapentaenoic acid (EPA, C20:5n−3) and docosahexaenoic acid (DHA, C22:6n−3), synthesized from alpha linolenic acid (ALA, 18:3n−3) (Yehuda et al., 2002). However, recent evidences showed that only 2 to 10% of ALA was converted to DHA or EPA, which suggested that EPA and DHA are likely to be dietary (Ross et al., 2007). Marine fish are the principal sources of DHA and EPA (Shahidi and Miraliakbari, 2004).
Abbreviations: AA, arachidonic acid; AD, Alzheimer’s disease; ADAS-cog, the cognitive portion of the Alzheimer’s Disease Assessment Scale; ALA, linolenic acid; CIBIC-plus, Clinician’s Interview-Based Impression of Change Scale; DHA, docosahex-aenoic acid; EPA, eicosapentdocosahex-aenoic acid; HDRS, Hamilton Depression Scale; LA, linoleic acid; MCI, Mild cognitive impairment; MMSE, Mini Mental Status Examination; PUFAs, polyunsaturated fatty acids.
⁎ Corresponding author. School of Nutrition and Health Sciences, Taipei Medical University, 250 Wu-Xin Street, Taipei 110, Taiwan. Tel.: +886 2 27361661~6550; fax: +886 2 27373112.
E-mail address:sihuang@tmu.edu.tw(S.-Y. Huang).
0278-5846/$– see front matter © 2008 Published by Elsevier Inc. doi:10.1016/j.pnpbp.2008.05.015
Contents lists available atScienceDirect
Progress in Neuro-Psychopharmacology &
Biological Psychiatry
Higher total intake of n−3 PUFAs, particularly DHA, has been found to be associated with reduced risk of AD (Morris et al., 2003). Lower levels of EPA, DHA, and total n−3 fatty acids in the plasma phosphatidylethanolamine fraction have been found in patients with AD and MCI (Conquer et al., 2000). In prospective studies, higher plasma DHA level associated with reduction in the risk of dementia and predicted less decline of sensorimotor speed and complex speed (Schaefer et al., 2006; Dullemeijer et al., 2007). Limited evidences seem to suggest a possible association between n−3 PUFAs and reduced risk of dementia (Issa et al., 2006).
In animal studies, DHA has been found to increase hippocampal acetylcholine levels and its derivative, neuroprotectin D1, may decrease apoptosis (Aid et al., 2005; Lukiw et al., 2005). EPA or DHA administration has been found to improve learning ability in experi-mental designs (Hashimoto et al., 2005a,b; Song and Horrobin 2004).
In open-label studies, improvement of Mini Mental Status Examination (MMSE) scores or life quality were found after supplementation of EPA or combination of n−3 and n−6 fatty acids in patients with AD (Otsuka 2000; Yehuda et al., 1996), however, another study found no clinical benefit of ethyl-EPA on cognition (Boston et al., 2004). A pilot study suggested combination of AA and DHA may improve the cognitive dysfunction in organic brain damages or MCI rather than AD (Kotani et al., 2006). Recently, one larger-sample size randomized double-blind placebo-controlled study found 1.6 g DHA and 0.7 EPA added to cholinesterase inhibitors did not delay cognitive decline in mild to moderate AD, but showed positive effects in patients with MMSEN27 (Freund-Levi et al., 2006). This supple-mentation did not result in marked effects on neuropsychiatric symptoms except for possible positive effects on depressive symp-toms and agitation sympsymp-toms in subgroups (Freund-Levi et al., 2008). Given these inconsistent findings, we carried out a preliminary double-blind placebo-controlled study to investigate the feasibility of using omega-3 PUFAs (EPA combined with DHA) monotherapy in patients with AD or MCI and to explore its effects on cognitive function and general clinical condition in these participants. Associations between changes in cognitive function and erythrocyte membrane composition of total n−3 PUFAs, EPA or DHA were also assessed. 2. Experimental procedures
2.1. Study subjects
This study had been approved by the institutional review board of Taipei City Hospital and registered at the Clinicaltrials.gov website (NCT00628017). Written informed consent was obtained from all participants and from their legal representatives before enrollment. Potential participants were recruited through newspaper advertise-ments on the basis of complaints of memory difficulties with respect to themselves or a cared-for other. All of them received a diagnostic interview, MMSE, and Logical Memory delayed-recall from the Wechsler Memory Scale III.
Participants were eligible if they fulfilled the diagnosis of AD according to the American Psychiatric Association, DSM-IV criteria
(American Psychiatric Association, 1994), with mild or moderate
severity, or amnesic MCI. Mild to moderate AD was defined by an MMSE score between 10 and 26, and a Clinical Dementia Rating (CDR) score of 1 or 2. Amnesic MCI was operationalised as: (Petersen
et al., 1999) (1). Subjective memory impairment by the patient and/
or an informant, (2) objective memory impairment falling at least 1.5 standard deviations or more below age- and education-specific norms (Logical Memory delayed-recall score from the Wechsler Memory Scale III) (Wecherler, 1997), (3) relatively normal perfor-mance in other cognitive domains, (4) no impairment in activities of daily living, and (5) failure to meet DSM-IV criteria for dementia. This definition is developed by the researchers at Mayo Clinic and is the most popular and widely used clinical criteria until now (Jelic et al.,
2006). The inclusion age range for participants was between 55 and 90 years old.
2.2. Enrolled procedure
Potential participants received an assessment by a psychiatrist or neurologist. Thorough medical, psychiatric and neurological assess-ments were performed. CT brain imaging was carried out to exclude vascular dementia. For participants without imaging data, the Hachinski’s Ischemic Scale was used (Hachinski et al., 1975). Blood tests including folic acid, vitamin B12, thyroid function, and routine biochemistry were also carried out to exclude primary causes of dementia or cognitive impairment.
Exclusion criteria were as follows: inadequate motor or sensory capacity to comply with testing; any ischemic lesion on brain CT reported by the radiologist; a modified Hachinski Ischemic Scale scoreN4; a 17-item Hamilton Depression Scale (HDRS) (Hamilton, 1960) scoreN13; abnormal levels of folic acid, vitamin B12, or thyroid function; severe cormobidity, including another neurodegerative diseases, another chronic debilitating neurological illness (e.g. cerebral palsy), brain trauma, tumors, severe pulmonary, renal, liver disease, cardiac disease, or autoimmune disease, or conditions expected to cause death within 1 year. Participants with a diagnosis of alcoholism, schizophrenia, and bipolar disorder were excluded. Participants receiving cholinesterase agents during the screen or taking NSAID on a long-term basis were also excluded. Concomitant medications such as anticholinergics, anticonvulsants, and antipsy-chotics were not administered during the course of study. Agents with potential neuroprotective effects, such as Vitamin E,fish oil supple-mentation, and Ginkgo biloba, were discontinued during the trial period if they were used before the study.
2.3. Randomization and intervention
A 24-week, randomized, double-blind study was carried out to compare the effects of omega-3 PUFAs against placebo. Eligible participants were randomly allocated to one of two groups. The randomization process was carried out by another member of staff independent of the study and blind to the assessment. Group 1 received omega-3 PUFAs as 3 capsules twice daily (total daily omega-3 fatty acid dosage of 1080 mg of EPA and 720 mg of DHA). Group 2 received three identical placebo capsules twice daily which contained olive oil esters. Identical gelatin capsules were used. Both treatment and placebo capsules were vacuum deodorized and supplemented with tertiary-butyl hydroquinone, 0.2 mg/g, and tocopherols, 2 mg/g, as antioxidants. The source of the omega-3 fatty acids was menhaden fish body oil concentrate.
2.4. Clinical assessments
Participants were assessed and the capsules replenished every 6 weeks after starting the trial. The primary outcome measures were the cognitive portion of the Alzheimer’s Disease Assessment Scale (ADAS-cog) (Rosen et al., 1984), and the Clinician’s Interview-Based Impression of Change scale which included caregiver-supplied information (CIBIC-plus) (Schneider et al., 1997). These two measure-ments were assessed at baseline and at weeks 6, 12, 18, and 24.
The ADAS-cog is a sensitive and reliable psychometric scale (Rosen
et al., 1984). It consists of 11 items, and scores range from 0 (no
impairment) to 70 (very severe impairment). To reduce the potential for practice effects at subsequent visits, different word lists were used. The CIBIC-plus uses information obtained during an independent clinical interview to assess disease severity and progression of illness. The change from baseline at subsequent visits is scored by the same interviewer using a 7-point Likert-type scale, in which 1 represents markedly improved; 4, no change; and 7, markedly worse. Secondary
outcome measurements were MMSE score and HDRS. They were measured at baseline and at week 24. The participants or their caretakers were asked to keep records on the number of the capsules taken for monitoring the adherence.
2.5. Laboratory assay
Erythrocyte membrane fatty acid compositions were measured at baseline, weeks 12, 18 and 24 and plasma fatty acids were assessed at baseline and week 24. Fasting blood samples were collected and analyzed for individual fatty acids with gas chromatography of methyl esters. Individual fatty acids were identified by comparison of gas chromatographically (Lipid Standards, FAMEs, Sigma Co., St. Louis, MO, USA). Detailed procedures have been described elsewhere (Chiu
et al., 2003). Laboratory measures were conducted on coded samples
by workers blinded to other data, including intervention group. 2.6. Statistical analyses
Participants who completed at least one outcome assessment were included in an intent-to-treat analysis. Considering repeated measure-ments in this design, the scores of ADAS-cog and CIBIC-plus at weeks 6, 12, 18, and 24 were modeled by use of linear regression with a random effect for participants (Everitt, 1998). These models included the baseline measure (to control for pretreatment differences), treatment group, time (since the week 6 assessment), and a group-by-time
interaction term. In addition, age, gender, years of education, and proportions of EPA, DHA, and total n−3 PUFAs were included in models. For secondary analyses, MMSE and HDRS scores at week 24 were treated as dependent variables, and group, age, gender, education year, and baseline scores as independent variables in linear regression models. For exploring the possible different effects of omega-3 fatty acids on group with AD or MCI, the CIBIC-plus was further divided to binary category, improvement (scores of CIBIC-plusb4) or no improvement (scores of CIBIC-plusNor =4) at endpoint (Thavichachart et al., 2006). Chi-square tests were used for the difference of omega-3 fatty acids and placebo in AD and MCI groups separately. The end outcome of ADAS-cog was treated as dependent variable and age, gender, educational year, baseline ADAS-cog and group as indepen-dent variables in a linear regression model for grouped AD and MCI. Changes in fatty acid levels were analyzed by linear regression: individual fatty acid levels at the 24th week in the two groups were compared with its baseline proportion as independent variables. All analyses were carried out using STATA software version 9.
3. Results
3.1. Overall sample description
111 potential participants attended the diagnostic screen, 49 or whom were potentially eligible for inclusion. Three withdrew informed consent, leaving 46 participants: 23 with a diagnosis of mild or moderate AD and 23 with mild cognitive impairment. The combined samples were randomized into the two intervention groups. In the end, there were 10 participants with AD and 14 with MCI assigned to omega-3 fatty acid group, and 13 participants with AD and 9 with MCI in placebo group. Among them, there is only one participant who had taken anti-AD medication and discontinued it for more than 6 months before initiation of the study. Thirtyfive completed at least one post-treatment assessment were included in the intention-to-treat analysis. Of these, 29 completed the full 24 week randomization period (Fig. 1). There were no significant differences between the two study groups in demographic data, proportion with a dementia diagnosis, and individual fatty acids (Table 1).
3.2. Adherence and safety assessment
Although loss to follow-up was higher in the placebo (31%) compared to the treatment group (23%), there was no significant difference (p = 0.44). The reasons for discontinuation were various. One participant in omega-3 fatty acids group and one in the placebo group discontinued the trial due to diarrhea. Another participant in omega-3 fatty acid group stopped the trial due to severe pruritus and Table 1
Baseline characteristics of the participants included in analyses Omega-3 group (n = 20)
Placebo group (n = 15)
Age (mean, 95% CI) 74.0 (70.1–77.8) 76.5 (71.8–81.1)
Gender (%female) 65.0 46.7
Years formal education (mean, 95% CI) 10.9 (8.6–13.2) 8.5 (5.2–11.7) Baseline MMSE (mean, 95% CI) 25.8 (24.0–27.6) 23.8 (20.8–26.8) Baseline ADAS-cog (mean, 95% CI) 9.1 (6.0–12.3) 9.9 (4.9–15.0) Baseline HDRS (mean, 95% CI) 2.8 (1.2–4.3) 3.7 (1.8–5.7)
Diagnosis (% AD / MCI) 40 / 60 60 / 40
Proportion of fatty acids
LA(C18:2n6) 6.62 (6.32–6.91) 6.92(6.51–7.33) AA(C20:4n6) 1.93 (1.65–2.20) 2.20 (1.82–2.57) ALA(C18:3n3) 5.48 (4.85–6.12) 5.80 (5.12–6.48) EPA(C20:5n3) 1.00 (0.82–1.18) 1.13 (0.85–1.42) DHA(C22:6n3) 3.18 (2.76–3.60) 4.01 (3.16–4.87) Total n3 6.11 (5.55–6.67) 7.34 (6.23–8.47) Total n6 16.00 (15.29–16.63) 16.64 (15.43–17.86)
The levels of fatty acids are presented as the proportion for the individual fatty acids. Fig. 1. Schematic view of participantflow.
the other in placebo group discontinued due to exacerbation of chronic bronchitis. The most common reason for loss to follow-up due to other reasons was that the caregiver was not able to accompany the person for the regular assessments. There were no significant group differences in baseline characteristics between those who did or did not complete the study (data not shown). The adherence of the participants who were included in the analyses was 92.4% infish oil group and 81.8% in placebo group, according to their record.
Adverse effects during the 24 week trial period are summarized in
Table 2. The most common side effects were gastrointestinal
problems, including soft stool or diarrhea, nausea, constipation or other gastrointestinal discomfort. Most of these were tolerated and self-limiting. However, one participant in the omega-3 group and one in the placebo group dropped out due to complaints of intolerable diarrhea.
3.3. Results of primary outcome
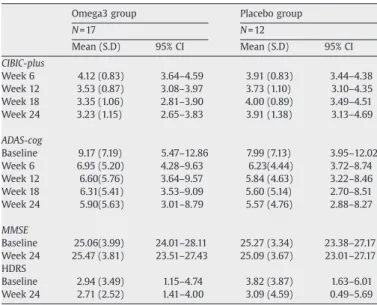
The unadjusted mean scores of our primary and secondary measurements of completers at baseline and follow-up are shown in
Table 3. The effective size for the CIBIC-plus is 0.54 (−0.22 to 1.29) and the ADAS-cog is 0.23(−0.5 to 0.98).
There were only age and group-by-time interaction showed significant findings in the mixed model for CIBIC-plus. There was a relative improvement in the CIBIC-plus score in omega-3 fatty acid group of−0.35 (−0.61 to −0.09, p=0.008) compared to placebo group every 6 weeks and increased CIBIC-plus score of 0.03 while age increased one year-old. We carried out a further secondary analysis on CIBIC-plus to explore which components may contribute to the group difference. The raw scores of the cognitive and behavior components from the patient interviews, and functional and behavior components from the caregiver interviews (CIBIC-plus) were treated as dependent variables individually in linear regression models with random effects. The group-by-time interaction in the cognitive (p = 0.06) and behavior components (p = 0.04) were stronger than in the function component (p = 0.1).
With regard to the changes in ADAS-cog, the effect of group-by-time was not significant in the mixed model. On the other hand, a higher proportion of EPA on the RBC membrane was associated with lower (better) ADAS-cog scores (−1.26 for each percentile increase, 95% CI−2.08 to −0.44, p=0.003). Proportions of total n−3 fatty acids and DHA at baseline were not significantly associated with the outcome (pN0.1). Age and baseline ADAS-cog scores were also showed associations with the ADAS-cog scores. Besides, the results are the same when only completers were included in the analyses.
3.4. Secondary analyses
In secondary analyses, neither the MMSE (p = 0.87) nor the HDRS (p = 0.76) were significantly different between the two groups at 24 weeks after adjusting for baseline scores. When the proportion of individual fatty acids between the two intervention groups were compared and adjusted for baseline levels, there was a significantly
greater increase in total n3 PUFAs for the omega-3 compared to the placebo group [mean (SD) values from 6.0 (1.2) to 7.2 (1.5) versus 6.8 (1.9) to 6.2 (1.2); p = 0.05] and a trend toward a greater increase in DHA levels for the omega-3 compared to the placebo group [ from 3.2 (0.9) to 3.6 (1.0) versus 3.7 (1.4) to 3.1 (0.7); p = 0.1]. No significant difference was found for the proportion of any other PUFAs between these two groups (Fig. 2). With regard to the proportion of plasma fatty acids, the omega-3 fatty acids group showed significant higher proportion of DHA [from 3.7 (1.0) to 5.1 (1.1) versus 3.8 (0.8) to 4.0 (1.4); p = 0.03] and total n3 PUFAs [from 6.0 (0.9) to 8.0 (1.3) versus 6.3 (1.1) to 6.8 (1.5); p = 0.03] compared to those of placebo groups, but no difference in the proportion of EPA. In addition, there was a trend toward lower proportion of plasma AA in omega-3 group compared to placebo group [from 12.8 (1.1) to 13.6 (1.4) versus 12.7 (1.1) to 14.4 (0.9); p = 0.1].
We further explored whether the omega-3 fatty acids had different effects in those with AD and MCI on the changes of CIBIC-plus and ADAS-cog. There was no significant difference in the proportions of participants with improved CIBIC-plus in omega-3 fatty acid and placebo group in those with AD (63% versus 33%) and those with MCI (58% versus 50%). Although the change of ADAS-cog was similar for the participants with AD between the treatment and placebo groups (mean (SD)−2.46 (2.63) versus −3.73 (4.29)), those with MCI showed better improvement in the omega-3 group than the placebo group (−3.23 (3.82) versus −0.37 (1.4), p=0.03) after adjusting for age, gender, and educational year.
4. Discussion
The preliminary results suggest that omega-3 PUFAs monotherpy were well tolerable for most of the participants with AD or MCI. This supplementation may improve global clinical function, as measured by the CIBIC-plus, relative to placebo (olive oil). No associations were found between randomization group and ADAS-cog, MMSE or HDRS scores. Levels of EPA on erythrocyte membrane, were associated with cognitive function, measured by ADAS-cog, in these patients.
Treatments with omega-3 fatty acids and olive oil did not show any unexpected severe adverse effects. Side effects, when they occurred, were generally tolerated and self-limiting. The most frequent adverse effect was diarrhea, although some complained of constipation. However, it should be borne in mind that two participants in this relatively small study discontinued their participation due to intoler-able diarrhea. When compared with a larger dosing-exploratory depression study by ethyl-EPA (1–4 g/day) in younger patients (Peet
Table 3
Changes in primary and secondary outcomes (completers analyses; unadjusted)
Omega3 group Placebo group
N = 17 N = 12 Mean (S.D) 95% CI Mean (S.D) 95% CI CIBIC-plus Week 6 4.12 (0.83) 3.64–4.59 3.91 (0.83) 3.44–4.38 Week 12 3.53 (0.87) 3.08–3.97 3.73 (1.10) 3.10–4.35 Week 18 3.35 (1.06) 2.81–3.90 4.00 (0.89) 3.49–4.51 Week 24 3.23 (1.15) 2.65–3.83 3.91 (1.38) 3.13–4.69 ADAS-cog Baseline 9.17 (7.19) 5.47–12.86 7.99 (7.13) 3.95–12.02 Week 6 6.95 (5.20) 4.28–9.63 6.23(4.44) 3.72–8.74 Week 12 6.60(5.76) 3.64–9.57 5.84 (4.63) 3.22–8.46 Week 18 6.31(5.41) 3.53–9.09 5.60 (5.14) 2.70–8.51 Week 24 5.90(5.63) 3.01–8.79 5.57 (4.76) 2.88–8.27 MMSE Baseline 25.06(3.99) 24.01–28.11 25.27 (3.34) 23.38–27.17 Week 24 25.47 (3.81) 23.51–27.43 25.09 (3.67) 23.01–27.17 HDRS Baseline 2.94 (3.49) 1.15–4.74 3.82 (3.87) 1.63–6.01 Week 24 2.71 (2.52) 1.41–4.00 3.09 (4.59) 0.49–5.69 Table 2 Adverse events
Omega-3 group (n = 24) Placebo group (n = 22)
Soft stool or diarrhea 8(33.3%) 6(27.7%)
Fish odour 3(12.5%) 1(4.5%)
Nausea 2(8.3%) 2(9.0%)
Constipation 2(8.3%) 1(4.5%)
Pruritis 1(4.1%) 0
Loss of body weight 1(4.1%) 0
Increased sleep 1(4.1%) 0
Increased body weight 0 1(4.5%)
General weakness 0 1(4.5%)
and Horrobin, 2002), the proportion of total adverse effects (61.5% versus 50% respectively) and adverse effects related to the gastro-intestinal system (38.5% versus 37.5% respectively) were similar. Combined ourfindings and the results of previous larger-sample size add-on study (Freund-Levi et al., 2006) , it seems feasible to conduct the studies offish oil supplementation in old people with cognitive deficit.
In the exploration analysis of CIBIC-plus, it appeared that the group difference of CIBIC-plus might be accounted for by the cognitive and behavioral components rather than the functional component. It has been suggested that omega-3 fatty acids may have beneficial effects on mood, although we feel that this is an unlikely explanation for our findings because of the stringent exclusion of people with significant depression and the lack of association with HDRS score (Peet and Horrobin 2002; Su et al., 2003). The relative improvement of general clinical condition might have resulted from improvement in other areas of health, such as cardiovascular or immunological systems on which beneficial effects of omega-3 PUFAs have been reported (Ruxton et al., 2004).
The negative results of cognitive assessments, ADAS-cog and MMSE, in the omega-3 fatty acids groups compared to placebo support the previous add-on study byFreund-Levi et al. (2006), and both of the studies showed there might be a positive effect of omega-3 fatty acids in subgroups with mild cognitive deficits. However, there may be some possible explanations of the negative results in ADAS-cog changes between the two groups. Clearly statistical power is an issue since the sample size was small. Considering small effective size (0.23) for the scores of ADAS-cog in this study, further study with the similar designs should enroll 210 participants given a 2-tailed
significance level of 0.05 and a power of 0.80 to detect the difference. The wide range of baseline cognitive function in our participants may have also reduced the likelihood of detecting a group difference. In our secondary analysis, participants with MCI showed more improvement of ADAS-cog than those with AD associated with omega-3 adminis-tration, which support recent two reports that people with very mild dementia (MMSEN27) or MCI are more beneficial in cognition than AD with PUFAs supplementation (Kotani et al., 2006; Freund-Levi et al., 2006). Besides, ADAS-cog scores were observed to improve to comparable extents in omega-3 (−2.9±3.4) and placebo group (−2.4 ± 3.8). Monounsaturated fatty acids (MUFAs), the principal constituent of olive oil, also have been reported with an inverse relationship with cognitive decline (Solfrizzi et al., 1999), raising the possibility that the ‘placebo’ may also have an active effect on the outcome. It is also possible that learning effects may relate to the observed improvement of ADAS-cog scores since both group showed improvement although the time interval, 6 weeks, for cognitive assessment in our study is similar to that of other studies (Rogers et al., 1998).
The PUFA components offish oil for the intervention also need consideration. In our study, a total daily omega-3 fatty acid dosage of 1080 mg of EPA and 720 mg of DHA was supplemented and higher erythrocyte membrane EPA proportions were independently asso-ciated with better cognitive outcomes in the random effect model. Recent dietary research (Morris et al., 2003) and animal studies have suggested associations between DHA levels and cognitive function (Lukiw et al., 2005; Hashimoto et al., 2005a;b). In addition, AA and DHA are selectively concentrated in gray matter and together account for approximately 20% of the synaptosomal membrane fatty acid rather than EPA (Fenton et al., 2000). However, ethyl-EPA was also Fig. 2. Changes of proportion of fatty acids in the completers of the two groups.
found to attenuate memory impairment induced by central IL-1β administration in rats (Song and Horrobin, 2004), and EPA may be superior to DHA as an augmentation in the treatment of schizophrenia and EPA alone or combined EPA and DHA rather than DHA monotherapy showed positive therapeutic effects in depression (Peet and Horrobin, 2002; Peet et al., 2001; Marangell et al., 2003). Nevertheless, the effects of EPA monotherapy on cognition in patients with AD have been inconsistent in previous open trials (Otsuka, 2000; Boston et al., 2004). Although increased total n3 PUFAs and DHA levels on RBC membrane and plasma levels were found in our study, EPA level, which might be important in cognitive function was not increased. In this study, changes on proportion of fatty acids in plasma are more sensitive to those on RBC membrane after omega-3 fatty acids supplementation. Plasma fatty acids seem to be a better indicator for assessment of compliance. In addition, the fatty acids changes (1.5 fold increase after 1.8 g/dayfish oil) in this study were lower than those in another study (2.4–3.6 fold increase after 2.4 g/ dayfish oil) (Freund-Levi et al., 2006). Compared to western countries, Taiwan is a highfish-consuming country (Hibbeln, 1998). Whether the dosage of n3 PUFAs needs higher to reach more prominent changes in fatty acids compositions and clinical effects in highfish-consuming countries and comparison between different combinations of EPA and DHA (such as pure EPA, pure DHA and placebo) should be considered. There are some limitations which should be considered in this study. First, as mentioned above, the sample size was small, due in part to relatively stringent exclusion and inclusion criteria. More practical criteria for enrollment should be considered in further research. Second, the relatively high drop out rate during the treatment period is also an important issue. Although the drop out rate was higher in the placebo group, it did not reach statistically significance and there were no substantial differences in the demographic and baseline clinical data between completers and non-completers. Since the most common reason for loss to follow-up is due to the non-compliance of caregivers rather than adverse effects in participants, regular phone call before visits may be considered in further studies. Third, the heterogeneity of our participants may have reduced the power of this study to detect group differences. Forth, the randomization was not stratified, which may make an unbalanced distribution of participants with AD and MCI in the two groups, especially in small sample size study although there was no significant difference in the distribution of patients with AD or MCI in the two groups at the post hoc comparison. Last, possible modifying factors were not measured such as frequency offish intake and apolipopro-tein E (APOE) genotype (Huang et al., 2005). The negative results of omega-3 fatty acids on cognitive assessments should be further proved by the studies with enough power and different dosage of omega-3 PUFAs.
In summary, our preliminary study suggests that conducting a study with omega-3 PUFAs monotherapy in patients with AD or MCI is feasible. Omega-3 fatty acids may improve general clinical function in patients with mild or moderate AD and MCI, but not their cognitive function. The cognitive effects of omega-3 fatty acids might be favored in patients with MCI rather than those with AD. Further studies should be considered with a larger-sample size, diet registration, higher dosage, comparison between different combinations of EPA and DHA (such as pure EPA, pure DHA and placebo), a more inert placebo, and greater homogeneity of participants, especially those with mild AD or MCI. Additional measurements focusing on explaining the changes of general function and a longer follow-up are also warranted.
Acknowledgement
This work is supported by the grants DOH 92, 93-TD-1095 from Department of Health, NSC 95-2314-B-532-010-MY3 and NSC 94-2320-B-038-008 from the National Science Council and Taipei City Hospital in Taiwan.
References
Aid S, Vancassel S, Linard A, Lavialle M, Guesnet P. Dietary docosahexaenoic acid [22: 6 (n−3)] as a phospholipid or a triglyceride enhances the potassium chloride-evoked release of acetylcholine in rat hippocampus. J Nutr 2005;135:1008–13.
American Psychiatric Association. Diagnostical and statistical manual of mental disorders. 4th edition. Washington, DC: APA; 1994.
Boston PF, Bennett A, Horrobin DF, Bennett CN. Ethyl-EPA in Alzheimer's disease—a pilot study. Prostaglandins Leukot Essent Fat Acids 2004;71:341–6.
Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, et al. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology 2005;64:2063–8.
Chiu CC, Huang SY, Su KP, Lu ML, Huang MC, Chen CC, et al. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol 2003;13:99–103.
Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer's disease, other types of dementia, and cognitive impairment. Lipids 2000;35:1305–12.
Dullemeijer C, Durga J, Brouwer IA, van de RO, Kok FJ, Brummer RJ, et al. n 3 fatty acid proportions in plasma and cognitive performance in older adults. Am J Clin Nutr 2007;86:1479–85.
Everitt BS. Analysis of longitudinal data. Beyond MANOVA. Br J Psychiatry 1998;172:7–10.
Fenton WS, Hibbeln J, Knable M. Essential fatty acids, lipid membrane abnormalities, and the diagnosis and treatment of schizophrenia. Biol Psychiatry 2000;47:8–21. Freund-Levi Y, Basun H, Cederholm T, Faxen-Irving G, Garlind A, Grut M, et al. Omega-3
supplementation in mild to moderate Alzheimer's disease: effects on neuropsy-chiatric symptoms. Int J Geriatr Psychiatry 2008;23(2):161–9.
Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol 2006;63:1402–8.
Hachinski VC, Iliff LD, Zilhka E, Du Boulay GH, McAllister VL, Marshall J, et al. Cerebral bloodflow in dementia. Arch Neurol 1975;32:632–7.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. Hashimoto M, Hossain S, Agdul H, Shido O. Docosahexaenoic acid-induced amelioration on impairment of memory learning in amyloid beta-infused rats relates to the decreases of amyloid beta and cholesterol levels in detergent-insoluble membrane fractions. Biochim Biophys Acta 2005a;1738:91–8.
Hashimoto M, Tanabe Y, Fujii Y, Kikuta T, Shibata H, Shido O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J Nutr 2005b;135:549–55.
Hibbeln JR. Fish consumption and major depression. Lancet 1998;351:1213. Huang TL, Zandi PP, Tucker KL, Fitzpatrick AL, Kuller LH, Fried LP, et al. Benefits of fatty
fish on dementia risk are stronger for those without APOE epsilon4. Neurology 2005;65:1409–14.
Issa AM, Mojica WA, Morton SC, Traina S, Newberry SJ, Hilton LG, et al. The efficacy of omega-3 fatty acids on cognitive function in aging and dementia: a systematic review. Dement Geriatr Cogn Disord 2006;21:88–96.
Jelic V, Kivipelto M, Winblad B. Clinical trials in mild cognitive impairment: lessons for the future. J Neurol Neurosurg Psychiatry 2006;77:429–38.
Kotani S, Sakaguchi E, Warashina S, Matsukawa N, Ishikura Y, Kiso Y, et al. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res 2006;56:159–64.
Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest 2005;115:2774–83.
Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry 2003;160:996–8.
Mazza M, Pomponi M, Janiri L, Bria P, Mazza S. Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: an overview. Prog Neuropsychopharmacol Biol Psychiatry 2007;31:12–26.
Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, et al. Consumption offish and n−3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 2003;60:940–6.
Otsuka M. [Analysis of dietary factors in Alzheimer's disease: clinical use of nutritional intervention for prevention and treatment of dementia]. Nippon Ronen Igakkai Zasshi 2000;37:970–3.
Peet M, Brind J, Ramchand CN, Shah S, Vankar GK. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr Res 2001;49:243–51.
Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry 2002;59:913–9.
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–8. Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind,
placebo-controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group Neurology 1998;50:136–45.
Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–64.
Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis 2007;6:21.
Ruxton CH, Reed SC, Simpson MJ, Millington KJ. The health benefits of omega-3 poly-unsaturated fatty acids: a review of the evidence. J Hum Nutr Diet 2004;17:449–59.
Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol 2006;63:1545–50. Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. Validity and
reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11(Suppl 2):S22–32.
Shahidi F, Miraliakbari H. Omega-3 (n−3) fatty acids in health and disease: Part 1— cardiovascular disease and cancer. J Med Food 2004;7:387–401.
Solfrizzi V, Panza F, Torres F, Mastroianni F, Del PA, Venezia A, et al. High monounsaturated fatty acids intake protects against age-related cognitive decline. Neurology 1999;52:1563–9.
Song C, Horrobin D. Omega-3 fatty acid ethyl-eicosapentaenoate, but not soybean oil, attenuates memory impairment induced by central IL-1beta administration. J Lipid Res 2004;45:1112–21.
Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 2003;13:267–71.
Thavichachart N, Phanthumchinda K, Chankrachang S, Praditsuwan R, Nidhinandana S, Senanarong V, et al. Efficacy study of galantamine in possible Alzheimer's disease with or without cerebrovascular disease and vascular dementia in Thai patients: a slow-titration regimen. Int J Clin Pract 2006;60:533–40.
Wecherler D. WAIS Memory Scale-III: The Psychological Cooperation; 1997. Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI. The role of polyunsaturated fatty acids
in restoring the aging neuronal membrane. Neurobiol Aging 2002;23:843–53. Yehuda S, Rabinovtz S, Carasso RL, Mostofsky DI. Essential fatty acids preparation (SR-3)