Intermetallic Reactions in Reflowed and Aged Sn-9Zn Solder
Ball Grid Array Packages with Au/Ni/Cu and Ag/Cu Pads
HSIU-JEN LIN1 and TUNG-HAN CHUANG1,2
1.—Institute of Materials Science and Engineering, National Taiwan University, Taipei 106, Tai-wan. 2.—E-mail: tunghan@ccms.ntu.edu.tw
During the reflowing of Sn-9Zn solder ball grid array (BGA) packages with Au/Ni/Cu and Ag/Cu pads, the surface-finished Au and Ag film dissolved rap-idly and reacted with the Sn-9Zn solder to form a #3-AuZn4/#-Au7Zn18 inter-metallic double layer and $-AgZn6 intermetallic scallops, respectively. The growth of #3-AuZn4is prompted by further aging at 100°C through the reaction of #-Au7Zn18 with the Zn atoms dissolved from the Zn-rich precipitates em-bedded in the "-Sn matrix of Sn-9Zn solder BGA with Au/Ni/Cu pads. No intermetallic compounds can be observed at the solder/pad interface of the Sn-9Zn BGA specimens aged at 100°C. However, after aging at 150°C, a Ni4Zn21 intermetallic layer is formed at the interface between Sn-9Zn solder and Ni/Cu pads. Aging the immersion Ag packages at 100°C and 150°C caused a #-Cu5Zn8intermetallic layer to appear between $-AgZn6intermetallics and the Cu pad. The scallop-shaped $-AgZn6intermetallics were found to detach from the #-Cu5Zn8 layer and float into the solder ball. Accompanied with the intermetallic reactions during the aging process of reflowed Sn-9Zn solder BGA packages with Au/Ni/Cu and Ag/Cu pads, their ball shear strengths de-grade from 8.6 N and 4.8 N to about 7.2 N and 2.9 N, respectively.
Key words: Intermetallic compounds, Sn-9Zn, Au/Ni/Cu pads, Ag/Cu pads, ball shear strength
INTRODUCTION
Out of environmental concerns, the development of Pb-free solders has become a key issue for the electronics industry. Compared to many other prom-ising Pb-free solders, such as Sn3.5Ag, Sn0.7Cu, and Sn-Ag-Cu, eutectic Sn-9Zn has the merits of a lower melting point (199°C) and better cost performance.1 However, further research efforts are required to ad-dress its disadvantages, such as poor wettability and oxidation resistance, so that the improvements made on eutectic Sn-9Zn can facilitate the develop-ment of an actual replacedevelop-ment for Sn-Pb solders. In addition, the reliability of Sn-9Zn solder joints on various surface-finished pads for electronic packag-ing should also be evaluated.
The Au/Ni surface finish has been widely used for printed circuit boards to protect Cu pads from oxi-dation and promote the wettability of liquid solder.2 During the reflow process, the Au thin film dissolves
rapidly into the solder matrix, triggering the occur-rence of further interfacial reaction between the sol-der and the Ni substrate. In the cases of traditional Pb solders and most Pb-free solders (such as Sn-Ag, Sn-Cu, and Sn-Bi), their reactions with Ni sub-strates lead to the formation of Ni3Sn4intermetallic compounds.3–5However, a liquid Sn-Zn solder alloy reacts with Ni to form Ni19.0Zn80.0Sn1.0 intermetal-lics due to the high activity of the element Zn.6 A soldering reaction between liquid Sn-8Zn-3Bi and Ni substrate was reported to result in a continuous in-termetallic layer of Ni5Zn21 at temperatures below 325°C and a double layer of Ni5Zn21/Ni35Zn22Sn43at above 325°C.7Harris also showed that a solid/solid interfacial reaction between Sn-Zn-Bi solder and Ni substrate at 125°C for 100 days caused the forma-tion of a Ni-85.9wt.%Zn intermetallic compound.8In addition, Shiue et al. aged a series of Sn-Zn based solders joined with Au/Ni-P/Cu substrates and re-ported an intermetallic compound of Zn-rich # phase (NiZn3) at the interface.9
For the Pb-free BGA packages with Au/Ni/Cu
(Received May 2, 2005; accepted August 30, 2005) 154
pads, various failures such as Au embrittlement and black pads have been reported.2In addition, the Au/ Ni electro- or electroless-plating process is quite costly and complex. Immersion silver is an effective alternative method for the surface finishing of Cu pads. The process involving immersion Ag takes about 7 min, and the cost is close to that of processes involving traditional Sn.3Immersion Ag can provide smooth surfaces and good wettability for liquid sol-ders on Cu pads. During reflow, the Ag metallization (about 0.2 %m in thickness) also dissolves rapidly into the solder matrix, causing the intermetallic re-actions at the interfaces between the solder alloy and the Cu pads.
For the interfacial reactions of Sn-Pb solders and most Pb-free solders with Cu pads, Cu6Sn5 and Cu3Sn intermetallic compounds have also been re-ported.10 However, a Sn-Zn/Cu interfacial reaction was reported to generate Cu-Zn intermetallics on account of the high activity of the element Zn. Chan et al. studied the soldering reactions between liquid Sn-9Zn and Cu substrates and found Cu33.4Zn66.5Sn0.1 at the interfaces.11 Suganuma et al. also investi-gated the soldering reactions between liquid Sn-Zn and Cu substrates at temperatures ranging from 230°C to 280°C for 5–35 min.12 Three layers of in-terfacial intermetallic compounds (#-Cu5Zn8, ""-CuZn, and an unknown thin-layer phase) were found. The total thickness of these intermetallics in-creased with the increasing Zn content in the Sn-Zn solders. Lin and Chuang further analyzed the inter-metallic reactions between liquid Sn-8Zn-3Bi solder and Cu substrates in a wide temperature range of 225–350°C for 30–90 min.13 The results indicated that a planar layer of Cu32.1Zn66.7Sn0.7Bi0.5 (# phase) along with a great number of scallop-shaped intermetallic compounds of Cu19.3Zn77.8Sn2.9 ($ phase) appeared at the interfaces at temperatures below 325°C. At temperatures higher than 325°C, the $-intermetallic scallops disappeared, and the planar #-intermetallics became cluster shaped. For
the solid/solid interfacial reactions between various Sn-Zn solders and Cu substrates, Lee et al.14 and Yoon et al.15,16 reported a #-Cu
5Zn8 intermetallic compound after aging at 100°C, 130°C, and 160°C. In addition, Harris showed that 13-%m-thick #-Cu5Zn8 intermetallics appeared at the Sn-Zn-Bi/ Cu interface after aging at 125°C for 100 days.8This study focused on the intermetallic reactions and re-lated bonding strengths of the eutectic Sn-9Zn sol-der joints in a ball grid array (BGA) package with Au/Ni/Cu and Ag/Cu pads after the reflow and aging processes.
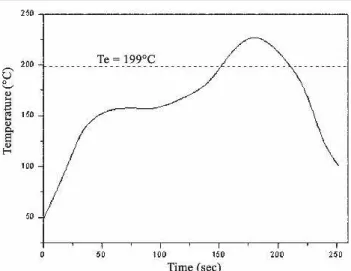
Fig. 1. Temperature profile of the reflow process for Sn-9Zn solder BGA packages in this study with Au/Ni/Cu and Ag/Cu pads.
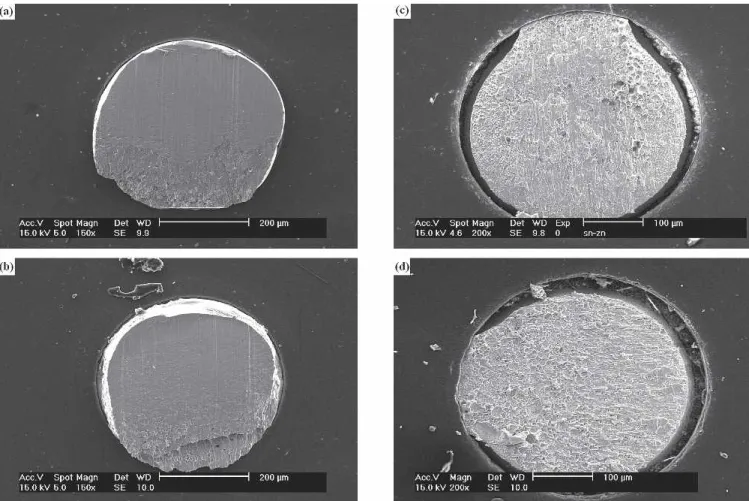
Fig. 2. Morphology of the floating intermetallic layers in Sn-9Zn sol-der BGA packages with Au/Ni/Cu pads after multiple reflows: (a) one time, (b) two times, and (c) three times.
Intermetallic Reactions in Reflowed and Aged Sn-9Zn Solder Ball Grid
EXPERIMENTAL PROCEDURE
Ball grid array packages with Au/Ni/Cu pads were electroplated with a 5-%m-thick layer of Ni and im-mersion plated with a 0.5-%m-thick layer of Au, while those with Ag/Cu pads were immersion depos-ited with a 0.2-%m-thick layer of Ag. Sn-9Zn (wt.%) solder balls of 0.4 mm in diameter were dipped in rosin mildly activated (RMA) flux, placed on the Au/ Ni and Ag surface finished Cu pads, and then re-flowed in a hot air furnace equipped with five heat-ing zones. Figure 1 shows the temperature profile adopted for the reflow process. During reflow, liquid Sn-9Zn solder reacted with Au/Ni/Cu and Ag/Cu pads to form intermetallic compounds at the inter-faces.
For further evaluation of the intermetallic reac-tions in Sn-9Zn BGA packages as electronic devices were in operation and the solder joints were being heated, certain reflowed specimens were aged at 100°C and 150°C for various time periods, and a solid/solid reaction was observed to occur at the sol-der/pad interface under this condition.
The BGA packages, after reflow and aging, were cross-sectioned through a row of Sn-9Zn solder balls. They were then cold-mounted, ground with 1500 grit SiC paper, and polished with 0.3-%m alumina pow-der. The morphology and chemical composition of intermetallic compounds were analyzed using a
scanning electron microscope equipped with energy-dispersive x-ray spectrometry (EDX). Finally, ball shear tests were conducted to measure the bonding strengths of the solder joints. For this purpose, a shear rate of 0.1 mm/s and a shear height of 80 %m (about 1/4 the reflowed ball height) were employed.
RESULTS AND DISCUSSION
The microstructure of the Sn9Zn solder BGA packages with Au/Ni/Cu pads after multiple reflows is shown in Fig. 2. The solder matrix contains many needle-shape Zn-rich precipitates embedded in the "-Sn matrix. The Zn content in the "-Sn matrix, as analyzed by EDX, is 2.2 wt.% (4 at.%). During the reflow process, the Au thin film on Au/Ni/Cu pads dissolves rapidly, and the resultant intermetallic compounds that are formed at the solder/pad inter-faces take the appearance of a planar double layer. The intermetallic layer tends to float away from the interfaces even after the first reflow (Fig. 2). In-creasing the reflow cycles causes the intermetallic compounds to float farther away. The composition (at.%) of the inner layer of the intermetallics, as ana-lyzed by EDX, is Au:Zn # 28.9:71.1, which corre-sponds to the # phase (Au7Zn18) in the Au-Zn equi-librium diagram. The outer intermetallic layer has a higher Zn content (Au:Zn # 18.7:81.3), which corre-sponds to the #3 phase (AuZn4) in the Au-Zn equi-librium diagram. The microstructure of the Sn-9Zn
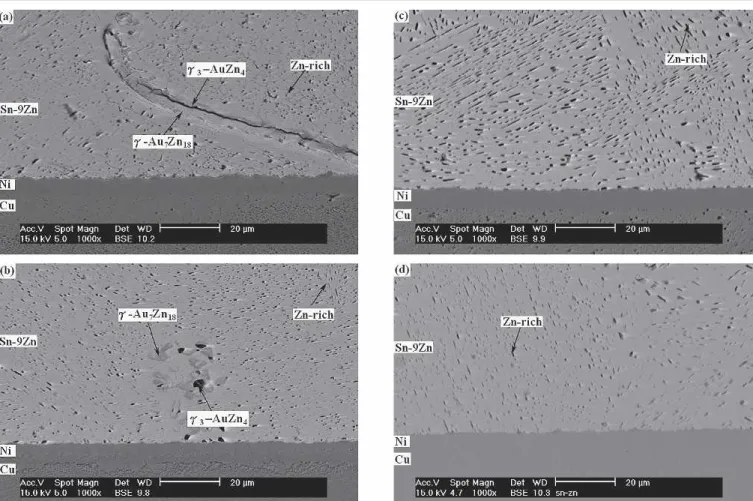
Fig. 3. Microstructure of the Sn-9Zn solder joints in BGA packages with Au/Ni/Cu pads after aging at 100°C for various times: (a) 100 h, (b) 500 h, (c) 700 h, and (d) 1000 h.
Fig. 4. Microstructure of the Sn-9Zn solder joints in BGA packages with Au/Ni/Cu pads after aging at 150°C for various times: (a) 100 h, (b) 500 h, (c) 700 h, and (d) 1000 h.
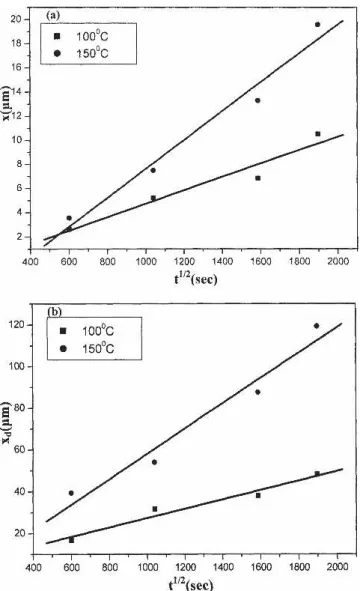
Fig. 5. Thickness (x) of the Ni4Zn21interfacial intermetallic layer and
precipitate-free zone (xd) in the solder matrix of Au/Ni surface
fin-ished Sn-9Zn BGA packages after aging at 150°C relative to the square root of time (t1/2).
Fig. 6. Morphology of (a) the as-reflowed Sn-9Zn solder ball with Ag/Cu and (b) the microstructure of the as-reflowed Sn-9Zn matrix.
solder joints remains unchanged after aging at 100°C, as evidenced by Fig. 3. However, the #3 -AuZn4intermetallic layer grows with the diminish-ing of #-Au7Zn18as the aging time increases, and the Zn content in the "-Sn matrix increases slightly to 2.3 wt.% (4.2 at.%). Following the floating away of the #3/# intermetallic layer, no further intermetallic reaction can be observed at the interface between the Sn-9Zn solder and the Ni/Cu pad even after a prolonged aging time of 1000 h.
However, aging at 150°C causes a planar interme-tallic phase to form at the solder/pad interface of Au/Ni surface finished Sn-9Zn BGA packages, as shown in Fig. 4. The EDX analysis indicates that the composition (at.%) of these interfacial intermetallics is Ni:Zn # 16.1:83.9, which corresponds to the Ni4Zn21phase; also, the Zn content in the "-Sn ma-trix is increased slightly further to 2.4 wt.% (4.3 at.%). Figure 4 reveals that the Ni4Zn21 intermetal-lic layer grows with the increase in aging time at 150°C. The thickness of the Ni4Zn21 intermetallic layer is measured and plotted against the square root of aging time (t1/2), as shown by Fig. 5a, where the linear relation of the curve indicates that the intermetallic growth is diffusion controlled. Figure 4 also indicates that the growth of Ni4Zn21 interme-tallic compounds causes the depletion of Zn-rich pre-cipitates in the solder balls near the Ni/Cu pads. The result indicates that the Ni4Zn21intermetallics are formed through the interfacial reaction of the Ni layer with the Zn atoms from the solder matrix. The consumption of Zn content in the Sn-9Zn solder caused the dissolution of Zn-rich precipitates near the solder/pad interfaces. Figure 5b shows that the thickness of precipitation-free zones increases para-bolically with the aging time, giving the implication that the reaction is diffusion controlled. The lattice diffusivity of Zn in the Sn matrix at 150°C, as cal-culated from the equation of Huang and Hunting-ton,17
D $m2"s% = 1.1 × 10−6exp
"
−50.2 kJ"molRT
#
,is 6.91 × 10−13 m2/s. The diffusion distances d = $
#
Dt% at this temperature for 100 h and 1000 h are 499 %m and 1577 %m, respectively. In comparison with Fig. 6, it is evidenced that the depletion thick-nesses of Zn-rich precipitates after aging at 150°C for durations of 100 h to 1000 h are about 15-fold smaller than the diffusion distances of Zn atoms in the Sn matrix. The results indicate that the kinetics-controlled mechanism for the dissolution of Zn-rich precipitates in the Au/Ni surface finished Sn-9Zn BGA packages is the interfacial reaction between Sn-9Zn solder and the Ni film on Cu pads, rather than the lattice diffusion of Zn atoms through the solder matrix.The microstructure of the as-reflowed Sn-9Zn sol-der ball in a BGA package with Ag/Cu pads, as shown in Fig. 6, also consists of many needlelike Zn-rich precipitates embedded in the Sn-rich
ma-trix. However, in addition to those fine precipitates, a small number of coarsened Zn-rich plates also ap-pear in the immersion Ag surface finished Sn-9Zn solder BGA packages. Figure 7 shows that the coars-ened Zn-rich plates display a much greater tendency to form near the as-reflowed solder/pad interface. Those fine precipitates are found to diminish around the coarsened Zn-rich plates. The reflow process also leads to the formation of a thin layer of scallop-shaped intermetallic compounds at the interface of the solder joints. The chemical composition (at.%) of these interfacial intermetallics, as analyzed by EDX,
Fig. 7. Morphology of the intermetallic compounds formed at the interface of Sn-9Zn solder BGA packages with Ag/Cu pads after multiple reflows: (a) one time, (b) two times, and (c) three times.
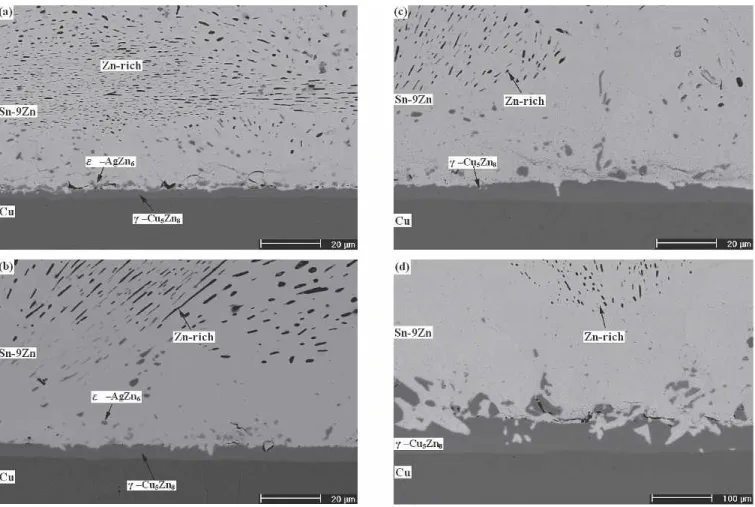
Fig. 8. Microstructure of intermetallic compounds formed in the Sn-9Zn solder BGA packages with Ag/Cu pads after aging at 100°C for various time periods: (a) 100 h, (b) 300 h, (c) 700 h, and (d) 1000 h.
Fig. 9. Microstructure of intermetallic compounds formed in the Sn-9Zn solder BGA packages with Ag/Cu pads after aging at 150°C for various time periods: (a) 100 h, (b) 300 h, (c) 700 h, and (d) 1000 h.
is Ag:Zn # 14.4:85.6, which corresponds to the $-AgZn6phase in the Ag-Zn equilibria diagram. Fig-ure 7 shows that the scallop-shaped $-AgZn6 inter-metallic has not altered much after multiple reflows. However, an additional intermetallic layer has formed between the $-AgZn6 scallops and the Cu pad. The EDX analyses show that the composition (at.%) of the new intermetallic layer is in the #-Cu5Zn8phase.
The #-Cu5Zn8 intermetallic layer also appears at the interface (Fig. 8a) after aging of the single-reflowed specimen at 100°C for 100 h. However, in Fig. 8a, the $-AgZn6scallops are found to have de-tached from the #-Cu5Zn8 intermetallic layer. Aged at 100°C for 300 h, the $-AgZn6 intermetallic scal-lops are observed (Fig. 8b) to have floated much far-ther away from the #-Cu5Zn8 intermetallic layer. With further increases of the aging time, the $-AgZn6 intermetallic compounds are dispersed in the solder matrix, and the #-Cu5Zn8 layer has grown, as revealed in Fig. 8b–d. In Fig. 8, depletion of the Zn-rich precipitates can also be observed in the solder region near the interfacial #-Cu5Zn8 in-termetallic layers. The result indicates that the growth of #-Cu5Zn8 intermetallics is caused by the reaction between the Cu pad and the Zn atoms from the solder matrix. The exhaust of the Zn content in Sn-9Zn solder appears to have caused the
dissolu-tion of Zn-rich precipitates near the solder/pad in-terfaces.
After aging at 150°C, many #-Cu5Zn8 intermetal-lic compounds are observed to strip off and float into the solder matrix of the Ag-surface finished Sn-9Zn BGA packages, as shown in Fig. 9. After prolonged aging for 1000 h, the thick #-Cu5Zn8 intermetallic layer becomes porous, with the solder penetrated into the Cu pad in scallop form (Fig. 9d). The pen-etration of Sn-9Zn solder into the Cu pad can be easily observed in Fig. 10d, as compared to the mor-phology of the solder balls after aging under other conditions listed in Fig. 10.
The growth thickness of the #-Cu5Zn8 intermetal-lic layer at the interfaces between the Sn-9Zn solder balls and the Cu pads after aging at 100°C and 150°C is plotted versus the square root of aging time. The linear relation in Fig. 11a implies that the growth kinetics of #-Cu5Zn8 interfacial intermetal-lics is diffusion controlled. Since the source for Zn atoms in the growth of #-Cu5Zn8is the dissolution of Zn-rich precipitates in the solder matrix, the widths of the precipitate depletion regions at the aging tem-peratures of 100°C and 150°C are measured and plotted in Fig. 11b versus the square root of aging time. The linear relation of both curves indicates that the reaction is also diffusion controlled. The lattice diffusivities of Zn in Sn matrix at 100°C and Fig. 10. Morphology of solder balls in Sn-9Zn BGA packages with Ag/Cu pads after aging at various temperatures and time periods: (a) 100°C, 300 h; (b) 100°C, 1000 h; (c) 150°C, 300 h; and (d) 150°C, 1000 h.
150°C, as calculated from the equation of Huang and Huntington,17
D $m2"s% = 1.1 × 10−6exp
"
−50.2 kJ"molRT
#
,are 1.01 × 10−13 m2/s and 6.91 × 10−13 m2/s. The diffusion distances $d =
#
Dt% at 100°C after aging times of 100 h and 1000 h are 191 %m and 650 %m, respectively. For the aging temperature of 150°C for 100 h and 1000 h, the diffusion distances of Zn at-oms in Sn matrix are 499 %m and 1577 %m, respec-tively. In comparison with Fig. 11a, it is evidenced that the depletion widths of Zn-rich precipitates during the aging treatments are about tenfold smaller than the diffusion distances of Zn atoms in Sn matrix. Similar to the former case, the Au/Ni surface finished Sn-9Zn BGA packages, the results indicate that the controlled mechanism for the dis-solution kinetics of Zn-rich precipitates in the Ag surface finished solder joints is the interfacial reac-tion between the Sn-9Zn solder and the Cu pad,rather than the lattice diffusion of Zn atoms through the solder matrix.
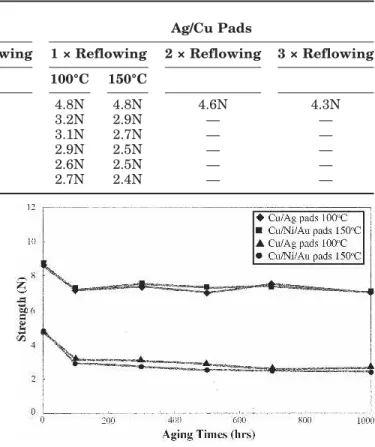
The ball shear strengths of solder joints in Sn-9Zn BGA packages with Au/Ni/Cu and Ag/Cu pads after various reflow and aging treatments are summa-rized in Table I. A single reflow cycle for Au/Ni sur-face finished packages results in a bonding strength of 8.6 N, which remains almost unchanged after multiple reflows, as shown in Fig. 12. For the Sn-9Zn solder joints with Ag/Cu pads, the bonding strength decreased slightly from the single reflowed condition (4.8 N) to the three times reflowed condi-tion (4.3 N). The results also indicated that the re-flowed Sn-9Zn solder BGA packages with Au/Ni/Cu pads possessed ball shear strengths twofold higher than those with Ag/Cu pads. Figure 13 compares the fractography of as-reflowed Au/Ni and Ag surface finished solder joints after the ball shear test. It is evidenced that the Sn-9Zn BGA packages with Au/ Ni/Cu pads fractured along the solder matrix with a fully ductile characteristic (Fig. 13a). However, Fig. 13b and 13c demonstrate that the as-reflowed Ag surface finished Sn-9Zn packages after the ball shear test fractured along the solder/pad interface, with the exposure of the interfacial $-AgZn6 inter-metallic layer (referring to Fig. 7a). It has been shown in Fig. 2 that the interface between the Sn-9Zn solder and the Ni/Cu pad after reflowing was free of intermetallic compounds. The much lower ball shear strength of the as-reflowed Ag surface fin-ished Sn-9Zn packages, in comparison with those with the Au/Ni surface finish, should be attributed to the existence of brittle $-AgZn6 interfacial inter-metallics in the former packages.
Aging at 100°C for 100 h causes the ball shear strengths of single reflowed Sn-9Zn packages with Au/Ni/Cu and Ag/Cu pads to decrease drastically to 7.2 N. With further increases of the aging time, the bonding strengths remain almost constant, as re-vealed in Fig. 13. The degradation of bonding strengths for both surface finished packages after aging at 150°C are similar to those of packages aged at 100°C. Fractography of all aged Sn-9Zn packages with Au/Ni/Cu and Ag/Cu pads after ball shear tests reveal a dimple characteristic (Fig. 15). The solder Fig. 11. Thickness (x) of the #-Cu5Zn8interfacial intermetallic layer
and precipitate-free zone (xd) in the solder matrix of immersion Ag
surface finished Sn-9Zn BGA packages after aging at 100°C and 150°C relative to the square root of time (t1/2).
Fig. 12. Ball shear strengths of Sn-9Zn solder BGA packages with Au/Ni/Cu and Ag/Cu pads after various reflow cycles.
Intermetallic Reactions in Reflowed and Aged Sn-9Zn Solder Ball Grid
joints have fractured along the solder matrix for those aged at either 100°C or 150°C. The result im-plies that the appearance of the Ni4Zn21and #-Cu5Zn8 intermetallic layers at the solder/pad in-terface of Au/Ni and Ag surface finished Sn-9Zn packages after aging at 150°C has not affected the bonding strength. To elucidate the degradation of bonding strengths in the aged solder joints, the hardness of the solder balls is measured and shown in Fig. 16. It is obvious that both tendencies of the ball strength (Fig. 14) and solder ball hardness (Fig. 16) are quite consistently related to the aging time. The results indicate that the strength degradation of solder joints in both surface finished packages after aging is caused by the softening of the solder matrix. The softening effect of Sn-9Zn solder balls with Au/ Ni/Cu pads after aging is attributed to the depletion of Zn-rich precipitates in both solder matrices due to the growth of the Ni4Zn21and #-Cu5Zn8 intermetal-lic layers, respectively. However, the depletion zones of Zn-rich precipitates due to the formation and growth of the Ni4Zn21intermetallic layer in the Au/ Ni surface finished packages after aging are much narrower than those for Sn-9Zn solder joints with Ag/Cu pads. From Fig. 14, the decrease in bonding strengths of Au/Ni and Ag surface finished Sn0-9Zn packages after aging processes are about 17% and 40%, respectively. The much more severe
degrada-Table I. Ball Shear Strengths of the Sn-9Zn Solder BGA Packages with Au/Ni/Cu and Ag/Cu Pads after Various Reflow and Aging Treatments
Surface
Finishing Au/Ni/Cu Pads Ag/Cu Pads
Reflowing 1 × Reflowing 2 × Reflowing 3 × Reflowing 1 × Reflowing 2 × Reflowing 3 × Reflowing
Aging 100°C 150°C 100°C 150°C 0 8.6N 8.6N 8.4N 9.1N 4.8N 4.8N 4.6N 4.3N 100 h 7.2N 7.2N — — 3.2N 2.9N — — 300 h 7.4N 7.5N — — 3.1N 2.7N — — 500 h 7.0N 7.3N — — 2.9N 2.5N — — 700 h 7.5N 7.4N — — 2.6N 2.5N — — 1000 h 7.0N 7.0N — — 2.7N 2.4N — —
Fig. 13. Fractography of solder joints after ball shear tests in the as-reflowed Sn-9Zn BGA packages with various surface finishes: (a) Au/Ni/Cu pads and (b) and (c) Ag/Cu pads.
Fig. 14. Ball shear strengths of Sn-9Zn solder BGA packages with Au/Ni/Cu and Ag/Cu pads after aging at 100°C and 150°C for various times.
tion of aged solder joints in Sn-9Zn BGA packages with Ag/Cu pads in comparison to those with Au/Ni/ Cu pads is consistent with the wider precipitate-free zones in the former case, which can be attributed to the higher growth rate of Cu-Zn intermetallics than Ni-Zn intermetallics at the solder/pad interfaces.11
CONCLUSIONS
The intermetallic compounds formed in Sn-9Zn solder joints of BGA packages with Au/Ni/Cu and Ag/Cu pads after the reflow and aging processes are
investigated. Experimental results show that the Au and Ag thin film dissolves rapidly during reflow and reacts with the Zn atoms of Zn-rich precipitates em-bedded in the Sn-9Zn solder matrix to form a #3 -AuZn4/#-Au7Zn18 intermetallic double layer and $-AgZn6 intermetallic scallops, respectively. Mul-tiple reflows cause the #3/# intermetallics in the Au/ Ni-surface finished Sn-9Zn packages to float away from the Ni/Cu pads. After aging at 100°C and 150°C, #3-AuZn4 grows as a result of the reaction between #-Au7Zn18and the Zn atoms dissolved from Zn-rich precipitates near the #3/# layer. At the in-terface between the Sn-9Zn solder and the Ni/Cu pad, an additional Ni4Zn21 intermetallic layer ap-pears at the aging temperature of 150°C. For the immersion Ag surface finished Sn-9Zn BGA pack-ages, it was observed that multicycle reflows caused an additional #-Cu5Zn8 intermetallic layer to form between $-AgZn6scallops and the Cu pad. #-Cu5Zn8 intermetallics also appeared after the aging of the reflowed specimens at 100°C and 150°C. However, the $-AgZn6 scallops were found to detach from the #-Cu5Zn8 intermetallic layer and float into the sol-der matrix. The growth of Ni4Zn21and #-Cu5Zn8 in-terfacial intermetallics causes the depletion of Zn-rich precipitates in the solder ball near the solder/ pad interfaces. Ball shear testing revealed that the as-reflowed Sn-9Zn BGA solder joints with Au/Ni/Cu Fig. 15. Typical fractography of ball shear tested solder joints in Sn-9Zn BGA packages with Au/Ni/Cu and Ag/Cu pads after aging at various temperatures: (a) Au/Ni/Cu pads, 100°C aging; (b) Au/Ni/Cu pads, 150°C aging; (c) Ag/Cu pads, 100°C aging; and (d) Ag/Cu pads, 150°C aging.
Fig. 16. Hardness of solder balls in Sn-9Zn BGA packages with Au/Ni/Cu and Ag/Cu pads after aging at 100°C and 150°C for various times.
Intermetallic Reactions in Reflowed and Aged Sn-9Zn Solder Ball Grid
and Ag/Cu pads possessed bonding strengths of 8.6 N and 4.8 N, respectively. The depletion of Zn-rich precipitates near the solder/pad interfaces after the aging process accounts for the softening effect of the solder matrix. This resulted in degradation of the ball shear strength of Au/Ni and Ag surface finished Sn-9Zn packages by about 17% and 40%, respec-tively.
ACKNOWLEDGEMENT
The authors gratefully acknowledge the financial support from the National Science Council, Taiwan, for this study under Grant No. 93-2216-E002-024.
REFERENCES
1. N.C. Lee, Adv. Microelectron. 26, 29 (1999).
2. H.J. Lau, C.P. Wong, N.C. Lee, and S.W. Ricky Lee,
Elec-tronics Manufacturing with Lead-Free, Halogen-Free & Conductive-Adhesive Materials (New York: McGraw-Hill
Handbooks, 2003).
3. Z. Chen, M. He, and G. Qi, J. Electron. Mater. 33, 1465 (2004).
4. J.W. Yoon, S.W. Kim, and S.B. Jung, J. Alloys Compounds 391, 82 (2005).
5. M.Y. Chiu, S.Y. Chang, Y.H. Tseng, Y.C. Chan, and T.H. Chuang, Z. Metallkd. 93, 248 (2002).
6. Y.C. Chan, M.Y. Chiu, and T.H. Chuang, Z. Metallkd. 93, 95 (2002).
7. M.Y. Chiu, S.S. Wang, and T.H. Chuang, J. Electron. Mater. 31, 494 (2002).
8. P. Harris, Soldering Surf. Mount Technol. 11, 46 (1999). 9. R.K. Shiue, L.W. Tsay, C.L. Lin, and J.L. On, Microelectron.
Reliab. 43, 453 (2003).
10. T.H. Chuang, H.M. Wu, M.D. Cheng, S.Y. Chang, and S.F. Yen, J. Electron. Mater. 33, 22 (2004).
11. Y.C. Chan, M.Y. Chiu, and T.H. Chuang, Z. Metallkd. 93, 95 (2002).
12. K. Suganuma and K. Niihara, J. Mater. Res. 13, 2859 (1998).
13. W.H. Lin and T.H. Chuang, Mater. Eng. Performance 12, 452 (2003).
14. H.M. Lee, S.W. Yoon, and B.J. Lee, J. Electron. Mater. 27, 1161 (1998).
15. S.W. Yoon, J.R. Soh, H.M. Lee, and B.J. Lee, Acta Mater. 45, 951 (1997).
16. S.W. Yoon, W.K. Choi, and H.M. Lee, Scripta Mater. 40, 327 (1999).
17. F.H. Huang and H.B. Huntington, Phys. Rev. B9 1479 (1974).