Agreement Assessment of Tigecycline Susceptibilities
Determined by the Disk Diffusion and Broth Microdilution
Methods among Commonly Encountered Resistant Bacterial
Isolates: Results from the Tigecycline In-vitro Surveillance
in Taiwan [TIST] Study, 2008-2010
Jien-Wei Liu
1, Wen-Chien Ko
2, Cheng-Hua Huang
3, Chun-Hsing Liao
4,
Chin-Te Lu
5, Yin-Ching Chuang
6, Shih-Ming Tsao
7, Yao-Shen Chen
8,
Yung-Ching Liu
8,9, Wei-Yu Chen
10, Tsrang-Neng Jang
10, Hsiu-Chen
Lin
11, Chih-Ming Chen
12, Zhi-Yuan Shi
13, Sung-Ching Pan
14, Chia-Ling
Yang,
14Hsiang-Chi Kung
14, Chun-Eng Liu,
15Yu-Jen Cheng
15, Yen-Hsu
Chen
16, Po-Liang Lu
16, Wu Sun
17, Lih-Shinn Wang
18, Kwok-Woon Yu
19,
Ping-Cherng Chiang
20, Ming-Hsun Lee
20, Chun-Ming Lee
21,
Gwo-Jong Hsu
22and Po-Ren Hsueh
23*
Division of Infectious Diseases, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University Medical College, Kaohsiung, Taiwan1; Division of Infectious Diseases, Department of Internal Medicine, National
Cheng Kung University Medical College and Hospital, Tainan, Taiwan2;Division of
Infectious Diseases, Department of Internal Medicine, Cathay General Hospital, Taipei, Taiwan3; Section of Infectious Diseases, Department of Internal Medicine,
Far Eastern Memorial Hospital, Taiwan4;Section of Infectious Diseases, Department
of Internal Medicine, Lotung Poh-Ai Hospital, Lotung, Taiwan5;Department of
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Diseases, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan7; Section of Infectious Diseases, Department of Internal
Medicine, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan8; Section of
Infectious Diseases, Shuang Ho Hospital, Taipei Medical University and School of Medicine, Taipei Medical University, Taipei, Taiwan9; Section of Infectious Diseases,
Department of Internal Medicine, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan10; Department of Laboratory Medicine, Taipei Medical University Hospital,
Taipei, Taiwan11; Section of Infectious Diseases, Department of Internal Medicine,
Tungs' Taichung MetroHarbor Hospital, Taichung, Taiwan12; Section of Infectious
Diseases, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan13; Section of Infectious Diseases, Department of Internal Medicine,
National Taiwan University Hospital Yun-Lin Branch, Taiwan14; Section of Infectious
Diseases, Department of Internal Medicine, Changhua Christian Hospital, Changhua, Taiwan15; Division of Infectious Diseases, Department of Internal
Medicine, Kaohsiung Medical University Hospital and Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan16;
Department of Infection Control, Pao-Chien Hospital, Taitung, Taiwan17;
Section of Infectious Diseases, Department of Internal Medicine, Buddhist Tzu Chi General Hospital, Hualein, Taiwan 18; Division of Infectious Diseases, Department of
25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43
Internal Medicine, Taipei Veterans General Hospital, Taipei, Taiwan19;
Division of Infectious Diseases, Department of Internal Medicine, Chang Gung Medical Foundation Linkou Branch, Linkou, Taiwan20; Section of Infectious
Diseases, Department of Internal Medicine, Mackay Memorial Hospital, Taipei, Taiwan21; Section of Infectious Diseases, Department of Internal Medicine,
Ditmanson Medical Foundation Chiayi Christian Hospital, Chiayi, Taiwan22;
Departments of Laboratory Medicine and Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei,
Taiwan23
*Corresponding author. Mailing address for Po-Ren Hsueh: Departments of Laboratory Medicine and Internal Medicine, National Taiwan University Hospital, No. 7 Chung-Shan South Rd., Taipei 100, Taiwan. Phone: 886-2-23123456, ext. 65355. Fax: 886-2-23224263. E-mail: hsporen@ntu.edu.tw. 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59
ABSTRACT
The Tigecycline In-vitro Surveillance in Taiwan (TIST) study, initiated in 2006, is a nationwide surveillance program designed to longitudinally monitor the in-vitro activity of tigecycline against commonly encountered drug-resistant bacteria. This study compared the in-vitro activity of tigecycline against 3,014 isolates of clinically important drug-resistant bacteria using the standard broth microdilution and disk diffusion methods. Species studied included methicillin-resistant Staphylococcus aureus (MRSA, n=759), vancomycin-methicillin-resistant Enterococcus faecium (VRE, n=191), extended-spectrum β-lactamase (ESBL)-producing Escherichia coli (n=602), ESBL-(ESBL)-producing Klebsiella pneumoniae (n=736), and Acinetobacter baumannii (n=726) that had been collected from patients treated between 2008 and 2010 at 20 hospitals in Taiwan. Minimum inhibitory concentrations (MICs) and inhibition zone diameters were interpreted according to the currently recommended US Food and Drug Administration (US FDA) criteria and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria. The MIC90 values of tigecycline against MRSA, VRE, ESBL-producing E. coli, ESBL-producing K. pneumoniae, and A.
baumannii were 0.5, 0.125, 0.5, 2, and 8 g/ml, respectively. The total error rates
between the two methods using the US FDA criteria were high: 38.4% for ESBL-60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78
producing K. pneumoniae and 33.8% for A. baumannii. Using the EUCAST criteria, the total error rate was also high (54.6%) for A. baumannii isolates. The total error rates between these two methods were <5% for MRSA, VRE, and ESBL-producing E. coli. For routine susceptibility testing of ESBL-producing K. pneumoniae and A. baumannii against tigecycline, the broth microdilution method should be used because of the poor correlation of results between these two methods.
Keywords: Agreement, antimicrobial susceptibilities, tigecycline, disk diffusion method, broth microdilution method, resistant bacteria, TIST
79 80 81 82 83 84 85 86 87 88 89
INTRODUCTION
Antibiotic resistance among clinical bacterial isolates is of major concern worldwide (1). The discovery of new antibiotics has lagged behind the demand for the coverage of emerging clinical multidrug-resistant (MDR) bacterial isolates (18, 21). Clinicians, therefore, are often forced to use agents that are inherently toxic or drugs for which there are no robust data regarding appropriate antibiotic dosing and therapeutic duration to treat patients with infections caused by MDR bacteria (1). Tigecycline, a minocycline derivate with potent activity against a wide range of MDR Gram-positive cocci and Gram-negative rods (15-17, 23) should be highly valued. However, it is very important to ensure that the prescribed antimicrobial is active in vitro against etiologic bacteria, especially when the pathogens are MDR that often cause infections in immunocompromised and critically ill patients (12, 13).
Antibiotic susceptibility can be tested using either a dilution or diffusion method. A number of such systems are commercially available; the major challenge, however, lies in the inflexibility of standard panels to test the in vitro susceptibilities of various bacteria to different antimicrobials (24). Disk diffusion susceptibility testing has been widely used in most clinical laboratories, even though many of those laboratories can afford to purchase automated or semi-automated systems for susceptibility testing. Despite the potential role played by tigecycline in the treatment of infections due to 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108
MDR bacteria, little is known about the interchangeability of the results of the disk diffusion and broth microdilution methods in testing the susceptibility of these MDR microbes to tigecycline.
This study compared the in vitro susceptibility of various MDR isolates to tigeclycline as measured by the disk diffusion method with the susceptibility of those isolates as measured by the broth microdilution method. The MDR isolates included methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant
Enterococcus faecium (VRE), extended-spectrum β-lactamase (ESBL)-producing Escherichia coli, ESBL-producing Klebsiella pneumoniae, and Acinetobacter baumannii isolates that had been collected from clinical specimens (blood, respiratory
secretion, pus, or urine) during the period 2008 to 2010. These clinical isolates were part of the bacterial collection in the Tigecycline In-vitro Surveillance in Taiwan (TIST) study, a program that commenced in 2006 to longitudinally monitor the in vitro activity of tigecycline against a variety of clinical bacteria in Taiwan (9).
109 110 111 112 113 114 115 116 117 118 119 120 121 122
MATERIALS AND METHODS
Bacterial isolates. A total of 3,014 isolates consisting of 759 MRSA, 191 VRE, 602 ESBL- producing E. coli, 736 ESBL-producing K. pneumoniae, and 726 A.
baumannii isolates were included in this study. The isolates were collected from
patients in 20 hospitals including district hospitals and medical centers distributed throughout Taiwan. The capacities of those institutions ranged from 450 to 2500 beds. All isolates were judged to be clinically significant by infectious a disease physician at the collecting hospital. To avoid duplication, each bacterial species was collected only once from an individual patient. Clinical isolates were identified by conventional methods (8, 11, 19, 20) or the automated ID 32GN system (bioMerieux, Vitek, systems, Hazelwood, Mo.) or both. ESBL-producing E. coli and ESBL-producing K.
pneumoniae were identified as recommended by the Clinical and Laboratory
Standards Institute (CLSI) (5). All A. baumannii isolates studied were considered multidrug resistant as they were resistant to 3 of the following antimicrobial agents: ampicillin/sulbactam, aztreonam, ceftazidime, ciprofloxacin, gentamicin, imipenem, and piperacillin. The collected isolates were stored at -70°C in skim milk or trypicase soy agar supplemented with 15% glycerol. Bacterial identifications were eventually confirmed at the central laboratory at Taiwan National University Hospital where disk diffusion and broth microdilution tigecycline susceptibility testing were carried out. 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141
This study was conducted with the ethical approval by the National Taiwan University Hospital Institutional Review Board (NTUH-IRB 9561709108).
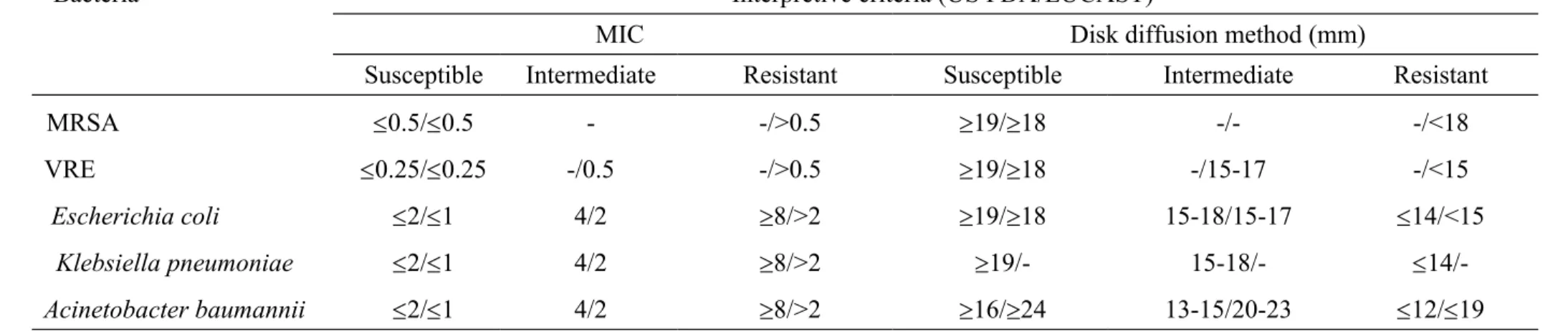
Antimicrobial susceptibility testing. Susceptibility to tigecycline was tested by the disk diffusion and broth microdilution methods as recommended by the CLSI (3, 4). Standard powder of tigecycline was obtained from Pfizer Inc., NY for broth microdilution testing. Tigecycline 15 g per disk was used for disk diffusion susceptibility testing. Mueller Hinton agar and broth (BBL Microbiology Systems, Cockeysville, Md.) for susceptibility tests were freshly prepared and then used within 12 hours (9). Quality control strains, including S. aureus ATCC 29213, E. faecalis ATCC 29212, and E. coli ATCC 25922 were used for tigecycline susceptibility testing as necessary. Interpretations of the minimum inhibitory concentrations (MICs) in the broth microdilution testing method and the diameters of the inhibitory zone in the disk diffusion testing method were based on the criteria proposed by the U.S. Food and Drug Administration (US FDA) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST), as summarized in Table 1 (6, 16, 17, 23). Some of the results of broth microdilution tigecycline susceptibility tests against these bacteria in this work will be published elsewhere (Chen et al., submitted for publication).
Agreement assessment of tigecycline susceptibility determined by the disk 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160
diffusion and broth microdilution methods. Agreement was defined as an identical result obtained by both the disk diffusion and broth microdilution tests. Non-agreement referred to errors which were categorized as very major error (VME), major error (MaE), and minor error (MiE). Using results of broth microdilution as standards, VME referred to a false-susceptible result by the disk diffusion test, while MaE indicated a false-resistant result by the disk diffusion test; MiE referred to an intermediate result by the disk diffusion test or the broth microdilution test and either a resistant or susceptible result by the other method (2). Acceptable inter-method errors rates were ≤1.5% for VME, ≤3% for MaE and ≤10% for MiE (2), which indicated that the disk diffusion and broth microdilution method were interchangeable for susceptibility of the tested microbes against tigecycline.
161 162 163 164 165 166 167 168 169 170 171
RESULTS
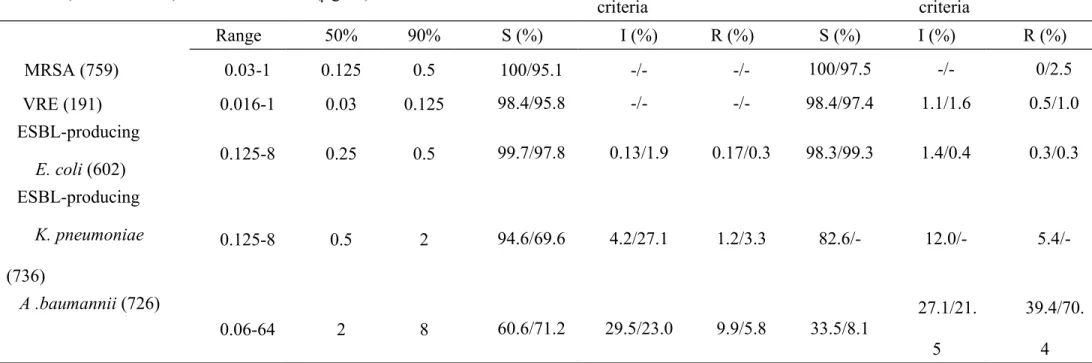
Tigecycline susceptibilities. Remarkably high tigecycline susceptible rates ( 95%) were found among the MRSA, VRE, and ESBL-producing E. coli isolates in the broth microdilution testing methods based on both US FDA and EUCAST interpretive criteria, among the ESBL-producing K. pneumoniae isolates in the broth microdilution method based on FDA interpretive criteria, and among the MRSA, VRE, and ESBL-producing E. coli isolates in the disk diffusion method based on US FDA and EUCAST interpretive criteria (Table 2).
Of note, using the FDA interpretive criteria, a high tigecycline susceptible rate of 94.6% in the broth microdilution testing method and a low tigecycline susceptible rate of 69.9% in disk diffusion testing method were found among the ESBL-producing K.
pneumoniae isolates (Table 2). Among the A. baumannii isolates, 68.2% were found
to be susceptible to tigecycline by the broth microdilution method and 71.7% were found to be susceptible to tigecycline by the disk diffusion method based on US FDA interpretive criteria; however, using the on EUCAST interpretive criteria, only 35% of the isolates were susceptible to tigecycline by both the disk diffusion and broth microdilution methods (Table 2).
Discrepancies between results of disk diffusion testing and broth microdilution testing. The total error rate of the disk diffusion method in comparison with the broth 172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190
microdilution method using the US FDA criteria was high: 38.4% for ESBL-producing K. pneumoniae and 33.8% for A. baumannii (Table 3). Using the EUCAST criteria, the total error rate was also high (54.6%) in A. baumannii isolates. The majority of these errors among ESBL-producing K. pneumoniae and A. baumannii belonged to MiE. The total error rates between these two methods for MRSA, VRE, and ESBL-producing E. coli were acceptable (all <5%).
191 192 193 194 195 196 197
DISCUSSION
The susceptibility rates of ESBL-producing K. pneumoniae isolates and A.
baumannii isolates to tigecycline differed markedly between the US FDA and
EUCAST criteria. The CLSI and US FDA criteria are based on clinical and bacteriological response rates in conjunction with population distributions and pharmacokinetics/pharmacodynamics to establish breakpoints in order to provide the best correlation between the in vitro test and clinical results (24). There are, however, a few breakpoint discrepancies between the CLSI and the US FDA criteria (24). The EUCAST takes a different approach to establishing susceptibility breakpoints by placing greater emphasis on the detection of emerging resistance through examination of microorganism population distributions (24).
Based on the US FDA interpretive susceptibility criteria, the lack of interchangeability between the disk diffusion method and broth microdilution method for tigecycline against our ESBL-producing K pneumoniae isolates was mainly due to the high percentage of MiE (26.5%); of note, there was a marked difference in the susceptibility rate of tigecycline among ESBL-producing K. pneumoniae isolates (69.9% vs. 96.3%) between the disk diffusion and broth microdilution methods. Taken together, these data indicate that the disk diffusion method for tigecycline susceptibility testing based on the US FDA interpretive susceptibility criteria may 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216
underestimate the rate of susceptibility to tigecycline among the ESBL-producing K.
pneumoniae isolates. Based on our findings, we suggest that when the disk diffusion
method shows that ESBL-producing K. pneumoniae isolates are susceptible to tigecycline based on US FDA interpretation-based category, tigecycline is the appropriate antibiotic for treating infection caused by these pathogens.
Given that previously published and current results on tigecycline susceptibility of A. baumannii isolates differ markedly between the disk diffusion method and the broth microdilution method, as well as between the agar-diffusion-based Etest and the broth microdilution method (14, 16), it is not surprising that the results from the disk diffusion and broth microdilution susceptibility testing methods are not interchangeable. The variability in magnesium and oxygen contents in the susceptibility media have been shown to interfere with the tigecycline susceptibility results against A. baumannii isolates (10, 22).
In summary, we found that the disk diffusion method and broth microdilution method both showed high rates of tigecycline susceptibility among MRSA, VRE, and ESBL-producing E. coli isolates based on both US FDA and EUCAST interpretive criteria, and among ESBL-producing K. pneumoniae isolates based on US FDA interpretive criteria. Our data suggest that the disk diffusion method, with its inherent flexibility in antibiotic selection and low cost, may be used as a substitute for the 217 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234 235
broth microdilution method in tigecycline susceptibility testing against MRSA and VRE and ESBL-producing E. coli isolates based on both US FDA and EUCAST interpretive criteria. For routine susceptibility testing of tigecycline against ESBL-producing K. pneumoniae and A. baumannii, the broth microdilution method, not the disk diffusion method, should be used due to the poor correlation of results between these two methods.
ACKNOWLEDGMENTS
This work was sponsored by Pfizer Ltd., Taiwan.236 237 238 239 240 241 242 243 244 245
REFERENCES
1. Boucher H. W., et al.2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12.
2. Clinical and Laboratory Standards Institute. 2001. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline, 2nd ed. NCCLS document M23-A2. NCCLS, Wayne, Pa.
3. Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial disk susceptibility tests: approved standard M2-A9. 9th ed. CLSI, Wayne, Pa.
4. Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A7. 7th ed. CLSI, Wayne, Pa.
5. Clinical and Laboratory Standards Institute. 2011. Performance standardsfor antimicrobial susceptibility testing; 21st informational supplement. M100-S21. CLSI, Wayne, Pa.
6. European Society of Clinical Microbiology and Infectious Diseases. 2011. Clinical breakpoints. In European Committee on Antimicrobial Susceptibility Testing. http://www.eucast.org/.
7. Facklam R. R., D. F. Sahm, and L. M. Teixeira. 1999. Enterococcus, p. 297-305. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken 246 247 248 249 250 251 252 253 254 255 256 257 258 259 260 261 262 263 264 265 266
(ed). Manual of Clinical Microbiology, 7th ed.ASM Press, Washington, DC. 8. Farmer J. J., III. 1999. Enterobacteriaceae: introduction and identification, p.
442-458. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed). Manual of Clinical Microbiology, 7th ed.ASM Press, Washington, DC.
9. Hsueh P. R. 2008. Tigecycline in-vitro surveillance in Taiwan (TIST). Int. J. Antimicrob. Agents 32 (Suppl. 3): 173.
10. Hope R, M. Warner M, S. Mushtaq, M. E. Ward, T. Parsons, and D. M. Livermore. 2005. Effect of medium type, age and aeration on the MICs of tigecycline and classicaltetracyclines. J. Antimicrob. Chemother. 56:1042-1046. 11. Kloos W., and T. L. Bannerman. 1999. Staphylococcus and Micrococcus, p.
244-282. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed). Manual of Clinical Microbiology, 7th ed.ASM Press, Washington, DC.
12. Lee C. H, L. H. Su, C. C Li, C. C. Chien, Y. F Tang, and J. W. Liu..
2010.Microbiologic and clinical implications of bacteremia due to
extended-spectrum- β-lactamase-producing Klebsiella
pneumoniae with or without plasmid-mediated AmpC. Antimicrob. Agents Chemother. 54: 5395-5398.
267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 284 285
producing Klebsiella pneumoniae bacteremia with carbapenems or flomoxef: a retrospective study and laboratory analysis of the isolates. J. Antimicrob. Chemother. 58:1074–1077.
14. Liao C. H., H. C. Kung, G. J. Hsu, P. L. Lu, Y. C. Liu, C. M. Chen, C. M. Lee, W. Sun, T. N. Jang, P. C. Chiang, Y. J. Cheng,H. C. Lin, Z. Y. Shi, L. S. Wang, Y. C. Chuang, S. M. Tsao, C. T. Lu, J. W. Liu, C. H. Huang, and P. R. Hsueh. 2006. In vitro activity of tigecycline against clinical isolates of
Acinetobacter baumannii in Taiwan determined by the broth microdilution and disk diffusion methods. Int. J. Antimicrob. Agents. 32 (Suppl. 3): 192-196.
15. Liu J. W., L. S. Wang, Y. J. Cheng, G. J. Hsu, P. L. Lu, Y.C. Liu, C. M. Chen, C. M. Lee, W. Sun, T. N. Jang, P. C. Chiang, Y. C. Chuang, H. C. Lin, Z. Y. Shi, H. C. Kung, C. H. Huang, S. M. Tsao, C. T. Lu, C. H. Liao, and P. R. Hsueh. 2008. In vitro activity of tigecycline against clinical isolates of
Acinetobacter baumannii in Taiwan. Int. J. Antimicrob. Agents 32 (Suppl. 3):
188-191.
16. Liu J. W., T. N. Jang, Y. J. Cheng, G. J. Hsu, W. Sun, C. T. Lu,P. R. Hsueh, and Tigecycline In-Vitro Surveillance in Taiwan (TIST) Group. 2010. Comparison of the Etest and broth microdilution methods for tigecycline susceptibility testing against clinical isolates of Acinetobacter baumannii from 287 288 289 290 291 292 293 294 295 296 297 298 299 300 301 302 303 304 305
Taiwan. Int. J. Antimicrob. Agents. 35: 201-202. (Letter.)
17. Lu C. T., Y. C. Chuang , W. Sun, Y. C. Liu, Y. J. Cheng, P. L. Lu, C. M. Chen, G. J. Hsu, T. N. Jang, C. M. Lee, Z. Y. Shi, L. S. Wang, H. C. Kung, H. C. Lin, C. H. Liao, J. W. Liu, C. H. Huang, S. M. Tsao, and P. R. Hsueh. 2008. Nationwide surveillance in Taiwan of the in vitro activity of tigecycline against clinical isolates of extended-spectrum β-lactamase-producing
Enterobacteriaceae. Int. J. Antimicrob. Agents 32 (Suppl. 3): 179-183.
18. Norrby S. R., C. E. Nord, and R. Finch. 2005. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect. Dis. 5: 115-119.
19. Ruoff K. L. 1999. Algorithm for identification of aerobic Gram-positive cocci, p. 262-264. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed). Manual of Clinical Microbiology, 7th ed.ASM Press, Washington, DC.
20. Schreckenberger P. C., and A. von Graevenitz. 1999. Acinetobacter,
Achromobacter, Alcaligenes, Moraxella, Methylobacterium, and other
nonfermentativeGram-negative rods, p. 539-560.In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed). Manual of Clinical Microbiology, 7th ed.ASM Press, Washington, DC.
306 307 308 309 310 311 312 313 314 315 316 317 318 319 320 321 322 323 324
J. Bartlett, and J. E. Edwards Jr., for the Infectious Diseases Society of America. for the Infectious Diseases Society of America. 2008. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 46:155-164.
22. Thamlikitkul V., and S. Tiengrim. 2008. Effect of different Mueller-Hinton agars on tigecycline disc diffusion susceptibility for Acinetobacter spp.. J. Antimicrob. Chemother. 62: 847-848.
23. Tsao S. M., H. C. Lin, C. M. Lee, G. J. Hsu, C. M. Chen, W. Sun, Y. C. Liu, T. N. Jang, Y. J. Cheng, P. L. Lu, P. C. Chiang, L. S. Wang, H. C. Kung, Y. C. Chuang, Z. Y. Shi, J. W. Liu, C. H. Huang, C. T. Lu, C. H. Liao, and P. R. Hsueh. 2008. Nationwide surveillance in Taiwan of the in vitro activity of tigecycline against clinical isolates of Gram-positive cocci. Int. J. Antimicrob. Agents 32 (Suppl. 3): 184-187.
24. Turnidge J. D., M. J. Ferraro, and J. H. Jorgensen. 2011. Susceptibility testing methods: general considerations, p. 1115-1121. In J. Versalovic, K. C. Caroll, G. Funke, J.H. Jorgensen, M. L. Landry, and D. W. Warnock (ed). Manual of Clinical Microbiology, 10th ed., vol. 1. ASM Press, Washington, DC.
326 327 328 329 330 331 332 333 334 335 336 337 338 339 340 341 342
TABLE 1. Interpretive minimum inhibitory concentration (MIC) and disk diffusion interpretive criteria for Gram-positive and Gram-negative bacteria applied in this study
Bacteria Interpretive criteria (US FDA/EUCAST)
MIC Disk diffusion method (mm)
Susceptible Intermediate Resistant Susceptible Intermediate Resistant
MRSA 0.5/0.5 - -/>0.5 19/18 -/- -/<18
VRE 0.25/0.25 -/0.5 -/>0.5 19/18 -/15-17 -/<15
Escherichia coli 2/1 4/2 8/>2 19/18 15-18/15-17 14/<15
Klebsiella pneumoniae 2/1 4/2 8/>2 19/- 15-18/-
14/-Acinetobacter baumannii 2/1 4/2 8/>2 16/24 13-15/20-23 12/19 The interpretations MIC in the broth microdilution testing and the diameter of the inhibitory zone in the disk diffusion testing were based on the criteria proposed by the U.S. Food and Drug Administration (US FDA)/European Committee on Antimicrobial Susceptibility Testing-2011 (EUCAST-2011) (6, 16, 17, 23).
MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci 343 344 345 346 347 348 349
“-” indicates that the interpretive criteria were not established. 350
TABLE 2. Susceptibilities to tigecycline as determined by the broth microdilution and disk diffusion methods Susceptibility results
(broth microdilution method/disk diffusion method)
Bacteria (no. of isolates) MIC (g/ml)
Categorized by US FDA interpretive criteria
Categorized by EUCAST interpretive criteria Range 50% 90% S (%) I (%) R (%) S (%) I (%) R (%) MRSA (759) 0.03-1 0.125 0.5 100/95.1 -/- -/- 100/97.5 -/- 0/2.5 VRE (191) 0.016-1 0.03 0.125 98.4/95.8 -/- -/- 98.4/97.4 1.1/1.6 0.5/1.0 ESBL-producing E. coli (602) 0.125-8 0.25 0.5 99.7/97.8 0.13/1.9 0.17/0.3 98.3/99.3 1.4/0.4 0.3/0.3 ESBL-producing K. pneumoniae (736) 0.125-8 0.5 2 94.6/69.6 4.2/27.1 1.2/3.3 82.6/- 12.0/- 5.4/-A .baumannii (726) 0.06-64 2 8 60.6/71.2 29.5/23.0 9.9/5.8 33.5/8.1 27.1/21. 5 39.4/70. 4 351 352
MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus faecium; S, susceptible; I, intermediate;
R, resistant; US FDA, US Food and Drug Administration; EUCAST, European Committee on Antimicrobial Susceptibility Testing. 353
TABLE 3. Error rates of tigecycline susceptibilities by the disk diffusion method in comparison with the broth microdilution method Bacteria
(no. of isolates)
Percentage of isolates with an indicated error based on US FDA interpretive
criteria
Percentage of isolates with an indicated error
based on EUCAST-2011 interpretive criteria
VME MaE MiE Total
errors
VME MaE MiE Total
errors MRSA (759) - - - - 0 2.5 0 2.5 VRE (191) - - - - 0.5 2.6 1.1 4.2 ESBL-producing E. coli (602) 0 0.2 1.7 1.9 0.2 0.2 1.3 1.7 ESBL-producing K. pneumoniae (736) 0 1.9 26.5 38.4 - - - -A. baumannii (726) 2.5 0.6 30.7 33.8 0.3 11.2 43.1 54.6 355
MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococus faecium; VME, very major error; MaE, major error; MiE, minor error; US FDA, US Food and Drug Administration; EUCAST, European Committee on Antimicrobial Susceptibility Testing. 356
357 358