Journal of Organornetallic Chemistry, 453 (1993) 201-206 201
JOM 23464
Coordination characteristics of three N,N ‘-di( azol-1-yl) methanes;
substituent effects on infrared absorption and structure
of the Group VI metal carbonyl derivatives *
Kom-Bei Shiu and Kuen-Song
Liou
Department of Chemistry, National Cheng Kung University, Tainan 70101 (Taiwan)
Yu Wang, Ming-Chu
Cheng and Gene-Hsiang
Lee
Department of Chemistry, National Taiwan University, Taipei 10764 (Taiwan)
(Received October 17, 1992; in revised form December 11, 1992)
Abstract
Compounds [M(H,CAz,)(CO),] (M = Cr, MO, or W), were obtained readily and similarly by refluxing the equivalent amounts of N,N’-di(azol-1-yl)methanes, H,CAz, (Az = pyrazol-1-yl (Pz), 3,5-dimethylpyrazol-l-y1 (Pz’) or 3,4,5-trimethylpyrazol-1-yl (Pz”) with [M(CO),] (M = Cr, MO, or W) in a mixed solvent system of 1,2-dimethoxyethane and tetrahydrofuran. Under phase transfer catalysis conditions this gives rise to the new nitrogen-bidentate ligand, HzCPz;‘, by reaction of HPz” with methylene chloride. IR absorption bands in the carbonyl region indicate that no regular trends can be found for different substituents on the pyrazolyl ring. This irregularity is attributed to flexibility of the boat conformation formed from the metal centre and the ligand, which is supported by comparative structure details of [Mo(H,CPz;) (CO),], reparked previously, [W(H,CPz;‘)(CO),] (6) and [W(H,CPz;)(CO),] (7): 6, triclinic, Pi, a = 8.403(3), b = 9.589(3), c = 13.310(3) A, a = 94.476(22), p = 102.837(21), y = 110.355 (:5)“, Z = 2, R = 0.024, R, = 0.023, based on 3070 with I > 2a (I). 7, monoclinic, P2,/c, a = 9.178(3), b = 15.885(9), c = 12.394(10) A, p = 108.64(9)“, Z = 4, R = 0.030, R, = 0.030, based on 2625 reflections with I > 2.5a(I).
1. Introduction
The syntheses and applications of neutral poly(azol- 1-yllalkanes, R.C(Az),_, (R = H, alkyl, or aryl; HAz = di-, tri- or tetra-azole; y1 = 2, 3, or 41, as multidentate ligands with either main-group or transition-metal atoms have been extensively studied [l-4]. Especially recently, ligands with Az = pyrazol-l-y1 (Pz) or 3,5-di- methylpyrazol-l-y1 (Pz’) have been the most vigorously developed. From the literature, it appears obvious, as one would expect, that ligands with more methyl-group substituents should have greater electron-donating ability, a feature previously reported for l,lO- phenanthroline (phen) (or 2,2’-bipyridine (bpy)) and the relevant substituted derivatives [5]. However, we
Correspondence to: Dr. K.-B. Shiu.
* Part of synthetic result is taken from K.-S. Liou, M. SC. Thesis,
National Cheng Kung University, Tainan, 1988.
0022-328X/93/$6.00
wish to present IR evidence that no regular trends are in fact observable for [Cr(H ,CAz *) (CO),], [Mo(H *- CAz,)i
(CO),l,
or [W(H,CAz,) (CO),]. This unex- pected irregularity can be rationalized by comparing the structural details of [Mo(H,CPz;)(CO),l, reported in 1989 [6], W(H,CPz~)(CO),] and [W(H,CPz;‘) (CO),], where Pz” is 3,4,5-trimethylpyrazol-l-y1 (Fig. 1).2. Experimental section
All operations were performed by the usual Schlenk techniques [7], using deoxygenated, dry solvents and gases. IR spectra, calibrated with polystyrene, were recorded on a Hitachi Model 260-30 instrument. Ab- breviations are as follows: vs, very strong; s, strong; m, medium and sh, shoulder. NMR spectra were obtained on a Bruker WP-100 (‘H, 100 MHz) FT-NMR spec- trometer. Chemical shifts (6 in ppm, J in herz) are
R4
K.-B. Shiu et al. / N.N ‘-di(azol-I-yornrthanes 203
2.6. Preparation of [W(H,CPz;)(CO),] (6)
This yellow compound was obtained in 83% yield by an analogous procedure (ca. 24 h). Anal. Found: C, 38.44; H, 3.81; N, 10.48. C,,H,,,N,O,W talc.: C, 38.65; H, 3.82; N, 10.61%. ‘H NMR (23°C CDCl,, 100 MHz): 3- and S-methyl groups, 6 2.31 (6H, s), 2.40 (6H, s); 4-methyl group, 1.90 (6H, s>; CH,, 6.31 (lH, s), 6.06 (lH, s>. IR (CH,Cl,): v(CO), 2003m, 1877~s 1867sh, 1822s and IR (KBr): u(CO1, 199Ss, 1874sh, 1857~s 1810s cm-‘.
2.7. X-Ray diffraction study of /W(H,CPz;)(CO), 1 (6)
and [W(H2CPz;)(CO),I (7)
Crystals of 6 and 7 were grown from CH,Cl J hexane at room temperature. General procedures and listings of programs were given previously [6]. Absorp- tion correction was performed on both structures using 4 scans. Related crystal data (Table 11, final coordi- nates of the non-hydrogen atoms (Table 2), and se- lected bond lengths and bond angles (Table 31 are reported. The anisotropic displacement coefficients of
TABLE 1. Crystal data for 6 and 7
the atoms, the H-atom coordinates and structural fac- tors are available from the authors. The ORTEP plots for 6 and 7 with the relevant numbering scheme are drawn in Figs. 2 and 3, respectively.
3. Results and discussion
We have prepared [M(H,CPz,)(CO),] and [M(H 2- CPz;‘)(CO),] (M = Cr, MO, or WI under similar reac- tion conditions to those used for [M(H,CPz;)(CO),] [lo]. These compounds are well characterized as cis- [M(n2-(H2CAz211(CO),] by elemental analysis, IR and NMR spectral results. Since the IR data of [M- (H,CPz2)(CO),] are quite similar to those reported previously by Lobia and Bonati [4], it is thus clear that our reaction condition can apply in the synthesis of the metal carbonyl derivatives of all three types of H,Az,, whether the substituents on the pyrazolyl ring are hydrogen atoms or methyl groups (the condition Lobia and Bonati used [4] applied only for H,CAz, = H2CPz2.) Compound Empirical formula Colour Crystal size (mm) Space group Unit cell dimensions
0 a, h, c, A a. P, Y, deg Volume, Aa Z Formula weight D,,,,, g/cm’ h, k, I ranges Abs car Abs coeff, mm ’ Transm range FCOOO) Diffractometer used Radiation; A, i Temperature (K) Scan type 20 range, deg Scan speed, deg/min Std rflns
Decay; % No. of unique rflns No. of rflns (N,) used No. of atoms refined No. of params (N,) refined Max A/a ratio
R.R ) w s;’
Resid peak; hole e/i’
6 C,,Hx,N,O,W yellow 0.30 x 0.36 x 0.42 triclinic,
pi
8.403(3X 9.589(3), 13.310(3) 94.476(22), 102.837(21), 110.355(25) 966.0(5) 2 528.21 1.816 -9 to 9,0 to 11. - 15 to 15 (1, scan 6.13 0.4799-0.9995 511.83 Nonius CAD4 MO Kn: 0.70930 297 0/2H 2-50 1.43-10 3 std/7200 set 51 34033070 with I> 2.0a(l) 46 236 0.347 0.024, 0.023, 2.77 0.96, - 0.96 7 C,sHn,N,O,W yellow 0.40 x 0.30 x 0.30 monoclinic. P2 ,/c 9.178(3), 15.885(9). 12.394(10) 90, 108.68(4), 90 1711.8(17) 4 500.16 1.941 - 10 to 10, 0 to 18. 0 to 14 1.94 0.543660.9984 951.66 1.66-10 51 2992 2625 with I > 2Sa(l) 40 218 0.012 0.030, 0.030, 4.54 0.94, - 1.60 ’
((1) (‘ompound 6
w
(i.Y03’)7(3) (.‘ I 0.8YXfA7) C2 I .OXW(8) C3 0.7774(8) C‘I I .OYOY(9) c5 1.41 lO(7) C6 i..llox77 (17 1..39.11(7) a 1.061X7) CY 0.X000(7) Cl0 0.71)30(7) Cl1 0.8Y33(7) Cl7 1.478X(X) Cl3 1.7067(X) (‘13 1.421 I(X) Cl5 0.7121(Y) Clfl 0.6083(Y) (‘17 0.‘)330~10) NI 1 .2300(6) N2 1.23OY(h) N3 O.Y557(5) N4 o.HY85t6) 01 0.X406(6) 02 l.lmKh) 03 1).6501(6) 04 l.l3Y-I(7) (hl C‘ornpoLtrlri 7 W’ O.Y695Y(4~ Nl 0.75239(7) N? OMYO(7) N.? 0.7139(7) N-l 0.782X7) <‘ 0.79 15(Y) Cl 1.1461(10) 01 1.2548(7) c2 1.1148(Y) 02 1.21 lO(7) C3 0.X656( IO) 03 O.X27Y(XJ C4 1 .04h‘X 10) 04 I .0937(7) Cl 1 0.6617(Y) Cl2 O.hjhl(Y) Cl3 05471(Y) Cl4 0.5874(Y) Cl5 0.5 15S( IO) c’21 0.X9.39( 1 I ) c’22 0.X279(Y) C23 0.7321( 10) C24 1).7037(10) (~25 0.6045( 13) O.iYl.3(4) 0.3547(4) 0.2 1 %(‘I) (I. 1506(l) O.i42(l(1) O..ii)l7(1) 0.3531(A) Ml iY(1) 0.0?47(1) O.OJb.~(.i) (i_1307(4) O..U2~(5) 0.3YKX.~ 1 O.i(lM5J 0.11104(1) 0.057X3) 0.1450~5) 0..32?5(.3) 0.32YY(.3 1 0.705X(3) 0. 171<%(.3) il.J6OY.?J 0.401?(.:J (l.l?v~‘(J) 0.1010(.3~O.O9riY30( 17) 0.284’Y5(‘4~ _ . 74SXIi)
O.O4SO(i 1 0.313X4) 2 ‘(3) (1.0777(?) 0.1 IYh(l) 2.T.3) ~O.O3hY(3) O.ZXhXi) 2.X(3) U.OJ62(3) 0.1 IXti(l) _‘.X(3) O.Oti(>3(4) 0.2241(6) 3. I(4) 0. I4X7(4) 0.247(6~ 3.‘)(J) 0.1x30(J) 0.2155(1) 5.5(?) 0.002~~ 4 1 (1.31YV6l 3.0(i) 0.04.51(3) O.iXhX5) d.YC3) 0.7103(4) 0.273X(h) 3.W) 0.‘762(33 O.lY30(5) h.6(4) 0.1505(3) O.‘mi(hl -$.0(T) 0.1792(i) 0.5735(JJ 5.X4) 0. I b.%(3) 0.39HK(O~ i 6(-t) 0.(1773(3) 0.3.%7~53 -. ,_ -’ 711) 0.01’4(4) il.3601(6) 3.X4) - 0.0563(4) 0.3 l-12(6) 3.0(3J -0.14X5) 0.2Y.iX7) 4.W) 0.12M.5) O.O2h4(h) 4.0(5) (J.O489(5) 0.00’).3(~1) 3..31) -0.011(5) o.oms(h) l.i(l) ~ 0.00’~4~5) 0.0100(7) 1.7(J) -O.i363(h) -0.01X5(7) tl..i((,) __- ” R,,,, is the mean of the principal axes of the thermal ellipsoid
In the literature, numerous cis-disubstituted te- tracarbonyl complexes of the group VI metal atoms, [M(L-L)(CO),]. are known [I 11. The four carbonyl stretching hands (i.e.. four IR-active vibrations). which
arc usually displayed in these compounds have been assigned by Cotton and Kraihanzcl on the basis of a simple group 1 hcory analysis [I?]: two vibrations associ- ated with the rl-0/r\-carbonyl groups (assigned a’r A I CO”’ and 13, CO”‘! clccur at IS(+) and lK5 cm ’ and two other \#ibration\ axacjciated with the cY.c-carhonyl ligands (assinncd . . ; 14 A, (‘0” and B, CO’“) occur at the higher wa\icnumhcl-5 of 2000 and 1X07 cm ’ in C’H ,(‘I? for I -1. -= hp! f3) comparing either only one or ail r*(C‘O) \;tlucs of [MtL,-I_)C(‘O),j [I I] OI‘ [c?s-M(L.- I,),(CO),] [I.?]. one can d~rfucc the relative 77-acceptor ability of chelating ligands. IA-I., giving an order such ;I\ bpy c: phell -: dmpc .\ dppm ‘., dppc L I’F: (two PI:; iigands take the place of one chclatc). whcrc tlmpc i\ I.;?-bis(dimcth!,ll,hosphinc?)cthan~: dppm is histdiphen- ylphclsphino)-mcthanc and dppc is I.?-bis(diphcnyl- phosphino)eth~tnc. Since the iigands NY used arc also symmctriL2l like dppm or bpy. \v~’ first list the i,((‘O) values l’<>r [M(lI ,(~Az-.~(C’O),] (M = (‘I. MO. or Mi: AZ = 19. I?‘. or Fl”) (Table 4) and then compare one or all ~((‘0) bands in the hope that et‘f‘ccts of diffcrcnt substituents uould 1~ rctlcctcd in the ~aluea, a feature: previously rcportcd lor phcn ior hpy) and the relevant substituted dtri\ ativca [5j, ln fact. as obscr~~cd from these ~alucs. no regular trend for /(‘r(H ,(‘Az,)(CO),], [Mo(Fi,C’Az,)(<‘O).,]. or ~W(H,C‘AL,)((~“O),]. can he inferred taking account of cspcrimcntal error. although tlnc can conclucic that the r4C’O) ~alucs of’ [Mo(H.- C’Az,)lCOJ,] XL similar to those of jM<~(bpy)(C‘O).~j and the 7”.acceptor abilit>~ t)f lj .(‘Ax , is similar to that _ of bpy (prcviou4y, Or0 (‘I trl. suggested the similaril)~ in r-acid ability between H -CPr-. and bpy on the basis 01 comparing the ELI v:~luc~ of [Rh(l.,-I_)((.‘O),]CIO, [IA]). Quite ob\~iousl~. \vhen the substitucnts on the pyrazolyl rings ;trc changed. the doncjr ability of the t&and is different from what woul~i tw cspectcd. Since donor ability can hc inferred from related structure\, WC decided to Lharactcriic at Icast two compounds with different substituents by .Y-rq cry+lllography and compare relatcc! structural features.
From Figs. 2 311~1 3 and Table 7. one can ohscrvc clearly that structure\ of both [W(H ,CPz; )(CO),] (6) and [W(~-l.Cl’z’~)(C‘O),] (71 arc LC‘Q similar to that of [M~(H,C’$Z~)((‘O)~] (8) ii,]. in having a six-mcmbercd boat metatlacyclc. formed from H >C’AI, and the metal atom, and two distorted c.i.\-carbonyls. I-lo~~~:c~i:r. if four different planes arc: &fined and calculated for 6-8, including two pyrazolyl-ring plane5 and two other ones (for example. IV. NC1 ). N(3) in ant: plane and (‘(81, N(2). N(3) in the other in 6 (Fig. 2)). different qtruc- tural features resulting from the methyl-group substitu- tion can be rccognizcd cvidcntly. Thcsc two planes form an angle of X2.‘)” in 6. S5.0’ in 7. and Sh. I’ in 8 white the two pyra~olyl-ring planes form an angle of
K.-B. Shiu et al. / N,N’-di(azol-I-yl)methanes 205 TABLE 3. Selected bond lengths (A) and angles (“) for 6 and 7 TABLE 3 (continued)
(a) Compound 6 (i) Bond lengths
W-C(l) 2.000(6) w-C(2) 1.935(5) W-C(3) 1.929(7) W-C(4) 2.015(6) W-N(l) 2.267(4) W-N(4) 2.270(4) C(l)-O(1) 1.149(7) C(2)-O(2) 1.170(7) C(3)-O(3) 1.178(8) C(4)-O(4) 1.157(7) C(5)-C(6) 1.393(9) C(5)-C(12) 1.484(8) C(5)-N(l) 1.328(7) C(6)-C(7) 1.368(8)
(ii) Bond angles
C(l)-W-C(2) 84.57(22) C(l)-W-C(S) 86.57(24) C(l)-W-C(4) 170.80(21) C(l)-W-N(l) 95.11(19) C(l)-W-N(4) 96.38(18) C(2)-W-C(3) 86.78(25) C(2)-W-C(4) 86.83(22) C(2)-W-N(l) 98.08(21) C(2)-W-N(4) 178.61(21) C(3)-W-C(4) 89.71(25) C(3)-W-N(l) 174.99(19) C(3)-W-N(4) 94.28(20) C(4)-W-N(l) 89.31(20) C(4)-W-N(4) 92.28(18) N(l)-W-N(4) 80.85(15) w-C(I)-O(1) 173.9(5) w-C(2)-O(2) 177.1(5) w-C(3)-O(3) 175.7(5) w-C(4)-O(4) 173.9(5) W(6)-C(5)-C(12) 126.6(5) C(6)-C(S)-N(1) 111.6(S) C(12)-C(5)kN(l) 121.8(5) C(5)-C(6)-C(7) 106.1(5) C(5)-C(6)-C(13) 126.5(6) C(7)-C(6)-C(13) 127.4(6) (h) Compound 7 0 Bond lengths W-N(l) 2.252(6) W-N(2) 2.270(5) W-C(l) 1.937(8) W-C(2) 2.018(7) W-C(3) 2.036(7) W-C(4) 1.966(7) N(l)-N(3) 1.375(7) N(l)-C(12) 1.339(9) N(2)-N(4) 1.367(8) N(2)-C(22) 1.347(9) N(3)-C 1.441(9) N(3)-C(14) 1.347(9) N(4)-C 1.432(9)
(ii) Bond angles
N(l)-W-N(2) 78.35(19) N(l)-W-C(l) 177.9(3) N(l)-W-C(Z) 97.91(24) N(l)-W-C(3) 93.7(3) C(6)-C(13) C(7)-C(14) ‘X-N(2) C(8)-N(2) C(8)-N(3) c@-c(10) C(9)-C(15) (X9)-N(4) c(1o)-c(ll) C(lO)-C(16) C(ll)-C(17) C(ll)-N(3) N(l)-N(2) N(3)-N(4) C(6)-C(7)-C(14) C(6)-C(7)-N(2) C(14)-C(7)-N(2) N(2)-(X-N(3) c(1o)-c(9)-c(15) C(lO)-C(9)-N(4) C(15)-C(9)-N(4) c@-c(10)-c(11) C(9)-C(lO)-C(16) C(ll)-C(lO)-C(16) c(1o)-c(ll)-c(17) C(lO)-C(ll)-N(3) C(17)-C(ll)-N(3) W-N(l)-C(5) W-N(l)-N(2) C(5)-N(l)-N(2) C(7)-N(2)-C(8) C(7)-N(2)-N(1) C(X)-N(2)-N(1) C(8)-N(3)-C(l1) C(S)-N(3)-N(4) C(ll)-N(3)-N(4) W-N(4)-C(9) W-N(4)-N(3) C(9)-N(4)-N(3) N(4)-C(24) C(l)-O(l) C(2)-O(2) C(3)-O(3) C(4)-O(4) C(ll)-C(12) C(12MX13) C(13)-C(14) C(14)-C(15) C(21)-C(22) C(22)-C(23) C(23)-C(24) C(24)-C(25) C-N(3)-C(14) N(2)-N(4)-C N(2)-N(4)-C(24) C-N(4)-C(24) 1.491(8) 1.493(8) 1.351(7) 1.434(7) 1.441(6) 1.395(7) 1.492(S) 1.335(6) 1.356(S) 1.493(7) 1.479(8) 1.342(6) 1.390(6) 1.370(6) 131.0(5) 106.9(5) 122.1(5) 112.4(4) 127.9(5) 110.4(5) 121.7(5) 106.1(4) 125.9(5) 128.0(5) 129.7(5) 107.4(4) 122.9(5) 133.7(4) 121.7(3) 104.3(4) 130.1(4) 111.2(4) 1 l&7(4) 130.2(4) 118.5(4) 111.2(4) 132.5(3) 122.2(3) 104.9(4) 1.360(9) 1.178(10) 1.144(9) 1.131(9) 1.134(9) 1.442(9) 1.407(10) 1.375(10) 1.502(10) 1.486(11) 1.394(11) 1.359(11) 1.492(12) 128.8(6) 119.6(5) 110.7(5) 129.7(6) N(l)-W-C(4) N(2)-W-C(l) N(2)-W-C(2) N(2)-W-C(3) N(2)-W-C(4) C(l)-W-C(Z) C(l)-W-C(3) C(l)-W-C(4) C(2)-W-C(3) ‘X2)-W-C(4) C(3)-W-C(4) W-N(l)-N(3) W-N(l)-C(12) N(3)-N(l)-C(Q) W-N(2)-N(4) W-N(2)-C(22) N(4)-N(2)-C(22) N(I)-N(3)-C N(l)-N(3)-C(14) 93.8(3) 101.1(3) 91.75(24) 94.6(3) 171.7(3) 84.1(3) 84.3(3) 86.9(3) 167.7(3) 86.7(3) 88.5(3) 121.6(4) 132.7(4) 105.7(5) 121.1(4) 132.5(5) 105.4(5) 119.3(5) 111.5(5) N(3)-C-N(4) w-c(1)-0(1) w-C(Z)-O(2) w-C(3)-O(3) w-C(4)-O(4) N(l)-C(12)-C(11) N(l)-C(12)-Cc131 C(ll)-C(12)-Cc131 C(12)-C(13)-C(14) N(3)-C(14)-C(13) N(3)-CU4)-C(15) C(13)-C(14)-C(15) N(2)-(X22)-C(21) N(2)-C(22)-C(23) C(21)-C(22)-C(23) C(22)-C(23)-C(24) N(4)-C(24)-C(23) N(4)-C(24)-C(25) C(23)-C(24)-C(25) 110.7(5) 173.7(6) 171.7(6) 170.4(7) 178.7(7) 123.5(6) 109.5(6) 127.0(7) 106.7(6) 106.5(6) 122.7(6) 130.8(7) 122.2(7) 110.2(6) 127.6(7) 106.4(6) 107.3(7) 123.3(7) 129.4(7)
120.8” in 6, 68.2” in 7 and 68.1” in 8. Apparently, the boat conformation does not change much whether the central metal atom is tungsten or molybdenum. This feature is to be expected from the similarity in the radii of the two atoms because of the lanthanide contraction [15]. However, when the hydrogen atom on the ring-4 position of Pz’ is replaced by a methyl group, the conformation changed greatly as observed in various angles for 6. We found that the net result from this conformational modification is best exemplified by one angle, formed by two cis-carbonyl groups and the cen- tral metal atom, which is 170.80(21)” in 6, 167.7(3)0 in 7, or 167.3(l)” in 8. As observed in Table 3, either boat conformation resulting from different substituents has
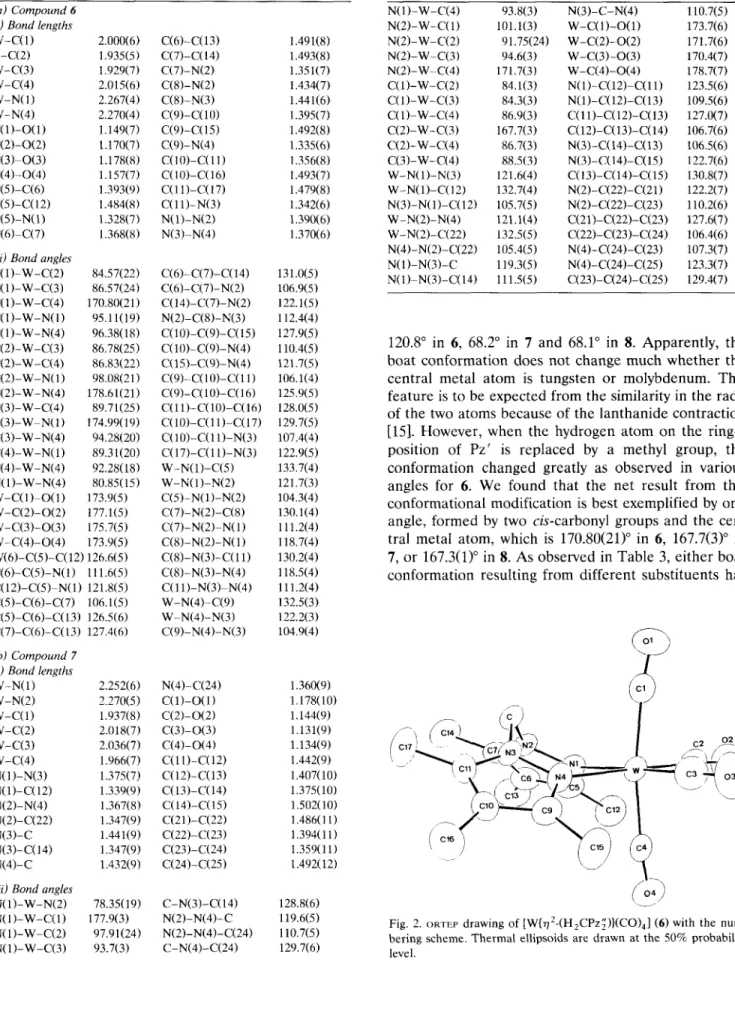
Fig. 2. ORTEP drawing of [W(~2-(H2CPz;))(CO),] (6) with the num- bering scheme. Thermal ellipsoids are drawn at the 50% probability level.
_t)( K -/:L ~,/I~l~ u<, ~:/ %',,%' '-di(a::(# / vlbn<t/mm'~ 0 2
\
C 2 0 1 ~ Ci ( } 4 O4 C~/
I O:t (3 N1 ~ ( " M Clf'~ i,:s
/"
C21 ~ Cli/
(}11Fig. 3. o l ~ H r drawing of [W{r/-(lt ,('Pz" I}(('()) i] (7)\~[th lhc tittlll bering scheme. ] t ] c r m a t ellipsoids are diavrn .:it the: 5f!'; mJ~abiiib, lcvct. c a u s e d s o m e b o n d a n g l e s , e s p e c i a l l y t h o s c f o r r n c d by c a r b o n y | s in t h e m o l e c u l e , a t t h e c o r r e s p o n d i n g l o c a - t i o n s 1o d i f f e r s i g n i f i c a n t l y f r o m e a c h o t h e r : f o r e x a m -
plc. C(3)-W--C(4)
g 0 . 7 1 ( 1 5 ) ° in 6 a n d ( 7 ( 3 ) - V v - C ( ] ) 8 4 . 3 ( 3 ) ° in 7 ( q / . F i g s . 2 a n d 3), a n d i n f l u e n c e s ( p r o b - a b l y in a c o m p l i c a t e d w a y ) il'tc r c l c x a i l t < a r b o n y l s t r c t c h i n g f r c q u c n c i c s s o t h a i n o s m ] p l c l r c n d ip, t h c M ( . ' O ) v a l u e s c a n h e f o u n d , l t o w c a n ~ c e x p l a i n i h c S | l U C t U l a l c h a n g eobserved
a s m o : - c s u b s t i t u c n i s a r c a d d e d ' ? C o n s t r u c t i n g a m o d e l s h o w s clcal+l> t h a t n t m - b o n d e d r e p u l s i v e i n t e r a c t i o n s e x i s t i,,c~\~ccn ct~c <411~- s i t u e n t s e s p e c i a l l y a t t h e ring-.3 ( a n d r i n g - S t t ) ~ s i t i o l > o f tv<o p y r a z o l y l r i n g s as vvel! a', h ~ t c r a c t i ~ m s b c t w c c ut h e s u b s l i t u e n t s ill: i h c r i n g - 3 ( o r t h u g - ) ) m d a t t h e
ring-4 positions
of l h c >,aiBc p y r a z o l ? S :~h~g. t h : n c c , w h e n t h e 1 - c p l a C e l l l C t l t b y m e t h y l g f t ) t t p ~fl: l h c h y d r o - 1,211 a t o m s a t t h e r i n g - 4 p o s i t i o m , <fl t l : , ( Pz:, tlccLtl-!%repulivc
i n l c r a c t h m , ~ , h K ' r c a s c . Cl'Cil{ill~Z ct p,t)o!t2l L n v i - r o n m e n t f o r t h e n o n b o n d c d i n l c r a c l i o n , , bct\~,ecn i\v<~T/\BI t{ 4. ('arbonY1 ,;trctchin~ froqucucic<, i~r [ b l ( t l . . . . .( LAx, t ( ( X ) ) i" +,
" , - , < . . . X 7 . . .
~,,]
...i 7 [
; < 2 . . .77
Cr Pz / t t l n I Xu(~ ~,' X ~, _ iS-<7_ (Tr Pz' 2I)(t) 18~7 i;<67 i ~ ~1) Cr P;<" 2()( I<1 l 8S 1 X(f.J i£27 =1,~4 INgh !x,? M o Px' 21 }l )6 I S<)3 t "<.72 ) >3{ Mo Pz" 2 0 i t I <'.;<)5 ]4h;t i ,<4YI Vv Pz 2(tl).1 J STcl ! ) X/7 W Pz' 2( II L'~ I ;-77 I ;46 :J I,~28 W [>z" I )( "~ ts77 Ig~)7 ISI/~'
tR ;i?7{}{,.7,,C~M<;~t] 2ii<;-.%dot ~ ,,,<t
~+l~ .c:t'~, ~!c<>7
,-L,:<7
HIC{LSLIFCd Ill MECH. 'Th<>c ol :ill o t h c l <tHllp]c:x¢', tkClt2 !]]cilbtllCd ill
(I1 X'I,. q' scc Icxi
n l c t h y l g r o u p s at l h o r i n p 3 ( o r r i n p - 5 ) p o s i t i o n s o f t w o d i f f e r e n t p y r a z o l ) J r h l g s in I t X£p:zi~. as i n d i c a t e d b ) t h e l a r g e i n g l c i l i ' i t , ~ ~ in 6. i o r m c d b } l ~ o p y r a z o l y l - r i n g p l a n e s c o m p a r e d , a i l h tt~osc ,>] c,<'4 2': i!l 7 '4nd 6 S . l ill 8.
I n c o l l c l t i s i o t l . {}I'. l ! c x i b i l i t v o f I h c ho<ll < ] x - l l l e l l l -
bored mcl',dllc)clc allow:.
~ t d j t i M I B C l ] t o l l h c c h c l a i c s t l t l C { t l r c {('~ H l i l l H I l i z c ( l i e llO]li~t'~i~.iot] : c p u l s i ~ c l l l l C ! i l C - l i o n s , l - h e M l u c i i ~ r l i l ! r i o c l { l i c 4 t b i i / h e n ailct-s l h c c l c c - I i O l ! tt.oIl'dli~!~L ~bili>, ,~> i~lltt i]o l c g t ! i m - i r c u d in c a r - I x m y t s l r c w h i n g \ i i l t l C ~- ltN { M i l l ( ' , \ z : ) ( ( ' O ) ~ ] is o h -%`21%'CtL {iS l]]clF<t F C p J L I C C I l l C I I [ t ) [ h_~diogc:~l itiOITIS b\'
n l e l h y l - g l o t ~ p , q l b s t J l t t c n i , , iLikes i ) l i i c c ~.,I l h o t ~ } l i i z o J k l r i n g , t h e c l c c l r . m <~iomllinb'_ M i l i I \ ol !I]i,: ~cxultin<4 l i e - {tI'lcJ t | , ( ' A 2 [ } ' i g . '~. disC:', 110t bcCt'qllC i}CCCSSHFi]\ ql'!tl1~2Cl i s 'A'ODIcI IIA\C !RkH{ OXI'ICCICd
A c k n o w l e d l l n l e n t
\ V c v+ish ic'~ l h : u ~ k t h e N a i i o n u l S c i e n c e ( ' o u n c i l o!: tilt., R e p u b l i c oH ( ' h i m ~ For f i n a n c i a l ' q i p p o r t o l t h i s i - e s e a r c t ! { C t m i r , l . c t N o , N ~ ( ' S I - ( l I ( ) R - M I ) ( ) 6 - 3 2 ) . R e f e r e n c e s ]1 i2 t3 i4 15
iuiS. 11 HimclIK~< ~ /u',u,. / & ; . i)<F'.]) l',?:{b)'D I m f i m c ! i k o .
Hr(<~ Imu%'. t / l v n : . :,J i9X6) 115
\ . R Kq~i / b ~ ' L i '\l~dc{ P,:ihm:m ) ) t I ca!>, :rod ( ) . . \ ~. Jcliil P %ahi ; ,'+,.i Nb,/,~, \I S;l+wi:io. t (),.ho;L .I. [!lgucto. i P. t:;,~<'! \ i ~ \q:Eiut / ! / , ' ~ / + n ~ / ¢ .:a'n~. /~ ( b S 2 )
i ! 4 1
( ] ( l . [ t i l I L i dlli] ] }iO!L!ij. l ( b ! ; U l c 3 ! ! / ! i t.!il't,;. {(1¢% (Jq,%l)) i['.i
~HhJ f C h ' ] ~. I l C ¢ . j r . d J]R: r<ciN
{J) (; ( ia~Hi <. /L:u.sm. sx c[~ ~md (i Mc,n,.mi. ln~,<.'. ~ 'h!n~ < hh,'.
,;// ( 9,',0 I{) ~ ( b } X t t n m , I ;rod <' ( k c ~ h u ! ,I (><k'a/t~;,l~( i'/i,'m 2#? {i<~5) i<:
( ~win, . % / ) ; , ? ~ / i ;o !l~SO) ' "
t ) . t S Ni',.cl tl d ) %ho:l/ni/a/ ~u: >/ I" .'Qu~,,H ~ ( om/.,<uu,'d~.
M~(M~,~ Bbli. ',,,~ "..,k i'm"
~g',t. FtNIN~,>; \ .f l i t { !i:licii! \ ktlDCIs. [ \~ .(7 %milh i N K ] - \ . R
l;dchci;. [ ,,,.!{', . ./~cl/~un~ , i /%o+,i~n' ()t.'..'~oJ, (/.'~m;,/~t all/! , ' J . i(]llgnllii',.. NO'> '~lik ig<xU I; 74{',!
1£. J~ h~,!itl . R ( 7 ('hlt~: / ( / u ( i : ' q % u {[}U/>viL %7 tiO~7) !~}-
1!~,. cxmupk~. ~,.>, (.,.J R \~ tLdk. I) t S'<ulkc]> aim .\. Oskam. hz+n~ < i i n ' R!~: 2'; t ieT"<) i.L:,: (b} ~ClCIClic:L' 4: (c) \I.N.
AckclIILmn, (. R l_<uh;! . ( J !)cxxknc. } M $pcchi. S/,. Kcdl!. \%. [{i. !',dN,.'ibc tilhi i i Ix.inl /n<<,~' i i:'m< .?h' t b),%)) 39 ? <1) { .\ (~lltu! ira! t S K~fitm]vcl. ] t i l t ( / n ' m 5<}(., <',4
CI()(32) l-![i,/k (i , t 7'> } ' [ l , ! { } t ; l l I l c ;ll]¢J i \ ( Hh~Q. IH<n'V ('A'CSN _7
\ l l i . ( hP;holm 1 ~, ( ~ n m u . t.( I lulhp, ali [ . ~ 1 Kobc~ amt ('.
(Xc~tom /n(,l,~ ('i~u., 27 ![0S4) 22o&
[, \, ()~c< X ] [])dCIX~I~ RX<I. ( [ i l i ; i l N ! i ! l ! . J. l{JOllclo. ( l ( ) C m ~ -
}:<c'o,;nd f l] ,.<.:r~, i (b~um,'mc; t h,'l*:. 25% t]0S4)71.
I S . BLI!Ic! i4cl I J i ! m ~ o d ( t d . ) /m,<~.uum ( /u'm',,9~. ]Jcniav, lln

![Fig. 3. o l ~ H r drawing of [W{r/-(lt ,('Pz" I}(('()) i] (7)\~[th lhc tittlll bering scheme](https://thumb-ap.123doks.com/thumbv2/9libinfo/8681248.197206/6.864.45.388.93.358/fig-drawing-w-pz-lhc-tittlll-bering-scheme.webp)