~
PergamonPII: S0277-5387(97)00367-7
Polyhedron
Vol. 17, No. 4, pp. 449 455. 1998 :( 1998 Elsevier Science Lid All rights reserved. Printed in Great Britain 0277 5387/98 $19.00+0.00Reinvestigation of the crystal structure and
cryomagnetic behaviour of copper(ll) propionates
Y. H.
C h u n g , aH. H. W e i ) * Y. H.
L i u , bG. H
L e e band Yu Wang ~
~ Department of Chemistry, Tamkang University, Tamsui, Taiwan
b Instrumentation Center, College of Science, National Taiwan University, Taipei, Taiwan
(Received l0 April 1997; accepted 3 September 1997)
Abstract--Two different complexes of copper(II) propionates, [Cu2(C2HsCO2)4] (1) and Na[Cu4 (C2HsCO2)9(H20)3] (2) have been isolated from the reaction of Cu(II)(NO023H20 with propionic acid in an aqueous solution of NaOH. X-ray structural analysis revealed that complex 1 consists of a one-dimensional chain of an apical-to-basal C u - - O - - C u linkage of pairwise binuclear anhydrous cupric propionates, which agrees with results previously reported. Compound 2 consists of a cation Na + which interacts with six oxygen atoms of carboxylate groups, and anionic
[Cu4(CzHsCOz)9(H20)3 ]
ion, which contains one isolated syn syn- carboxylato dimeric copper(lI) complex [Cu2(C2HsCO2)4(H:O):], and a linear chain containing one syn syn- carboxylato dimeric [Cu2(C2HsCO2)4] and its apical position linked by a carboxylate bridge with a unit of [Cu(C2HsCO2)(H20)]. Cryomagnetic susceptibilities 1 and 2 have been measured over the temperature range 4~300 K. In 1, the spins are coupled through an alternating one-dimensional antiferromagnetic interaction. In 2, the cryomagnetic exchange interaction has been interpreted in terms of a strong antiferromagnetic interaction associated with the two paired dinuclear units of C u ( I I ) . . . C u ( I I ) , and one mononuclear Cu(III unit which complies with a Curie Weiss law. (() 1998 Elsevier Science Ltd. All rights reservedKeywords:
crystal structure: magnetic property: copper complexes: polynuclear complexes: propionate complexes.The chemistry of the copper complexes with various carboxylates has been investigated for long time. A large number of binuclear copper(II) carboxylate adducts, [Cu(RCO2)L]2, have extensively been inves- tigated in an effort to clarify the factors influencing the
magnitude of the intramolecular magnetic exchange interaction which occurs between two Cu(II) ions in
these compounds [1 7]. In m o s t [Cu(RCO2)2L]2
complexes, two copper(lI) atoms are bridged in pairs by four
syn-zvn-carboxylato
groups with two additional unidentate ligand (L) occupying the apical position.Relatively few studies have been reported on the
magneto-structural corrections in the non-adduct complex of copper(II) carboxylates such as
[Cue(RCO2)4] [4]. Although an X-ray crystallographic structure [8] and cryomagnetic data [9] of the dimeric
* Author to whom correspondence should be addressed.
copper(II) propionate [Cu2(C2H5CO2)4] have been reported in the past, the precise cryomagnetic behav- iour, especially with regard to the X-ray structural data for this compound has, however, been rather controversial.
The contrasting structures of dimeric adduct [Cu2(RCO2)aL2], and polymeric non-adduct [Cu2(RCO~)4] prompted us to reinvestigate in par- ticular the cryomagnetic behaviour of the non-adduct copper(II) propionate [Cu2(C2H~CO2)4] compound. However, in the course of our synthetic inves- tigations of the copper(II) propionic acid system, the products of a blue-green complex [Cu2(C2HsCO2)4] (1) and an emerald-green species Na[Cu4(C2HsCO2),(H20)3] (2) are isolated from the mixted aqueous solution of Cu(NO3)2" 3H~O, pro- pionic acid, and N a O H depending on their isolation conditions. The preparation, structure, EPR, and cryomagnetic behaviours of these two compounds
are described in this paper. 449
450
E X P E R I M E N T A L
Preparation o f [Cu2(C2HsCO2)4] (1) and Na[Cu4 (C2HsCO2)9(H20)3] (2)
In the earliest report [9], unhydrated copper(II) pro- pionate was prepared from excess of the diluted acid and copper carbonate and then dehydrated at 100°C over posphoric oxide. Here we modified the procedure as following: propionic acid (10 mmol) was dissolved in 50 cm 3 of water, and the resulting solution was adjusted to pH = 5.8 with ca 100 cm 3 of 0.25M NaOH. To this solution 25 cm 3 of aqueous Cu(NO3)2" 3H20 (60 mmol) was added under stirring. The resulting blue-green solution was allowed to stand in air at room temperature for one week and the earlier blue-green crystals of the compound 1 were filtered, after which the filtrate was concentrated (ca 3 weeks) to one third of its volume and the emerald-green crys- tals suitable for X-ray analysis of compound 2 were collected, washed with water, and air-dried at room temperature. Anal. Calc. (found) for C~2H20Cu208 (1) : C, 33.40 (33.48) H, 4.60 (4.62)%. IR(cm-~) : 1628 (s), 1590, 1556 (s). Calc. (found) for C27H51Cu4NaO2~ (2): C, 32.77 (32.56); H, 5.16 (5.21)%. IR (cm-~): 1619 (s), 1592 (s), 1540.
measured at 25°C on an Enraf-Nonius CAD4 diffractometer by using monochromated Mo-K~ radi- ation. Crystal parameters and the selected data of collection and refinements of the complexes are sum- marized in Table 1. The structure was solved by heavy- atom method and subsequent difference Fourier maps, followed by full-matrix least-squares refinement based on F with the NRCVAX computer program [10]. The hydrogen atoms were placed at calculated positions with isotropic thermal parameters.
Physical measurements
Infrared spectra were recorded on a Bio-Rad FTS- 40FTIR spectrophotometer as KBr pellets in the 400ff400 c m - ~ region. X-band EPR spectra at 300 K for the complexes were recorded on a Bruker ECS-106 spectrometer. Temperature dependence of magnetic susceptibilities of the polycrystalline sample were mea- sured between 4.2 and 300 K at a field of 1 T using a Quantum Design Model MPMS computer-controlled SQUID magnetometer. Diamagnetic corrections were made using Pascal's constants [11].
RESULTS AND DISCUSSION X - r a y crystallography Synthesis and structural characterizations o f 1 and 2
Intensities and lattice parameters of the blue-green The reaction of Cu(NO3)2" 3H20 and NaOH with crystal of 1 and the emerald-green crystal of 2 were propionic acid in aqueous solution, on the time of
Table 1. Crystallographic data for compounds 1 and 2
1 2
Formula CI2HzoCu208 C27HsICU4NaO21
Formula weight 419.37 988.85
Size, mm 0.05 × 0.13 × 0.40 0.40 x 0.50 x 0.50
T (K) 298 298
Crystal system Triclinic Monoclinic
Space group PT P21/n a, (A) 5.1846(19) 13.470(6) b, (,~) 8.4373(16) 26.370(14) c, (A) 9.523(3) 13.540(6) ct, C) 91.732(20) fl, (°) 91.79(3) 114.06440(0) 7, (°) 105.06(3) V, .A) 401.76(21) 4391(3) Z 1 4 D,., gcm 3 1.73 1.496 F(000) 215 2038 2[Mo K~],/~, 0.7107 0.7107 #, mm ~ 26.875 19.789 N 1415 6019 No (I > 2a(/)) 1235 3942 R ~ 0.024 0.046 Rw h 0.023 0.050
crystal growing, deposited the initial product blue- green crystal 1 from the mixed solution, and then deposited the emerald-green compound 2 after con- centration of the solution.
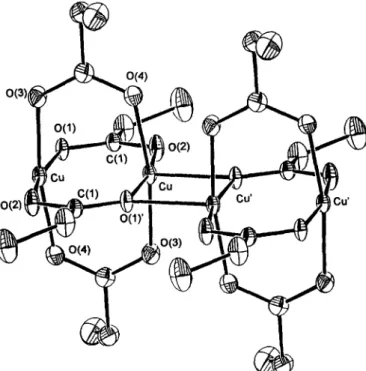
The crystal structure of 1 is in good agreement with that previously reported [8] which shows the structure of the polymeric chain of
[Cue(CzHsC02)4]
(as shown in Fig. l). Thesyn-syn
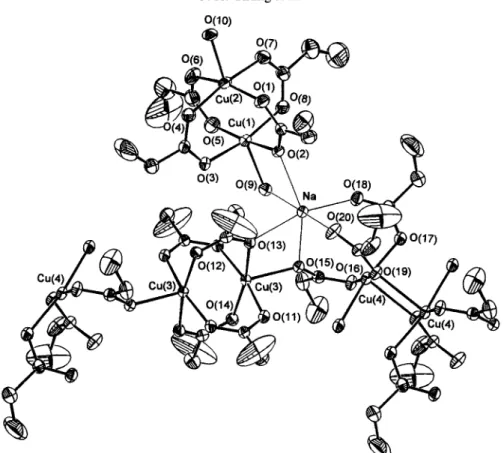
propionate bridged Cu(II) dimer is self-assembly oxygen-bridged (apical) to the nearest dimers. This bridging arrangement gives rise to a C u . " Cu' (apical) distance of 3.2442(3) A, sub- stantially longer than C u " . C u of 2.5826(12) A associated with the Cu2(C2HsCO2)4 core. There was no doubt that the original C u . ' - C u distances of 2.578(4) A [8] are not significantly different from our refinement (2.5826(12) A). The original C u - - O (basal) distance of 1.94 A is shown to be somewhat shorter than the 2.0024(19) A from our refinement, whereas the present C u - - O (apical) distance of 2.2259(19) A is somewhat shorter at 2.28(1) A in the original struc- ture. The most important part of the previous deter- mination was the geometry of their bridging propionate groups, the mean O ~ C - - O angle (113) was clearly quite different from our refinement value of 124.2.The crystal structure of 2 is shown in Fig. 2. Rep- resentative bond distances and angles are compiled in Table 2. The structure is composed of three units of formula
Na[Cu4(C2HsCO2)9(H20)3]
(2); (i) the dis- crete hydrated binuclear[CH2(C2H5CO2)4(H20)2 ] (a),
where two Cu(II) atoms are bridged by four pro- pionate groups in
syn-syn
conformation; (ii) Thebridged Cu(1)- • • Cu(2) distance, 2.5777(16) A, which is close to that of 1 or to that of the well-known copper(I1) acetate monhydrate dimer [12] and (iii) the polymeric chain of [Cu4(C2HsCO2)5(H20)] moiety, which consists of one unhydrated copper(II) pro- pionate [Cuz(C2HsCO2)4] dimer (b), and its apical pos- itions which are bridged by two additional
syn-anti-
propionate groups [O(15)---C(19)--O(16)] from the axial position of the central copper(II) [Cu (C2HsCO2)2(H20)] (c) moiety. In c, the Cu(4) atom is coordinated by one oxygen atom [O(16)] from the bridged
syn anti-propionate
group, two oxygen atoms of O(17) and O(19) from monodentate propionate groups, one oxygen atom O(21) from an aqua ligand, and one oxygen atom O(19) from the neighbour c moiety, thus forming a distorted square pyramidal structure. In addition, the oxygen O(19) is self- assembling and bridged to the next Cu(4) of the neigh- bour c unit. These bridged C u . . - C u distances for C u ( l ) . . - C u ( 2 ) , C u ( 3 ) " . Cu(3), and C u ( 4 ) - . . C u ( 4 ) in a, b, and c moieties are 2.5777(6), 2.6092(20), and 3.2146(20)/k, respectively.Finally, it is noteworthy that Na + cation is six- coordinated with six oxygen atoms [0(2), 0(9), O(13), O(15), O(18), and 0(20)] of the subunits of a, b, and c, respectively, as shown in Fig. 2. The mean N a - - O interatomic distance is 2.40/k.
Magnetic properties
The room-temperature X-band (9.8 GHz) EPR spectra of the samples of 1 and 2 show a broad signal
452
o(lo)
0(6)
oc,)
\
) o(17)
Cu(4)
Fig. 2. ORTEP stereoview of 2 (30% probability thermal ellipsoids).
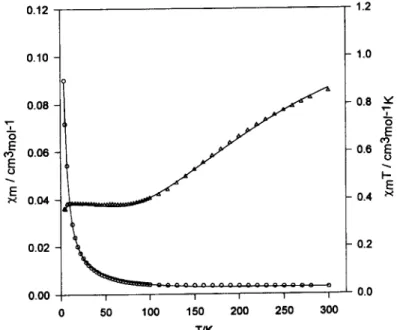
with a pronounced hump at 9 = 2.07 for 1 and 2.05 for 2 respectively. The results of the temperature dependence of magnetic measurements for [Cu2(C2HsCO2)4] 1 and Na[Cu4(C2HsCO2)9(H20)3] 2 are illustrated in Figs 3 and 4, respectively.
The solid lines in Fig. 3 of compound 1 represent a best fit of data. As already mentioned in the molecular structure section, the bond distance of the two cop- per(II) ions C u . . . Cu (basal) is shorter than that of the self-assembly oxygen-bridged C u ' . . . Cu' (apical) in this polymeric chain. Thus there are two magnetic interactions present in the system. Consequently, the data were analyse by use of an analytical expression for the exchange interaction in an alternating chain of S = 1/2 ions developed by Hatifield [13,14] which has the following form :
)~m = Ng2u~[(A + Bx+C~)/(1 + Dx+ EZ~ + F3)] (1) with x = J/kT. The coefficients A - F are functions of the alternation parameter ~ defined by the Ham- iltonian
n/2
H = - 2 J ~ [ $ 2 , $ 2 , , +~S2iSz,+,] (2)
i = 1
where J and ~J are the coupling constants of C u . . . Cu and C u ' " C u ' respectively. The data fit of this expression resulted (the solid lines in Fig. 3) in
J = - 2 2 0 cm-~, 9 = 2.07 (from EPR) and the alter- nation parameter 7 = 0.10. It is noteworthy that the original reported 2J value of 300 c m - 1 for compound 1 [9], obtained from the simple Bleaney-Bowers equa- tion of a two spin system with H = 2JS~$2, is smaller than that of 2J obtained by us.
The cryomagnetic susceptibility of compound 2 is illustrated in Fig. 4, in the form of gm(4Cu) and Zm T vs T plots. Upon lowering the temperature from 300 K to about 90 K, the ZmT value decreases from 0.86 cm 3 mol -~ K (#e~ = 2.61 #B) to a value of 0.39 cm 3 mol ~ K(#en = 1.75 /zB) indicating a strong anti- ferromagnetic interaction, whereas on reaching an extended plateau between 90 and 8 K, the values of Zm T remain practically constant, and finally decreases below 8 K. In view of the crystal structure of 2, mag- neto-structural correlation is complicated in the pre- sent system, nevertheless, the shorter distance between copper(II) atoms with bridging a syn-syn carboxylate dimer, the exchange coupling is always larger and antiferromagnetic in nature [4]. Clearly, as mentioned above in the structural analysis, this strong anti- ferromagnetic interaction should occur between C u ( 1 ) " ' C u ( 2 ) and C u ( 3 ) ' " C u ( 3 ) . The magnetic interaction between the self-assembly oxygen-bridged Cu(4).. • Cu(4) is also rather smaller in compound 1. These become virtually diamagnetic at low tem- perature and Cu(4) dominates the magnetic response
Table 2. Selected bond distances (A) and bond angles (~) of 1 and 2 453 Compound 1 Cu--Cu 2.5826(12) Cu--Cu' 3.2442(13) Cu--O(1) 2.0024(19) Cu--O(2) 1.9632(20) Cu--O(3) 1.9378(21) Cu---O(4) 1.9330(21) Cu--O(l)' 2.2259(19) O(1)--Cu--O(2) 169.56(7) O(3)--Cu--O(4) Cu--O(l )--Cu' 100.08(8) O(1)~C(1)--O(2) O(3)--C(4)--O(4) 124.9(3) Cu---O(2)--C(1) Cu(--O(1)--C(I) 124.41(17) C u ' - - O ( 1 ) ~ ( 1 ) Compound 2 Cu(l)---Cu(2) 2.5777(16) Cu(3)--Cu(3) Cu(4)--Cu(4) 3.2146(20) Cu(1)---O(2) Cu(1)---0(3) 1.958(5) Cu(l)--O(5) Cu( 1 )--0(9) 2.175(5) Cu(2)--O(4) Cu(2)--O(6) 1.982(7) Cu(2)---O(7) C u(2)--O(10) 2.102(6) Cu(3)--O(l 1 ) Cu(3)--O(12) 1.987(5) Cu(3)--O(l 3) Cu(3)--O(15) 2.120(5) Cu(4)-4)(16) Cu(4)--O(17) 1.969(5) Cu(4)--O(19) Cu(4)~(19) 2.299(5) Cu(4)--O(21) Na--O(2) 2.420(6) Na--O(9) Na--O(13) 2.387(6) Na--O(15) Na--O(18) 2.301 ((6) N a--O(20) O(3)--Cu( 1 )--0(8) 167.39(23) O(2)--Cu( 1 )---0(9) 87.90(22) O(3)--Cu(1 )--0(9) 96.02(22) O( 1 }--Cu(2)--4)(4) 91.5(3) O(I)--C(1 )--0(2) 124.3(7) O(5)--C(7)--O(6) 125.4(9) O(11 )--Cu(3)--4)(12) 168.85(21) O( 11 )--C( 13)--0(12) 124.4(7) Cu(3)--O(15)--C(19) 134.4(5) Cu(4)--O(16)--C(19) 112.3(5) O(17)--Cu(4)--O(21) 169.85(21) 169.69(8) 123.5(3) 122.40(18) 135.31(17) 2.6092(20) 1.953(5) 1.929(6) 1.947(6) 1.932(6) 1.989(5) 1.968(5) 1.934(5) 1.943(5) 2.043(6) 2.591(7) 2.428(6) 2.294(6) O(2)--Cu(1)--O(5) 174.0(3) O(5)--Cu(1)--O(9) 98.0(3) O( 1 )--Cu(2)--O(6) 164.6(3) O(4)--Cu(2)--O(7) 171.0(3) O(3)--C(4)--O(4) 125.5(8) O(7)---C(10)---0(8) 125.2(8) O(13)--Cu(3)--O(14) 168.77(21 O(13)--C(16)--O(14) 125.1(7) O(15)--C(19)--O(16) 122.4(7) Cu(4)--O(19)--Cu(4) 98.29(19) O(17)--C(22)--O(I 8) 125.1(7)
of the compound 2. Accordingly, we attempted to fit the cryomagnetic data, however, an appropriate known theoretical model is not available, and after we tested a variety of models, the only one that gave a reasonable data fit involved two different dinuclear centres and one mononuclear centre in equal pro- portions, with different exchange constants. The mag- netic susceptibility can be described by eqn (3),
)~m = X~ + ( 1 / 2 ) Z 2 + ~ 3 + N ~ (3) where X,, ~2, and Z3 are the temperature-dependent magnetic susceptibilities for the units of Cu(1)...Cu(2), Cu(3)...Cu(3), and Cu(4) respec- tively, and N~ is the temperature-independent sus- ceptibility. Assuming that the Cu(4) unit conforms to the Curie-Weiss law, with Weiss constant 0 as an intermolecular exchange interaction, then Z3 =
C/(T--0).
The sum of the exchange Hamiltonian of ZI and Z2 is H =-2JIZSIS2-2J2~,StS 2
whereS, = $2 = 1/2, has the Bleaney-Bowers expression (4) [15]
Z, + (1/2)2: =
(2NgZ#~/kT){[3
+exp ( -2J,/kT)]
+ 1/213 + exp ( -
2J2/kT)]-
'} (4) where J~ and dr2 are the exchange interaction constants associated with Cu(1)...Cu(2) and Cu(3)...Cu(3) units respectively. With expression (3), and letting Jl > J2 [2-4], the best fit for 2 resulted in J, = - 2 2 5 c m - ' , J2 = - 160 cm-% # = 2.0, C = 0.39 cm 3 m o l - ' K, 0 = - 0.49 K, and N0~ = 60 x 10 -6 cm 3 m o l - ~. The solid line in Fig. 4 is calculated using the parameters derived above. The constant C value of 0.39 cm 3 m o l - ' K is close to the value of 0.38 cm 3 mol -~ K for the noncoupled one spin S = 1/2 system ; and the smaller 0 value of - 0 . 4 9 K indicates that a weak inter- molecular antiferromagnetic interaction contributes to the bulk susceptibility at low temperature.1.0 0.006 0.005 0.004
i
.003
E 0.002
0.001
0.000
0.9 0 50 100 150200
250
300
T/K
0.8 0.30.7 v
0.6 ~ 0.5 ~ I- 0.4 E 0.2 0.1 0.0 1.2Fig. 3. Thermal variation of the molar magnetic susceptibility for 1 in the form Xm and XmT vs T, the solid line is the best theoretical fit (see text).
0.12
0.10
1.0 v 0.08 o.8!
--o
E 0.06 0.6 ~ 0.04 ...0.4
0.02 ~
0.2
0 . 0 0 I I I I I I U , U0
50
100
150
200
250
300
TIKFig. 4. Thermal variation of the molar magnetic susceptibility for 2 in the form Zm and xmT vs T.
454 Y . H . C h u n g
et al.
Finally, it is of interest to note that to our knowl- edge, c o m p o u n d 2 is the first example of a polymeric copper(II) c o m p o u n d built from a discrete copper(II) carboxylate dimer, carboxylato-bridged polymic chain, a n d the six-coordinated sodium cation.
S U P P L E M E N T A R Y M A T E R I A L
Tables containing atom positions, anisotropic dis- placement parameters, hydrogen atom location, a n d
b o n d lengths a n d angles have been deposited a n d are also available from the authors on request.
Acknowledgement--This
work was supported by a grant of National Science of Council of Taiwan (NSC86--2113-M032- 005).R E F E R E N C E S