O S W A L D B A U M G A R T N E R
1725
sind. Der mittlere C 1 - O Abstand und die O - C 1 - O
Winkel stimmen sehr gut mit jenen anderer Perchlorate
/iberein (Beveridge, Bushnell & Kirk, 1985; Dauter,
Hansen, Mawby, Probitts & Reynolds, 1985; Figgis,
Wadley & Graham, 1972; Garland, Le Marouille &
Spodine, 1985; International Tables f o r X-ray Crystal-
lography, 1968; Thompson, Calabrese & Whitney,
1985). Die Temperaturfaktoren der Perchloratsauer-
stoffatome sind relativ hoch. GroBe Beweglichkeiten
der Perchlorationen bis zu verschiedenen Disorder-
Ph~inomenen aufgrund der offensichtlich schwachen
Wechselwirkung der Perchlorationen mit den umgeben-
den Molekiilen sind auch in anderen Perchloraten zu
finden (Dauter et aL, 1985; Figgis et al., 1972; Garland
et al., 1985). Die Mobilit/it der C104-Gruppen ist in
[Fe(C3H7NS)6](C104) 2 wesentlich kleiner als in [Fe(C 3-
HTNO) 6](CIO4) 2 (Baumgartner, 1986), m6glicherweise
aufgrund der gr6f3eren Acidit/it der H-Atome der
Methylgruppen in der N/ihe der Perchlorationen im
D M T F und damit wegen der etwas st/irkeren D o n o r -
Akzeptor Wechselwirkung zwischen den Perchlorat-
sauerstoffatomen und den Liganden (kleinster C -
H . . . O Abstand: 3,39 A).
Herrn Doz. Dr. H. V611enkle danke ich f'tir wertvolle
Diskussionen, dem Interfakult~en Rechenzentrum der
Technischen Universit~it Wien f/Jr die Rechenzeit und
dem Fonds zur F6rderung der wissenschaftlichen
Forschung in Osterreich f/ir die finanzielle Unter-
st/itzung (Projekt-Nr. 2178).
Literatur
]]AUMGARTNER,
O. (1986). Z. Kristallogr. 174, 253-263.
BEWgtDGE, K. A., BUSHNELL, G. W. & KINK, A. D. (1985). Acta
Cryst. C41, 899-902.
DAUTER, Z., HANSEN, L. K., MAWaV, R. J., PRoma-rs, E. J. &
REYNOLDS, C. D. (1985). Aeta Cryst. C41, 850-855.
FIGGIS, B. N., WADLEY, L. G. B. & GRAHAM, J. (1972). Acta
Cryst. B28, 187-192.
GARLAND, M. T., LE MAROUmLE, J. Y. & SPODINE, E. (1985). Acta
Cryst. C41, 855-858.
GRrrZNER, G., LINERT, W. & GtrrMANN, V. (1981). J. lnorg. Nucl.
Chem. 43, 1193-1199.
International Tables for X-ray Crystallography (1968). Bd. III.
Birmingham: Kynoch Press. (Gegenw~tiger Verteiler D. Reidel,
Dordrecht.)
International Tables for X-ray Crystallography (1974). Bd. IV.
Birmingham: Kynoch Press. (Gegenw/irtiger Verteiler D. Reidel,
Dordrecht.)
KIRFEL,
A.
(1977). Acta Cryst.
B33,
2788-2790.
LINERT, W., GUTMAr~, V., BAUMGARTNER, O., WIESINGER, G. &
KIRCHMAVR, H. (1983). Inorg. Chim. Acta, 74, 123-130.
MAIN, P., HULL, S. E., LESSINGER, L., GERMAIN, G., DECLERCQ,
J.-P. & WOOLFSON, M. M. (1978). MULTAN78. A System of
Computer Programs for the Automatic Solution of Crystal
Structures from X-ray Diffraction Data. Univ. York, England.
MOTHERWELL,
W. D. S. &
CLEGG,
W. (1978). PLUTO. Program
for Plotting Molecular and Crystal Structures. Univ. Cambridge,
England.
SHELDRICK,
G. M. (1976). SHELX76. Program for Crystal
Structure Determination. Univ. Cambridge, England.
STEWART, J. M., KRUGER, G. J., AMMON, H. L., DICKINSON, C. &
HALL, S. R. (1972). XRA Y72 System - Version Juni 1972. Tech.
Ber. TR-192. Computer Science Center, Univ. Maryland,
College Park, Maryland.
THOMPSON,
J. S.,
CALABRESE,
J. C. & WHn'NEY, J. F. (1985). Acta
Cryst. C41, 890-892.
Acta Cryst. (1986). C42, 1725-1731
Structure of Cun and C o II
Clusters of 7-Azaindolate: [Cu2(CTHsN2)4(dmf)2] (I),
[Cu4(OCH3)4(CTHsN2)4(dmD2] (II) and [C040(CTHsN2)6].CHCI3 (III)
BY SHIE-MING PENG*
Department o f Chemistry, National Taiwan University, Taipei, Taiwan and Institute o f Chemistry, Academia
Sinica, Taipei, Taiwan
AND Yn-NAN LtN
Department o f Chemistry, National Taiwan University, Taipei, Taiwan
(Received 7 April 1986; accepted 8 July 1986)
Abstract.
(I)
Tetrakis (7-azaindolato)bis (dimethyl-
formamide)dicopper (II),
[Cu2(C 7HsNe)4(C3HTNO)2],
M r = 741.84, monoclinic, P2~/n, a = 9.377 (6), b =
13.854(3),
c = 1 2 . 9 2 8 ( 4 ) A, f l = 9 4 . 1 0 ( 4 ) ° ,
V =
1675.07 A 3, z = 2, D m = 1.48, D x = 1.47 Mg m -3,
* To whom correspondence should be addressed.
0108-2701/86/121725-07501.50
gt = 1-318 mm -l,
2(Mo Ka) = 0.7093 A,
F ( 0 0 0 ) - -
764, final R = 0 . 0 4 4
for 1712 observations. (II)
Tetrakis(7-azaindolato)bis(dimethylformamide)tetra-
(methanolato)tetracopper (II), [Cu4(C 7HsN2)4(CH30)4-
(C3HTNO)2], M r = 993.01, triclinic, P i , a = 9.189 (3),
b = 9 . 5 1 1 ( 2 ) ,
c = 1 2 . 7 0 4 ( 2 ) A ,
a = 8 8 . 0 9 ( 2 ) , f l =
83.26(3),
) , = 6 6 . 7 5 ( 3 ) °,
V = 1 0 1 2 . 9 1 A 3, Z = l ,
© 1986 International Union of Crystallography
1726
Cu u A N D Co n CLUSTERS OF 7 - A Z A I N D O L A T E
D m = 1.63,
D x = 1.63 Mg m -3,
# = 2.135 mm -1,
2(Mo Ks) = 0.7093 A, F(000) = 508, final R = 0.041
for 4217 observations. (III) Hexakis(7-azaindolato)-
oxotetracobalt(II)-trichloromethane
(1/1),
[Co4(C 7- Cu
H5N2)6OI.CHC13, M r = 1073"90, triclinic, P i , a =
N
N(IA)10.165(3),
b = 1 0 . 3 8 9 ( 3 ) ,
c = 2 0 . 6 0 3 ( 3 ) A ,
a =
N(2A)
98.21 (2),
f l = 90-83 (2),
y = 96"04 (2) °,
V =
rq(IS)
N(2B)2140.71A a, Z = 2 ,
D m = l . 6 6 , D x = 1 . 6 7 M g m -a, o
# = 1.567 mm -I,
;t(MoKs) = 0.7093 A,
F(000) =
C(l)
c(211080, final R = 0.062 for 2846 observations. All three
c(3)
C(IA)
of the title compounds exhibit ligand disorder. In (I), the
c(zA)
Cu-Cu distance is 2.782 (2) A; C u - O 2.325 A. In (II) c(4A)
C(5A)
Cu ions occur in square-pyramidal [ C u - N 1.993 (2), C(6A)
Cu-O(methoxide) 1.939 (2), C u - O ( d m f ) 2.422 (3) A]
c(7A)
C(1B)and
square-planar
[ C u - N
1.966 (2),
C u -
c(2s)
O(methoxide) 1.926 A] coordination geometries. The c(3s)
C(4B)C u - C u distances, 2.999 (1) and 3.014 A, are long. In c(ss)
C(6B)(III), the C o - C o distance, 3.156 (1)A, is also long. c(7s)
The presence of superexchange magnetic interactions is
c(3A) (0.76)*indicated in (H) and (III).
c(3A') (0.27)*
Introduction.
Transition-metal complexes with un-
saturated electron-rich ligands are of interest, owing to
their unusual spectroscopic, magnetic, redox and
structural properties (Balch & Holm, 1966; Lauher &
Ibers, 1975; Peng & Goedken, 1976; Peng, Liaw,
Wang & Simon, 1985; Peng, Chen, Liaw, Chen &
Wang, 1985). The anion of 7-azaindole, which is similar
to the dpt (1,3-diphenyltriazene) and carboxylate ions,
has a potential capacity to coordinate on two metals to
form - M - L - M - links.
Ph... ~N-..NtPh
L = 7-azaindolate 1.3-diphenyltriszenateR
IM
carboxylateA series of complexes containing L
(Co4OL6,
Ni2L 4
and Cu2L4L' 2 complexes) has been reported (Brookes
& Martin, 1974, 1975a,b). The three title compounds
were prepared and their structures were determined in
order to study the extent of n-electron delocalization of
the 7-azaindolate anion and the metal-metal inter-
action in the metal clusters.
Experimental. Crystals of compounds (I), (II) were
obtained by mixing Cu(OAc)2 , 7-azaindole and meth-
anolic K O H in dimethylformamide (dmf) solution.
X-ray analysis: CAD-4 diffractometer. D m measured
by flotation. Absorption correction according to the
experimental tD rotation. For (I)" crystal size 0.10 x
0.10 x 0.30 mm, 20ma x = 50 °, - 1 1 _< h _< 9, 0 < k ___
16, 0 < l < 15. Unit-cell parameters from 25 reflections
( 1 8 < 2 0 < 2 5 ° ) .
Three standard reflections, 2944
unique reflections measured, 1712 reflections con-
sidered observed with I > 3tr(/). R = 0 . 0 4 8 , wR =
Table 1. Atomic positional parameters and equivalent
isotropic temperature factors of ICu2(C 7H5 N 2)4(dmf)2]
x
y
z
Biso(A 2)
0-38762 (6) 0.05328 (5) 0.03546 (5) 3.74 (2) 0.0151 (5) 0.2226 (3) 0.1554 (4) 4.7 (2) 0.4941 (4) -0.0489 (3) 0.8285 (3) 4.6 (2) 0.3083 (4) 0.0458 (3) 0.8884 (3) 4.2 (2) 0.4956 (5) 0-1726 (3) -0.0034 (4) 4.2 (2) 0.6834 (4) 0.0786 (3) -0.0622 (4) 4.4 (3) 0.2027 (4) 0.1448 (3) 0.0954 (3) 6.3 (3) 0.0845 (7) 0.1477 (4) 0.1247 (5) 5.8 (4) 0.0780 (8) 0.3146 (5) 0.1527 (6) 8.5 (6) -0.1264 (7) 0.2176 (5) 0.1912 (6) 7.7 (5) 0.5347 (7) -0.0900 (5) 0.7411 (5) 6.0 (4) 0.4498 (8) -0.0787 (5) 0.6506 (6) 7.6 (5) 0.2915 (8) 0.0046 (5) 0.7163 (5) 7.6 (6) 0.1519 (6) 0.0722 (5) 0.7440 (5) 6.2 (4) 0.1853 (6) 0.0877 (4) 0.8488 (57 5.2 (4) 0.3715 (57 -0.0009 (4) 0.8154 (4) 3.7 (3) 0.4486 (6) 0.2623 (4) 0.0046 (5) 5.6 (4) 0.5239 (7) 0.3406 (4) -0.0264 (5) 6.1 (4) 0.6535 (6) 0.3308 (4) -0.0696 (5) 5.9 (4) 0.7059 (6) 0.2417 (4) -0.0783 (5) 5.1 (3) 0.8307 (6) 0.1966 (5) -0.1144 (6) 6.4 (5) 0.8098 (6) 0.1002 (4) -0.1033 (6) 6.5 (5) 0.6208 (5) 0.1631 (3) -0.0448 (4) 3.7 (3) 0.3297 (10) -0.0313 (7) 0.6388 (8) 8.0 (4) 0.1999 (20) 0.0321 (14) 0.6678 (151 5.7 (7) * O c c u p a n c y factors o f d i s o r d e r e d atoms.0.044, S = 1.93 based on F. 218 variables refined.
w=I/[G2(Fo)+(O'O1Fo)2]. For
(II): crystal size
0.10 x 0.10 x 0. 30 mm,
20max=60 o,
- 1 2 < h <
12,
- 1 2
< k _< 13, 0 _< l < 17. Unit-cell parameters from 25
reflections (12 < 20 < 23°). Three standard reflections,
5885 unique reflections measured, 4217 reflections
considered observed with I > 2tr(/). R = 0 . 0 4 1 , wR
= 0.035, S = 3.11 based on F. 274 variables refined.
W = 1/[0"2(/'o) + (0.01Fo)2]. Structures were solved by
Patterson synthesis. H atoms found on difference
Fourier synthesis were fixed by least-squares process.
(A/tr)ma × =.0.13, peak on final difference Fourier map
< 0.51 e A -3 for (I). (A/G)max = 0"01, peak on final
difference Fourier map < 0 . 6 2 e A -3 for (II). Com-
pound (III) was prepared by mixing Co(OAc)2 ,
7-azaindole and K O H in MeOH solution. Crystal
(0.03 x 0.03 x 0.10mm) was obtained by dissolving
compound (III) in CHC13 solution followed by slow
evaporation. X-ray analysis: CAD-4 diffractometer.
D m measured by flotation. Absorption correction by tD
rotation.
20ma x = 50 °,
- 1 2 < h < 12, - 1 2 < k < 11,
0 < l < 24. Unit-cell parameters from 25 reflections
(14 < 2 0 < 21°). Three standard reflections, 7521
unique reflections measured, 2846 reflections con-
sidered observed with I > 20(/). R = 0 . 0 6 2 , wR =
0.0517, S = 2.04 based on F. 300 variables refined.
w = 1/[a2(Fo) + (0.01Fo)2]. Structure obtained by Pat-
terson synthesis. H atoms found on difference Fourier
synthesis
were
fixed
by
least-squares
process.
(A/a)max=O'02, peak on final difference Fourier map
< 0.66 e A -3. Atomic scattering factors calculated by
the
analytical
form
using
the
coefficients
in
International Tables f o r X-ray Crystallography (1974).
Programs from N R C C PDP-11 package (Gabe & Lee,
1981).
SHIE-MING PENG AND YII-NAN LIN
1727
Discussion. Atomic positional parameters and equiva-
lent isotropic temperature factors are listed in Table 1
for (I), Table 2 for (II), Table 3 for
(III).*
OR TEP
(Johnson, 1965) drawings and labeling schemes are
given in Fig. 1 for (I), Fig. 2 for (II), Fig. 3 for (III). The
bond lengths and angles are tabulated in Tables 4, 5, 6
respectively. All three structures suffer ligand disorder.
It was found that there were peaks ( 1 - 2 e A -3)
remaining between C(4) and C (5) atoms in some of the
ligands. They were assigned as C(3') atoms and the
occupancy factors of C(3) and C(3') atoms were
refined.
C(3L C(3') c(2} / ~c(4) c(s) c~5,) c(4,) ~ ~ c ( 2 ' )I
I
I
I
I
I
C17'1 C11'1C11'~
, ~ ( 7 ) / C ( 6 ) C(6')~ / ~ l N(1) N(2) N(2') N(I')Another indication of the disorder of the ligands is
the temperature factors of the atoms. N(1), N(2) and
C(7) atoms have Bis o values around 3 to 4 A 2 and the
remaining atoms of the ligand have higher B~s o values
(5-8 ./k 2).
* Lists of anisotropic thermal parameters, structure factors and H-atom coordinates have been deposited with the British Library Document Supply Centre as Supplementary Publication N o . S U P 4 3 2 3 0 ( 1 1 4 pp.). Copies may be obtained through The Executive Secretary, International Union of Crystallography, 5 Abbey Square, Chester C H 1 2 H U , England.
Table 2.
Atomic positional parameters and equivalent
isotropic temperature factors of
[Cu4(CTHsN2) 4-
(OCH3)4(dmf)2]
x y z Blso(A') Cu(1) 0.39871 (5) 0.07936 (5) 0.17520 (4) 2.93 (2) Cu(2) 0.29301 (5) 0.03420 (5) -0.03234 (4) 2.95 (2) O(1) 0.3387 (3) -0.0622 (3) 0.1024 (2) 3.6 (1) 0(2) 0.3039 (3) 0.1900 (2) 0.0542 (2) 3.5 (I) 0(3) 0.1629 (3) 0.1755 (3) 0.3027 (3) 5-5 (2) N -0.0541 (4) 0.1381 (4) 0.3762 (3) 4.2 (2) N(IA) 0.5275 (3) 0.7632 (3) 0.7841 (2) 3.1 (1) N(2A) 0.2844 (3) 0.8552 (3) 0.9001 (2) 3.5 (2) N(IB) 0.5297 (3) 0.9273 (3) 0.2739 (2) 3.2 (1) N(2B) 0.7702 (3) 0.8350 (3) 0.1559 (2) 3.3 (I) C(I) 0.0715 (5) 0.1115 (5) 0-3064 (3) 4.3 (2) C(2) -0.1023 (6) 0.2578 (5) 0.4539 (4) 6. l (3) C(3) -0.1579 (5) 0.0581 (5) 0.3727 (4) 5.7 (3) C(4) 0.3625 (5) -0-2129 (4) 0.1329 (4) 5.1 (3) C(5) 0.2592 (5) 0.3468 (4) 0.0350 (4) 5.0 (3) C(IA) 0.6100 (4) 0.6515 (4) 0.7121 (3) 3.6 (2) C(2A) 0.5501 (6) 0.5460 (5) 0.6900 (4) 5.6 (3) C(3A) (0.76)* 0.5781 (7) 0.4631 (6) 0-2705 (5) 5.8 (4) C(4A) 0.3442 (6) 0.6410 (4) 0.7958 (4) 6.0 (3) C(5A) 0-1659 (5) 0.6879 (5) 0-8698 (4) 5.7 (3) C(6A) 0.1580 (5) 0.8140 (4) 0.9228 (3) 4.2 (2) C(7A) 0.3902 (4) 0.7565 (4) 0.8278 (3) 3.0 (2) C(1B) 0.4747 (5) 0.9072 (5) 0.3723 (3) 4.1 (2) C(2B) 0.5714 (6) 0.8036 (5) 0.4380 (4) 5.7 (3) C(3B) (0.79)* 0.7141 (7) 0.7186 (6) 0.4180 (4) 5.0 (3) C(4B) 0.7699 (5) 0.7361 (4) 0.3250 (3) 5.0 (3) C(5B) 0.9500 (5) 0.6572 (5) 0.2548 (4) 5.5 (3) C(6B) 0.9220 (5) 0.7306 (4) 0.1613 (3) 4-0 (2) C(7B) 0.6844 (4) 0.8378 (4) 0.2492 (3) 2.9 (2) C(3A') (0.33)* 0.7500 (15) 0.4056 (14) 0.1908 (11) 4.6 (4) C(3B') (0.34)* 0.8917 (14) 0.6467 (14) 0.3340 (10) 4.6 (4)* Occupancy factors of disordered atoms.
The first, dimeric, structure, (I), which is similar to
that of [Cu2(OAc)4(H20)2], contains four 7-azain-
dolate bridges with two Cu ions and two coordinated
dmf molecules with Cu-Cu distance 2.782 (2)A,
C u - N bond length 2.003 (4)A, C u - O 2.325 (4)A.
The Cu-Cu
distance is longer than those of
[Cu2(dpt) 4] (2.40 A), [Cu2(OAc)4(H20)] (2.64 A), and
shorter than those of [Cu(aP)2(H20) 4] (2.95A),
Table 3.
Positional and thermal parameters of
[Co 4-
(C7HsN2)60].CHC13
X y zBiso(A 2)
Co(l) 0-1489 (2) 0-2413 (2) 0-1856 (1) 3.2 (1) Co(2) 0.9093 (2) 0.3634 (2) 0-2584 (1) 3.5 (1) Co(3) 0.0369 (2) 0-1272 (2) 0.3126 (1) 3.3 (1) Co(4) 0.1926 (2) 0-4082 (2) 0.3275 (1) 3.8 (1) O 0.0735 (8) 0.2844 (8) 0.2713 (4) 2.9 (2) N(IA) 0.0961 (12) 0.5212 (12) 0.3929 (6) 4.4 (3) N(2A) --0-1071 (11) 0.5085 (12) 0.3311 (6) 3.9 (3) C(IA) 0.1545 (17) 0.5791 (17) 0.4498 (9) 5.8 (4) C(2A) 0.0936 (19) 0.6709 (19) 0.4922 (10) 7.1 (5) C(3A) (0.8)* -0.0162 (23) 0.7016 (23) 0.4815 (12) 6.7 (6) C(4A) -0.0828 (17) 0.6525 (17) 0.4305 (9) 5.6 (4) C(5A) -0.2209 (18) 0.6553 (18) 0.3970 (9) 6.2 (5) C(6A) -0.2217 (16) 0.5695 (17) 0.3411 (8) 5.3 (4) C(TA) -0.0253 (15) 0.5578 (15) 0.3845 (8) 4.3 (4) NOB) 0.3154 (1 l) 0.3206 (11) 0.3795 (6) 4.0 (3) N(2B) 0.1737 (ll) 0-1245 (11) 0.3839 (6) 3.8 (3) C(IB) 0-4342 (16) 0.3834 (16) 0-4026 (8) 5.0 (4) C(2B) 0.5140 (19) 0.3355 (19) 0.4430 (10) 7.4 (5) C(3B) 0.4762 (20) 0.2257 (20) 0.4642 (10) 8. l (6) C(4B) 0.3662 (16) 0-1503 (16) 0.4460 (8) 4.7 (4) C(5B) 0.2962 (17) 0.0290 (18) 0.4547 (9) 6.2 (5) C(6B) 0.1804 (16) 0-0131 (16) 0.4160 (8) 5.2 (4) C(7B) 0.2823 (14) 0.2058 (14) 0.4005 (7) 3.3 (3) N(IC) 0.0197 (ll) 0.2483(11) 0.1118(6) 3.7(3) N(2C) -0.0948 (12) 0.4158 (12) 0.1692 (6) 4.2 (3) C(IC) 0.0420 (16) 0.1913 (16) 0.0516 (8) 4.8 (4) C(2C) -0.0141 (17) 0.2307 (18) -0.0007 (9) 6.1 (5) C(3C) (0.8)* -0-0785 (22) 0.3297 (21) 0.13000 (11) 5-8 (6) C(4C) -0.1057 (18) 0.3891 (17) 0.0545 (9) 6.1 (5) C(5C) --0.1835 (17) 0.5003 (17) 0.0837 (9) 5.9 (5) C(6C) -0.1709 (16) 0.5089 (16) 0.1500 (8) 5.4 (4) C(7C) -0.0579 (14) 0.3459 (14) 0.1133 (7) 3.6 (3) N(ID) 0.0643 (11) 0.9676 (11) 0-2496 (6) 3.5 (3) N(2D) 0-2046 (11) 1.0623 (11) 0.1749 (6) 3.5 (3) C(ID) 0-0176 (15) 0.8517 (15) 0.2662 (8) 4.5 (4) C(2D) 0.0579 (18) 0.7352 (18) 0.2365 (9) 6.1 (5) C(3D) 0.1420 (18) 0.7313 (18) 0.1873 (9) 6-3 (5) C(4D) 0.1935 (15) 0.8381 (15) 0.1676 (8) 4.2 (4) C(5D) 0.2833 (16) 0.8798 (16) 0.1201 (8) 4.8 (4) C(6D) 0.2859 (15) 1.0119 (15) 0.1259 (7) 4.0 (4) C(7D) 0-1489 (14) 0.9599 (14) 0.2008 (7) 3.2 (3) N(IE) 0-7524 (11) 0.2341 (l 1) 0.2663 (6) 3.6 (3) N(2E) 0.8527 (12) 0.1120 (12) 0.3417 (6) 4.4 (3) C(IE) 0.6362 (15) 0.2464 (16) 0.2354 (8) 4.7 (4) C(2E) 0.5189 (16) 0.1774 (16) 0.2492 (8) 5.1 (4) C(3E) 0.5182 (16) 0.0891 (16) 0.2911 (8) 5.4 (4) C(4E) 0.6282 (16) 0.0696 (16) 0.3234 (8) 4.6 (4) C(5E) 0-6674 (16) -0.0071 (16) 0.3700 (8) 5-1 (4) C(6E) 0.7997 (15) 0.0227 (15) 0.3792 (8) 4.5 (4) C(7E) 0.7454 (14) 0.1441 (14) 0.3071 (7) 3-6 (3) N(IF) 0.3069 (12) 0.5206 (12) 0.2758 (6) 4.6 (3) N(2F) 0.2952 (12) 0.3788 (12) 0.1730 (6) 4.7 (3) C(IF) 0.3713 (19) 0.6383 (19) 0.3049 (10) 7.1 (5) C(2F) 0.4639 (22) 0.7011 (22) 0.2702 (11) 9.1 (6) C(3F) (0.8)* 0.4893 (30) 0-6642 (30) 0.2165 (16) 11.0 (10) C(4F) 0.4433 (18) 0.5648 (18) 0.1823 (9) 6.7 (5) C(5F) 0.4465 (22) 0.4832 (22) 0.1088 (1 I) 9.5 (7) C(6F) 0.3515(19) 0.3765(19) 0.1126(9) 7.0(5) C(7F) 0.3440 (14) 0.4866 (14) 0.2151 (7) 3.5 (3) C(3A') (0.2)* 0.8370 (73) 0.6944 (73) 0.4395 (37) 3.9 (17) C(3C') (0.2)* 0.8381 (80) 0.4457 (81) 0.0274 (41) 5.1 (21) C(3F') (0.2)* 0.4929 (56) 0.5732 (56) 0.1350 (28) 1.5 (12) C 0.6572 (19) 0.9540(19) 0.1031 (10) 7.1 (5) CI(1) 0.6804 (7) 1.0956 (7) 0.0713 (4) 11.6 (6) C1(2) 0-6150 (6) 0.8242 (6) 0.0456 (3) 9.0 (4) C1(3) 0.7856(6) 0.9348(8) 0.1535(3) 11.1 (6)1728
C u llA N D
C o I1C L U S T E R S OF 7 - A Z A I N D O L A T E
[CUE(ad)a(H20)2]
(2.95 ,/~),
[Cu2(OAc)4(P-tol) 2]
(3.2 A) (Table 7). The direct magnetic interaction of
this complex is comparable to those in Table 7.
I •
C2 C3
Fig. 1.
ORTEP(Johnson, 1965) plot of the [Cu2(CTHsNz)4(dmt')2]
molecule with labeling scheme. The thermal ellipsoids are drawn
at the 50% probability level.
C 2 ~ N C3
01 Nt~ c2~ C3A(
C/d
;SA C58C2B
Fig. 2.
ORTEPplot of the
[ C u 4 ( C v H s N 2 ) 4 ( O C H 3 ) a ( d m 0 2]molecule
with labeling scheme, The thermal ellipsoids are drawn at the
50% probability level.
C3A "CIA~~~~i~
c2 a '-
I
c 3 F - * - ~
T ~ U / " N , ~
"~c4c
' Ct, F r ~ C~" C2CFig. 3. OR TEP plot o f the [ C o 4 ( C v H s N 2 ) 6 0 ] m o l e c u l e with labeling
s c h e m e . T h e t h e r m a l ellipsoids are d r a w n at the 5 0 % p r o b a b i l i t y
level.
Table
4.
Bond
Cu-Cu Cu-N(IB) N - C ( I ) N(IA)-C(IA) N(2A)-C(7A) N(2B)-C(6B) C(IA)-C(2A) C(4A)-C(7A) C(SA)-C(6A) C(2B)-C(3B) C(4B)--C(7B) Cu-N(IA) Cu-N(2B) N-C(2) N(IA)-C(7A) N(IB)-C(IB) Cu-Cu-N(1A) C u - C u - N ( I B ) C u - C u - O N(1A)--Cu-N(1B) N(IA)--Cu--O N(2A)-Cu-N(2B) N(l B)-Cu-N(2B) N(2B)-Cu-O C ( I ) - N - C ( 3 ) 2.782 2.021 1.303 1.345 1.319 1.367 1.377 1.439 1.384 1.380 1.435 2.012 1.984 1.405 1.328 1.325lengths
[Cu(C 7H5N2)4(dmf)2]
(A) and bond angles (o)for
(!) N(2B)-C(TB) (4) C(2A)-C(3A) (7) C(4A)-C(3A)
(7)
C(SA)-C(3A')
(6) C(3B)--C(4B) (7) C(5B)--C(6B) (9) Cu-N(2A) (8) C u - O (9) N-C(3) (8) N(2A)-C(6A) (7) N(1B)-C(TB) (4) O--C(1) (4) C(4A)--C(5A) (8) C(4A)-C(3A') (6) C(1B)-C(2B) (6) C(4B)--C(5B) 83.6 (1) Cu-Cu-N(2A) 87.0 (1) Cu-Cu-N(2B) 178.9 (1) N(1A)--Cu-N(2A) 89.3 (1) N(IA)-Cu-N(2B) 95.9 (1) N(2A)--Cu-N(1B) 90.7 (1) N(2A)-Cu-O 167.8 (1) N(1B)-Cu-O 100-2 (1) C ( I ) - N - C ( 2 ) 123.5 (5) C(2)-N-C(3) 1.335 (6) 1.30 (l)1.20(1)
1.24 (2) 1.337 (8) 1.359 (8) 1.994 (4) 2.325 (3) 1.438 (7) 1.358 (6) • 331 (6) • 197 (7) .67(1) • 09 (2) • 369 (8) -433 (8) 84.1 (1)80.8
(i) 167-6 (1) 89.5 (1) 88.0(1) 96.3 (1) 92-0 (1) 119.7(5)
116.8 (5) Cu-N(IA)--C(IA) 123.4 (3) C(1A)-N(IA)-C(TA) 113.6 (4) Cu-N(2A)-C(7A) 123.4 (3) Cu-N(IB)-C(1B) 125.0 (3) C(1B)-N(IB)-C(7B) 115.6 (4) Cu-N(2B)-C(TB) 128.4 (3) C u - O - C ( I ) 148.5 (4) N(IA)-C(1A)-C(2A) 119.0 (5) C(5A)-C(4A)-C(7A) 101.9 (4) C(5A)-C(4A)-C(3A') 48. (l) C(7A)-C(4A)-C(aA') 150. (1) C(4A)-C(5A)-C(6A) 99.9 (4) C(6A)-C(5A)-C(3A') 141. (1) N(IA)-C(7A)-N(2A) 125.7 (4) N(2A)-C(7A)-C(4A) 112.2 (4) C(IB)--C(2B)--C(3B) 121.9 (5) C(3B)-C(4B)---CC5B) 138.0 (5) C(5B)-C(4B)-V(7B) 104.6 (4) N(2B)-C(6B)--C(5B) 113.1 (5) N(IB)-C(7B)-C(4B) 124.9 (4) C(2A)-C(3A)-C(4A) 114.8 (9) Cu-N(IA)--C(TA) 123.0 (3) Cu-N(2A)-C(6A) 125.9 (3) C(6A)--N(2A)---C(TA) 110.7 (4) Cu-N(1B)-C(TB) 119-4 (3) Cu-N(2B)-C (6B) 125-5 (3) C(6B)--N(2B)-C(TB) 106-1 (4) N - C ( I ) - O 128. I (5) C(1A)-C(2A)--C(3A) 126.8 (7) C(5A)-C(4A)--C(3A) 134.4 (7) C(7A)--C(4A)--C(3A) 123.7 (8) C(3A)--C(4A)--C(3A') 86. (1) C(4A)---C(5A)-C(3A') 41.0 (9) N(2A)--C(6A)-C(5A) 115-4 (5) N(IA)--C(TA)--C(4A) 122.1 (5) N(1B)--C(1B)-C(2B) 122.6 (5) C(2B)--C(3B)--C(aB) 117.7 (5) C(3B)-C(4B)--C(7B) 117.4 (5) C(4B)-C(5B)---C(6B) 105.5 (4) N(IB)---C(TB)-N(2B) 124.4 (4) N(2B)-C(7B)--C(4B) 110.7 (4) C(4A)-C(3A')--C(5A) 91. (1)Table
5.
Cu(l)-Cu(2)Cu(1)-o(2)
Cu(I)-N(1B) Cu(2)--N(2A) O(2)-C(5) N-C(2) N(IA)-C(7A) N(1B)-C(1B) N(2B)-C(7B) C(3A)-C(4A) C(4A)-C(3A') C(IB)-C(2B) C(4B)-C(5B) C(5B)-C(6B) Cu(l)-Cu(2) Cu(l)-O(3) Cu(2)-O(1) Cu(2)-N(2B) O(3)-C(1) N-C(3) N(2A)-C(6A)Bond lengths
(A)
and angles
[Cu4(OCH3)2(C 7HsN2)a(dmf)2]
3.0141 (8) N(IB)--Ct7B) 1.342 (4) 1.927 (2) C(IA)--C(2A) 1.371 (5) 1.993 (2) C(4A)-C(5A) 1.691 (7) 1.965 (2) C(5A)-C(6A) 1.368 (5) !.403 (4) C(2B)-C(3B) 1.239 (7) 1.428 (5) C(4B)-C(7B) 1.413 (4) 1.342 (4) C(5B)-C(3B') I. 10 (l) 1.330 (4) Cu(1)-O(1) 1.939 (2) 1.339 (4) Cu(I)-N(IA) 1.976 (2) 1.248 (7) Cu(2)-O(2) 1.915 (2) l . l l (1) O(l)-C(4) 1.410 (4) 1.373 (5) N - C ( I ) 1.315 (5) 1.678 (6) N(IA)-C(1A) 1.344 (4) 1.357 (6) N(2A)-C(7A) 1.340 (4) 2.999 (1) N(2B)-C(6B) 1.363 (4) 2.422 (3) C(2.4)-C(3A) 1.260 (7) 1.926 (2) C(4A)-C(7A) 1.410 (4) 1.966 (2) C(5A)--C(3A') 1.16 (1) 1.212 (4) C(3B)--C(4B) 1.264 (7) 1.440 (5) C(4B)-C(3B') I. 12 (1) 1.363 (4) 77.97 (3) Cu(2)-Cu(l)-O(l) 38.59 (7) 38.18 (6) Cu(2)-Cu(l)-O(3) 107.22 (7) 129.18 (8) Cu(2)-Cu(I)-N(IB) 130.61 (8) 87.78 (8) Cu(2)-Cu(l)-O(2) 86.02 (8) 174.76 (7) Cu(2)-Cu(I)-N(1A) 83.52 (9) 82.60 (9) O(l)--Cu(l)-O(2) 75.30 (9) Cu(2)-Cu(l)-Cu(2) Cu(2)-Cu(1)-O(2) Cu(2)-Cu(1)-N(IA) Cu(2)-Cu(l)-O(1) Cu(2)-Cu(l)-O(3) Cu(2)-Cu(I)-N(IB)(o) for
SHIE-MING PENG A N D YII-NAN LIN
1729
Table 5
(cont.)
Table 6
(cont.)
O(l)-Cu(l)-O(3) 96.8 (1) O(l)-Cu(1)-N(1B) 96.3 (1) O(2)-Cu(I)-N(1A) 94.2 (1) O(3)-Cu(1)-N(IA) 92.4 (I) N(IA)-Cu(1)-N(IB) 92.4 (I) Cu(l)--Cu(2)-O(1) 38.92 (6) Cu(1)-Cu(2)-N(2A) 132.78 (9) Cu(l)-Cu(2)-O(l) 106.69 (8) Cu(I)-Cu(2)-N(2A) 78.57 (9) O(1)-Cu(2)-O(2) 75.88 (9) O(I)-Cu(2)-N(2B) 170.3 (1) O(2)-Cu(2)-N(2B) 95.4 (1) Cu(l)-O(1)-Cu(2) 102.5 (l) Cu(2)-O(1)-C(4) 129.4 (2) Cu(1)-O(2)-C(5) 127.2 (2) Cu(1)-O(3)-C(I) 117.4 (2) C(1)--N-C(3) 122.0 (3) Cu(1)-N(IA)--C(IA) 123.8 (2) C(IA)-N(IA)--C(7A) 113.5 (2) Cu(2)-N(2A)-C(TA) 129.5 (2) Cu(I)-N(IB)-C(IB) 123.6 (2) C(IB)-N(IB)-C(7B) 112.8 (2) Cu(2)-N(2B)-C(TB) 128.8 (2) O(3)--C(I)-N 126.1 (3) C(IA)-C(2A)-C(3A) 128.3 (4) C(3A)-C(4A)-C(5A) 133.0 (4) C(3A)-C(4A)-C(3A') 90.1 (7) C(5A)-C(4A)-C(3A') 43.0 (7) C(4A)-C(5A)-C(6A) 100.2 (3) C(6A)-C(5A)-C(3A') 141.0 (7) N(1A)-C(7A)-N(2.4) 125.7 (2) N(2A)--C(TA)--C(4A) 114.2 (3) C(1B)-C(2B)-C(3B) 127.9 (4) C(3B)-C(4B)-C(5B) 134.1 (3) C(3B)-C(4B)-C(3B') 93.4 (7) C(5B)-C(4B)-C(3B') 40.7 (6) C(4B)-C(5B)-C(6B) 101.3 (3) C(6B)-C(5B)-C(3B') 142.8 (7) N(IB)-C(7B)-N(2B) 125.4 (3) N(2B)-C(7B)-C(4B) 114.6 (3) O(l)-Cu(l)-N(IA) 166.8 (1) O(2)-Cu(1)-O(3) 97.6 (1) O(2)-Cu(1)-N(IB) 166.1 (1) O(3)-Cu(1)-N(IB) 94.3 (1) Cu(l)-Cu(2)-Cu(l) 102.03 (3) Cu(l)-Cu(2)-O(2) 38.46 (7) Cu(1)-Cu(2)-N(2B) 133.30 (8) Cu(1)-Cu(2)-O(2) 104.96 (8) Cu(I)-Cu(2)-N(2B) 79.42 (9) O(1)-Cu(2)-N(2A) 94.9 (1) O(2)--Cu(2)-N(2A) 170.7 (1) N(2A)-Cu(2)-N(2B) 93.6 (I) Cu(l)-O(l)-C(4) 126.4 (2) Cu(1)-O(2)-Cu(2) 103.4 (1) Cu(2)-O(2)-C(5) 129.4 (2) C ( I ) - N - C ( 2 ) 121.3 (3) C(2)-N-C(3) 116.5 (3) Cu(I)-N(IA)--C(7A) 122.7 (2) Cu(2)-N(2A)-C(6A) 121.8 (2) C(6A)-N(2A)-C(7A) 108.7 (2) Cu(I)-N(IB)-C(7B) 123.6 (2) Cu(2)-N(2B)-C(6B) 122.7 (2) C(6B)-N(2B)--C(7B) 108.3 (3) N(IA)-C(IA)-C(2A) 119-6 (3) C(2A)-C(3A)-C(4A) 112.3 (4) C(3A)-C(4A)-C(7A) 126.1 (4) C(SA)-C(4A)-C(7A) 100.8 (3) C(7A)-C(4A)-C(3A') 143.8 (8) C(4A)-C(5A)-C(3A') 40.9 (6) N(2A)-C(6A)-C(5A) 116.1 (3) N(IA)-C(7A)-C(4A) 120.1 (3) N(IB)--C(IB)-C(2B) 121.2 (3) C(2B)-C(3B)-C(4B) 112.2 (4) C(3B)-C(4B)-C(TB) 125.9 (4) C(5B)-C(4B)-C(TB) 100.0 (3) C(7B)-C(4B)-C(3B') 140.6 (7) C(4B)-C(5B)--C(3B') 41.5 (6) N(2B)-C(6B)-C(5B) 115.8 (3) N(IB)-C(7B)-C(4B) 119.9 (3) C(4A)-C(3A')-C(5A) 96.1 (9)
Table 6.
Co(l)-O Co(1)-N(2F) Co(2)-N(2C) Co(3)-N(2B) Co(4)-O Co(4)--N(IF) N(2A)-C(6A) C(2A)-C(3A) C(4A)-C(7A) C(SA)-C(3A') N(2B)-C(6B) C(2B)-C(3B) C(4B)-C(7B) N(IC)-C(7C0 C (1C)-C (2C-") c ( 4 c ) - c ( 5 c ) C(5C)-C(6C) N(ID)-C(7D) C ( 10)--C (2D) C(4D)-C(5D) N(IE)-C(IE) N(2E)-C(7E) C(3E)-C(4E) C(5E)-C(6E) N(2F)-C(6F) C(2F)-C(3F) C (4F)-C (7F) C(5F)-C(3F') C-C1(3) Co(l)-N(IC) Co(2)-O Co(2)-N(IE) Co(3)-N(ID) Co(4)-N(IA)Bond lengths
(A)
and angles ( o ) f o r
[ C o 4 ( C T H 5 N 2 ) 6 0 ] . C H C 1 3 1.917 (8) C(2C)-C(3C) 2.00 (1) C(4C)-C(7C) 1.96 (I) C(5C)-C(3C') 1.99 (1) N(2D)-C(6D) 1.905 (8) C(2D)-C(3D) 1-98 (1) C(4D)-C(TD) 1.39 (2) N(1E)--C(7E) 1.22 (2) C(IE)-C(2E) i.43 (2) C(4E)-C(5E) 1.04 (7) N(1F)-C(IF) 1.4 ! (2) N(2F)-C(7b0 1.30 (2) C(3F)-C(4F) 1.46 (2) C(4F)-C(3F') 1.35 (I) C-Cl(1) 1.33 (2) Co(I)-N(2D) 1.52 (2) Co(2)-N(2A) 1.33 (2) Co(3)-O 1.32 (I) Co(3)-N(2E) 1.38 (2) Co(4)-N(1B) 1.42 (2) N(IA)-C(7A) 1.35 (i) C(IA)-C(2A) 1.38 (1) C(4A)-C(5A) 1.34 (2) C(5A)-C(6A) 1.35 (2) N(IB)--C(TB) 1.35 (2) C(IB)--C(2B) 1.14 (3) C(4B)-C(5B) 1.46 (2) N(IC)-C(IC) 1.07 (6) N(2C)-C(7C) 1.69 (1) C(3C)-C(4C) 1.99 (1) C(4C)-C(3C') 1.965 (8) N(ID)-C(ID) 2.00 (1) N(2D)-C(TD) 1.99 (1) C(3D)-C(4D) 1.98 (1) C(5D)-C(6D) 1.28 (2) 1.42 (2) 1.23 (8) 1.38(1) 1.32 (2) 1.46 (2) 1.33(1) 1.38 (2) 1.4o (2) 1.37 (2) 1.35(1) 1.20 (3) I. I0 (6) 1.68 (2) 1.98 (1) 1.97 (1) 1.942 (8) 1.97 (1) 1.99 (1) 1.34 (1) 1.39 (2) !.56 (2) 1.33 (2) 1.33(1) 1.32 (2) 1.42 (2) • 31 (I) • 33 (l) • 23 (2) • 06 (8) .34(1) .33(1) • 29 (2) • 36 (2) N(IA)-C(IA) 1.32 (2) N(2A)-C(7A) 1.36 (1) C(3A)-C(4A) 1.24 (2) C(4A)-C(3A') 0.97 (7) N(IB)-C(IB) 1-35 (1) N(2B)-C(7B) 1-33 (1) C(3B)-C(4B) 1.31 (2) C(5B)-C(6B) 1.39 (2) N(2C)-C(6C) 1.39 (2) O-Co(1)-N(IC) I l l .3 (4) O-Co(1)-N(2F) 109.4 (4) N(1C)-Co(I)-N(2F) 103.8 (5) O-Co(2)-N(2A) ll0.1 (4) O-Co(2)-N(IE) I I0. I (4) N(2A)-Co(2)-N(IE) 104.9 (4) O-Co(3)-N(ZB) 109.6 (4) O-Co(3)-N(2E) 109.9 (4) N(2B)-Co(3)-N(2E) 115.3 (4) O--Co(4)-N(1A) 111.2 (4) O-Co(4)-N(IF) 112.1 (4) N(IA)-Co(4)-N(1F) 108.7 (5) Co(1)-O-Co(2) 108.5 (4) Co(1)-O-Co(4) 110.1 (4) Co(2)-O-Co(4) 108.6 (4) Co(4)-N(IA)-C(IA) 121. (1) C(1A)-N(1A)-C(7A) 112. (I) Co(2)-N(2A)-C(7A) 130.5 (9) N(IA)-C(1A)-C(2A) 121. (1) C(2A)-C(3A)-C(4A) 122. (2) C(3A)-C(4A)-C(TA) 117. (1) C(5A)-C(4A)-C(7A) I02. (1) C(7A)-C(4A)-C(3A') 143. (4) C(4A)-C(5A)---C(3A') 38. (4) N(2A)-C(6A)-C(5A) 115. (1) N(IA)--C(7A)-C(4A) 124. (1) Co(4)-N(1B)--C(1B) 121- (l) C(1B)-N(IB)-C(7B) ll5. (1) Co(3)-N(2B)-C(7B) 129.2 (9) N(IB)--C(IB)-C(2B) 123. (I) C(2B)-C(3B)-C(4B) 126. (1) C(3B)-C(4B)-C(7B) 113. (1) C(4B)-C(5B)-C(6B) 108. (1) N(IB)-C(7B)-N(2B) 127- (1) N(2B)-C(TB)-C(4B) 110. (1) Co(1)-N(IC")--C(7C) 121.8 (9) Co(2)-N(2C)--C(6C) 125. (1) C(6C)-N(2C)-C(7C) 107. (1) C(IC)-C(2C)-C(3C) 127. (1) C(3C)-C(4C)-C(5C) 140. (1) C(3C)-C(4C)-C(3C') 87. (4) C(5C)-C(4C)-C(3C') 53. (4) C(4C)-C(5C)-C(6C) 106. (1) C(6C)-C(5C)-C(3C') 150. (4) N(IC)-C(7C)-N(2C) 124. (1) N(2C)-C(7C)-C(4C) 113- (1) Co(3)-N(ID)-C(7D) 126.3 (9) Co(I)-N(2D)-C(6D) 125.3 (9) C(6D)-N(2D)-C(7D) 106. (1) C(ID)-C(2D)-C(3D) 121. (1) C(3D)-C(4D)--C(5D) 140. (1) C(5D)-C(4D)-C(7D) 104. (l) N(2D)-C(6D)-C(5D) l l 3 - ( l ) N(ID)-C(7D)-C(4D) 125. (I) Co(2)-N(IE)-C(IE) 119.5 (9) C(1E)-N(IE)-C(7E) 115. (1) Co(3)-N(2E)-C(7E) 125.3 (9) N(IE)-C(1E)-C(2E) 122. (1) C(2E)-C(3E)-C(4E) 122. (1) C(3E)-C(4E)-C(7E) 115. (1) C(4E)-C(SE)-C(6E) 106- (1) N(1E)-C(7E)-N(2E) 124. (1) N(2E)-C(7E)-C(4E) 110. (I) Co(4)-N(IF)-C(7F) 125. (1) Co(I)-N(2F)-C(6F) 119. (1) C(6F)-N(ZF)-C(VF) 111. (1) C(IF)-C(ZF)-C(3F) 124- (2) C(3F)-C (4F)--C(5F) 143. (2) C(3F)-C(4F)---C(3F') 101. (3) C(5F)-C(4F)-C(3F') 41. (3) C(4F)-C(5F)-C(6F) 102. (1) C(6F)-C(5F)-C(3F') 145. (3) N(IF)-C(7F)-N(2F) 126. (1) N(2F)-C(7F)-C(4F) II0. (I) C(4C)-C(3C')-C(5C) 83. (5) Cl(1)-C-Cl(2) 114. (i) N(2E)-C(6E) 1.35 (2) C (2E)-C(3E) 1.33 (2) C(4E)-C(7E) 1.42 (2) N(1F)-C(7F') 1.30 (1) C(IF)-C(2F) 1.35 (3) C(4F)-C(5F) 1.60 (2) C (5F)-C(6F) 1.40 (2) C-C1(2) 1.66 (1) O - C o ( I ) - N ( 2 D ) 111.0 (4) N(IC)-Co(I)-N(2D) 107.8 (4) N(2D)--Co(I)-N(2F) 113-3 (4) O-Co(2)--N(2C) 109.3 (4) N(2A)-Co(2)-N(2C) 113.4 (4) N(2C")---Co(2)-N(I,E') 108.9 (4) O-Co(3)-N(ID) 111.5 (4) N(2B)--Co(3)-N(ID) 101.6 (4) N(ID)-Co(3)-N(2E) 108.7 (4) O-Co(4)-N(IB) 111.6 (4) N(1A)-Co(4)-N(IB) 107.2 (4) N(IB)--Co(4)-N(IF) 105.7 (5) Co(1)-O-Co(3) 109.7 (4) Co(2)-O-Co(3) 110.4 (4) Co(3)-O-Co(4) 109.6 (4) Co(4)-N(1A)-C(7A) 127- (1) Co(2)-N(2A)-C(6A) 123.1 (9) C(6A)-N(2A)---C(7A) 106. (1) C(IA)-C(2A)-C(3A) 124. (I) C(3A)-C(4A)-C(5A) 140. (l) C(3A)-C(4A)-C(3A') 99. (4) C(5A)--C(4A)-C(3A') 41. (4) C(4A)--C(5A)-C(6A) 104. (1) C(6A)--C(5A)-C(3A') 142. (4) N(IA)-C(7A)-N(2A) 123. (1) N(2A)-C(7A)--C(4A) 113. (I) Co(4)-N(IB)-C(7B) 123.5 (9) Co(3)-N(2B)-C(6B) 121.2 (9) C(6B)-N(2B)-C(7B) 108. (1) C(IB)-C(2B)-C(3B) 119. (1) C(3B)-C(4B)-C(5B) 142. (1) C(5B)-C(4B)-C(7B) 105. (1) N(2B)--C(6B)--C(5B) 109- (I) N(1B)-C(TB)-C(4B) 123. (I) Co(I)-N(IC")-C(1C) 119.0 (9) C(1C")-N(1C~C(7C) 114- (1) Co(2)-N(2C)--C(7C) 125.4 (9) N(1C)-C(1C)-C(2C) 119. (I) C(2C)-C(3C)-C(4C) 119. (2) C(3C)-C(4C)-C(7C) 118. (1) C(5C)-C(4C)-C(7C) 102. (1) C(7C)-C(4C)-C(3C') 155. (4) C(4C)-C(5C)-C(3C') 44. (3) N(2C)-C(6C)--C(5C) 112. (1) N(IC)-C(7C)-C(4C) 123. (1) Co(3)-N(ID)-C(1D) 117.9 (9) C(ID)-N(ID)--C(7D) 114. (1) Co(1)-N(2D)-C(7D) 127.2 (9) N(ID)-C(ID)-C(2D) 123. (l) C(2D)-C(3D)-C(4D) 121. (1) C(3D)-C(4D)-C(7D) 116. (I) C(4D)-C(5D)-C(6D) 107- (l) N(ID)-C(7D)-N(2D) 124- (l) N(2D)-C(7D)-C(4D) ! 1 I- (I) Co(2)-N(IE)-C(7E) 125-3 (9) Co(3)-N(2E)-C(6E) 125.0 (9) C(6E)-N(2E)-C(TE) 104. (l) C(IE)-C(2E)-C(3E) 120. (l) C(3E)-C(4E)-C(5E) 139. (l) C(5E)-C(4E)-C(7E) 106. (1) N(2E)--C(6E)-C(5E) l l4. (1) N(1E)-C(7E)-C(4E) 126- (1) Co(4)-N(I F)-C(IF) 122. (1) C(IF)--N(IbO-C(7bO 112. (1) Co(I)-N(2F)-C(7F) 130. (1) N(IF)--C(IPO--C(2F) I19. (I) C (2F)-C(3F)-C (4F) 127. (3) C(3F)-C(4F)-C(7F) 114. (2) C(5F)-C(4F)-C(TF) 103. (1) C(7F).-C(4F)-C(3F') 144- (3) C(4F)--C(5F)-C (3F') 43. (3) N(2F)---C(6F)-C(5F) 114. (1) N(IF)-C(TF)-C(4F) 123. (1) C(4A)-C(3A')-C(5A) 101. (6) C (4F)-C(3F')-C(5F) 96. (4) C](I)-C-CI(3) 112. (1)
1730
Cu ll A N D Co II C L U S T E R S O F 7 - A Z A I N D O L A T E
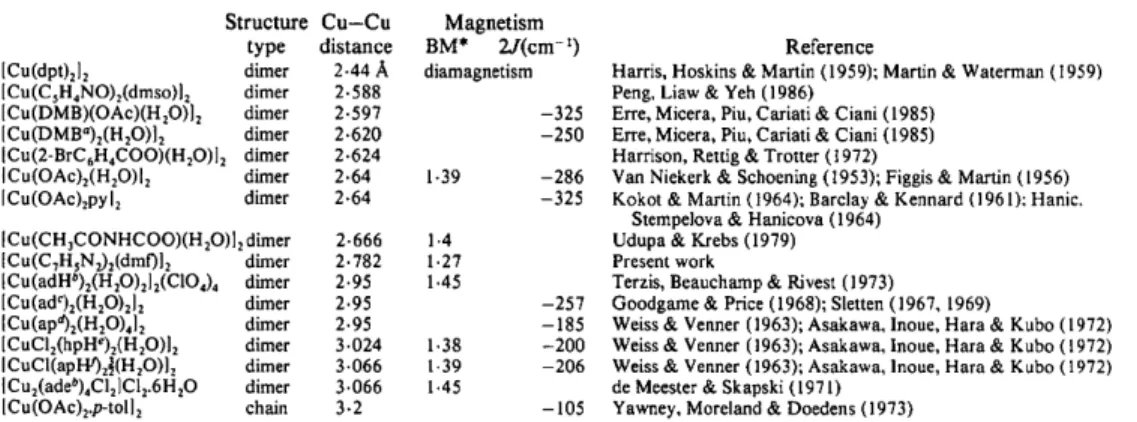
Table 7.
Comparison of the structure and magnetic interaction of dinuclear copper complexes
Structure Cu-Cu Magnetism type distance BM* 2J(cm-~) [Cu(dpt)212 dimer 2.44 A diamagnetism ICu(CsH4NO)2(dmso)l 2 dimer 2.588 ICu(DMB)(OAc)(H20)] 2 dimer 2.597 -325 ICu(DMB°)2(H20)]2 dimer 2-620 -250 ICu(2-BrC6H4COO)(H20)I 2 dimer 2.624 ICu(OAc)2(H20)I 2 dimer 2.64 1.39 -286 ICu(OAc)2pyl 2 dimer 2-64 -325 [Cu(CH3CONHCOO)(H20)]2 dimer 2.666 1.4 [Cu(CTHsNz),(dmf)] 2 dimer 2.782 1.27 [Cu (adHa)2(H,O)212(CIO4)4 dimer 2.95 1.45
ICu(ad02(H20)212 dimer 2.95 -257 [Cu(apa)2(H20)4]2 dimer 2.95 -185 [CuClz(hpHe)2(H20)12 dimer 3.024 1.38 -200 ICuCI(apW)2½(H20)I 2 dimer 3.066 1.39 -206 I Cu2(adeb)4CI2lCl2.6 H20 dimer 3.066 1.45 [Cu(OAc)vp-toll 2 chain 3.2 -105 Reference
Harris, Hoskins & Martin (1959); Martin & Waterman (1959) Peng, Liaw & Yeh (1986)
Erre, Micera, Piu, Cariati & Ciani (1985) Erre, Micera, Piu, Cariati & Ciani (1985) Harrison, Rettig & Trotter (1972)
Van Niekerk & Schoening (1953); Figgis & Martin (1956) Kokot & Martin (1964); Barclay & Kennard (1961); Hanic.
Stempelova & Hanicova (1964) Udupa & Krebs (1979) Present work
Terzis, Beauchamp & Rivest (1973) Goodgarne & Price (1968); Sletten (1967. 1969)
Weiss & Venner (1963); Asakawa. Inoue, Hara & Kubo (1972) Weiss & Venner (1963); Asakawa, Inoue, Hara & Kubo (1972) Weiss & Venner (1963); Asakawa, Inoue, Hara & Kubo (1972) de Meester & Sk apski ( 1971)
Yawney, Moreland & Doedens (1973)
Notes: a DMB = 2,6-methoxybenzoato, b adH =adenine=ade, c ad=adeninato, d a p = 6-aminopurinato, e apH = 6-aminopurine, f hpH = 6-hydroxypurine.
* 1BM-- 9.27 x 10-24JT -t.
The second, tetrameric, structure, (II), contains four
methoxide bridges, four 7-azaindolate bridges and two
coordinated dmf molecules. Two kinds of Ca ions were
found in the structure; one has square pyramidal
coordination geometry with C u ( 1 ) - N 1.993 (2)A,
Cu(1)-O(methoxide)
1.939 (2)A,
Cu(1)-O(dmf)
2.422 (3) A, the other has square planar geometry with
C u ( 2 ) - N
1.966 (2) A,
Cu(2)-O (methoxide)
1.926 (2)A. The geometry and long Cu(1)-Cu(2)
distances, 2.999 (1) and 3.014(1)A, of this complex
indicate the presence of a superexchange magnetic
interaction.
The third, tetrameric, structure, (III), contains six
7-azaindolate bridges, four tetrahedral Co ions, and an
O ion. The central O atom is tetrahedrally surrounded
by the four Co atoms and each Co is tetrahedrally
coordinated by one O and three N atoms of 7-
azaindolate. The structure is similar to those of
[Be40(OOCR) 6] and [Zn40(OOCR)6] but is the first
structure of this type observed for a cobalt complex.
The average C o - C o distance, 3.156 (1) A, is long and
indicates the superexchange interaction of this cluster.
In the ligand moiety, the C - N and C - C bond
lengths of azaindolate are between those of single-bond
and double-bond values. The three structures suffer
ligand disorder. It is not possible to estimate quanti-
tatively the extent of n-electron delocalization in the
7-azaindolate ligand.
The authors would like to express their appreciation
for the support of this work by the National Science
Council.
References
ASAKAWA, T., INOUE, M., HARA, K. & KUBO, M. (1972).
Bull.
Chem. Soc. Jpn,
45, 1054-1057.BALCH, A. L. & HOLM, R. H. (1966).
J. Am. Chem. Soc.
88,
5201-5209.BARCLAY, G. A. & KE~,~ARO, C. H. L. (1961).
J. Chem. Soc.
pp. 5244-5251.BROOKES, R. W. & MARTIN, R. L. (1974).
Aust. J. Chem.
27, 1569-1571.BROOKES, R. W. & MARTIN, R. L. (1975a).
Aust. J. Chem.
28, 1363-1366.BaooKEs, R. W. & MARTIN, R. L. (1975b).
Inorg. Chem.
14, 528-536.EgRE, L., MICERA, G., PItJ, P., CAmATI, F. & CLAM, G. (1985).
lnorg. Chem.
24, 2297-2300.FmGlS, B. & MARTIN, R. L. (1956).
J. Chem. Soc.
pp. 3837-3846. GABE, E. J. & LEE, F. L. (1981).Acta Cryst.
A37, $399.GOODGAME, D. M. L. & PmCE, K. A. (1968).
Nature (London),
220, 783-784.
HANtC, K., STEMPELOVA, D. & HANICOVA, K. (1964).
Acta Cryst.
17, 633-639.
HAmUS, C. M., HOSKINS, B. F. & MAaTrN, R. L. (1959). J.
Chem.
Soc.
pp. 3728-3837.HARmSON, W., RE'rnG, S. & TROTTER, J. (1972).
J. Chem. Soc.
Dalton. Trans.
pp. 1852-1856.International Tables for X-ray Crystallography
(1974). Vol. IV. Birmingham: Kynoch Press. (Present distributor D. Reidel, Dordrecht.)JOHNSON, C. K. (1965).
ORTEP.
Report ORNL-3794. Oak Ridge National Laboratory, Tennessee.KOKOT, E. & MARTIN, R. L. (1964).
lnorg. Chem.
3, 1306-1312. LAUHER, L. W. & IBERS, J. A. (1975).Inorg. Chem.
14, 640-645. MARTIN, R. L. & WATERMAN, H. (1959). J.Chem. Soc.
pp.1359-1370.
MEESTER, P. DE & SKAPSKI, A. C. (1971).
J. Chem. Soc. A,
pp. 2167-2169.PEYG, S., CHEN, C., LIAW, D., CHEY, C. & WANG, Y. (1985).
Inorg. Chim. Acta.
pp. L 3 1 - L 3 3 .PENG, S. & GOEDKEY, V. L. (1976). J.
Am. Chem. Soc.
98, 8500-8510.PENG, S., LIAW, D., WAYG, Y. & SIMON, A. (1985).
Angew. Chem.
Int. Ed. Engl.
24, 210-211.PENG, S., LIaw, D. & YEH, S. (1986). Unpublished results. SLErT~N, E. (1967).
Chem. Commun.
pp. 1119-I 120. SLE'rrEY, E. (1969).Acta Cryst.
25, 1480-1491.TERZIS, A., BEAUCHAMP, A. L. & RIVEST, R. (1973).
![Table 1. Atomic positional parameters and equivalent isotropic temperature factors of ICu2(C 7H5 N 2)4(dmf)2]](https://thumb-ap.123doks.com/thumbv2/9libinfo/8655980.194416/2.882.375.720.92.410/table-atomic-positional-parameters-equivalent-isotropic-temperature-factors.webp)
![Table 3. Positional and thermal parameters of [Co 4- (C7HsN2)60].CHC13 X y z Biso(A 2) Co(l) 0-1489 (2) 0-2413 (2) 0-1856 (1) 3.2 (1) Co(2) 0.9093 (2) 0.3634 (2) 0-2584 (1) 3.5 (1) Co(3) 0.0369 (2) 0-1272 (2) 0.3126 (1) 3.3](https://thumb-ap.123doks.com/thumbv2/9libinfo/8655980.194416/3.882.433.778.303.1000/table-positional-thermal-parameters-c-hsn-chc-biso.webp)
![Fig. 2. ORTEP plot of the [ C u 4 ( C v H s N 2 ) 4 ( O C H 3 ) a ( d m 0 2] molecule with labeling scheme, The thermal ellipsoids are drawn at the 50% probability level](https://thumb-ap.123doks.com/thumbv2/9libinfo/8655980.194416/4.882.98.307.427.668/ortep-molecule-labeling-scheme-thermal-ellipsoids-probability-level.webp)