Polymer functionalized ZnO nanobelts as oxygen sensors with a significant response
enhancement
This article has been downloaded from IOPscience. Please scroll down to see the full text article. 2009 Nanotechnology 20 065503
(http://iopscience.iop.org/0957-4484/20/6/065503)
Download details:
IP Address: 140.112.113.225
The article was downloaded on 06/02/2009 at 04:33
Please note that terms and conditions apply.
The Table of Contents and more related content is available HOME | SEARCH | PACS & MSC | JOURNALS | ABOUT | CONTACT US
Nanotechnology 20 (2009) 065503 (6pp) doi:10.1088/0957-4484/20/6/065503
Polymer functionalized ZnO nanobelts as
oxygen sensors with a significant response
enhancement
J H He
1, C H Ho and C Y Chen
Institute of Photonics and Optoelectronics, and Department of Electrical Engineering, National Taiwan University, Taipei 10617, Taiwan, Republic of China
E-mail:jhhe@cc.ee.ntu.edu.tw
Received 18 September 2008, in final form 28 November 2008 Published 15 January 2009
Online atstacks.iop.org/Nano/20/065503
Abstract
A plasma-polymerized acrylonitrile (PP-AN)/ZnO nanobelt (NB) nanosensor reveals a better oxygen-sensing response than a bare ZnO NB nanosensor due to the sorption nature of the polymer. With the aid of UV light, significant response enhancements of PP-AN/ZnO NB nanosensors at low temperature have been observed since the effects of oxygen
desorption/adsorption in PP-AN on the electron depletion region in the ZnO are significant. The minimum sensitivity at 150◦C is 16.6 ppm. This work permits its feasibility in areas where it is impossible to work at higher temperatures since lowering the working temperature of the sensor can avoid the structural deterioration, causing instability in the response.
(Some figures in this article are in colour only in the electronic version)
1. Introduction
Hybrid nanomaterials can combine the features of different nanomaterials and tailor the properties to achieve the desired material performance. They have been used for various applications, such as active antifouling films [1], light-emitting diodes [2–4], solar cells [5,6], memory devices [7], photodetectors [8,9] and gas sensors [10,11].
Due to the ultrahigh surface-to-volume ratio and reduced dimensionality of the active area in the one-dimensional nanostructures, such as carbon nanotubes (CNTs) [10, 11], nanowires (NWs) [12–15] and nanobelts (NBs) [16–18], chemical sensing based on these nanostructures has attracted enormous attention, as this is widely perceived as one of the most promising areas for nanotechnology to generate significant impact. Moreover, the unique structural and electrical properties of ZnO nanostructures [18–21] have encouraged scientists to develop ZnO nanostructured gas sensors since the device responds to the change in the electrical conductance occurring in the surface-surrounding atmosphere [18,22,23].
Commercial sensors based on metal oxides operate at 300–500◦C to enhance the surface molecular sorption kinetics. 1 Author to whom any correspondence should be addressed.
Although unmodified ZnO nanostructure sensors clearly have a role to play in gas sensing, the high-temperature operation of these oxide sensors is energy-consuming and not favorable in many cases, particularly in an explosive environment. In addition, lowering the working temperature of the sensor can avoid the structural deterioration and the diffusion of the contact atoms in the sensing layers, causing instability in the response. As a result, it is worth investigating whether it is possible to utilize other functional nanomaterials to enhance the properties of gas sensing at low working temperature. The concept of the sorption of gas in the polymer has been utilized in mass-sensitive resonant frequency sensors [24], in which transduction involves measuring mass changes, volume changes, conductance changes or capacitance changes induced by sorption of gaseous analytes in the polymer. Typically, the sensitivity of these sensors does not exceed 10–100 ppm for common analytes [24]. Reaching a level below 10 ppm is seen only in some cases [25]. Accordingly, the sorbent polymer-coated ZnO nanostructures may exhibit a substantial improvement in the sensitivity of the gas sensor performance as compared with unmodified ZnO nanostructures.
In this work, we presented a bilayered polymer/ZnO gas sensor based on ZnO NBs and a plasma-polymerized acrylonitrile (PP-AN) nanoscale surface coating with better oxygen-sensing response than unmodified ZnO NBs due to
Nanotechnology 20 (2009) 065503 J H He et al oxygen sorption in PP-AN. We also demonstrated that UV light
illumination can enhance the oxygen sensing of PP-AN/ZnO NBs significantly by modifying the surface potential since the effects of oxygen desorption/adsorption of the polymer on the electron depletion region of the ZnO is significant under UV light. The sensing behaviors of the bilayered nanomaterial demonstrated encouraging performance aspects including reduced operating temperature, reduced power consumption and enhanced sensitivity.
2. Experimental details
ZnO NBs were synthesized by catalytically activated vapor phase transport and a condensation deposition process in a horizontal tube furnace. Morphological studies of grown NBs have been performed with a transmission electron microscope (TEM) and a scanning electron microscope (SEM). TEM examination was carried out by using a JEOL 3000F field emission TEM operating at 300 kV with a nominal point-to-point resolution of 0.17 nm. SEM observations were conducted by using a JEOL JSM-6500 field emission SEM operating at 15 kV with a nominal point-to-point resolution of 1.5 nm. An energy-dispersive spectrometer (EDS) attached to a TEM was utilized to determine the ratio of the chemical composition. From the EDS analysis, the electron beam can be focused down to a diameter of 1.5 nm. The photoluminescence (PL) measurement on synthesized ZnO NBs was studied at room temperature using an He–Cd laser in the spectral range of 350–800 nm with a wavelength of 325 nm as the excitation source. The ZnO NB device for electrical measurement was fabricated with the aid of a focused ion beam (FIB) microscope. Details about the device fabrication via an FIB microscope have been reported elsewhere [12]. By taking advantage of the rectangular cross section of NBs, after the ZnO NB device fabrication, the 20 nm PP-AN was deposited as a nanoscale surface layer by employing plasma-enhanced chemical vapor deposition (PECVD) technology adapted to microfabricated structures [26,27]. To explore the oxygen-sensing properties of ZnO NB-based devices, the electrical transport was characterized in the quartz tube with oxygen flow with a concentration of 150 ppm. Before measurements, the quartz tube was pumped down to 10−2Torr and then baked at 100◦C for 1 h to remove any adsorbed O2on the NB surface. To explore the effect of UV light on gas sensing, the light source used in the experiments was a 100 W UV lamp with a wavelength of 365 nm, which corresponds to an energy greater than the ZnO bandgap.
3. Results and discussion
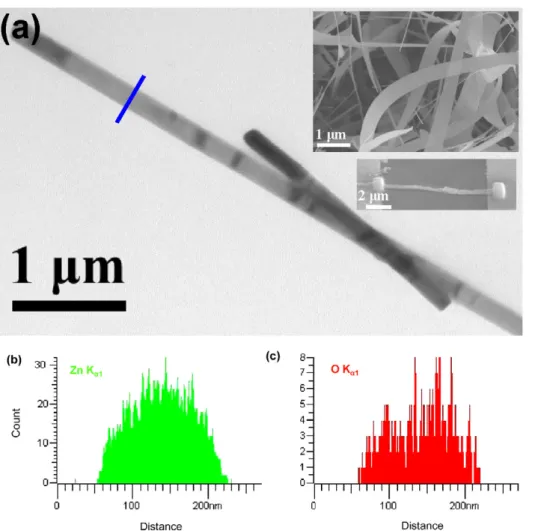
After the growth process, the substrate-bound NBs were mechanically scraped and sonicated in ethanol and deposited on carbon-coated copper grids for TEM characterization. Figure 1(a) shows a typical TEM image of a ZnO NB. A representative SEM image of the grown ZnO NBs is shown in the top right inset of figure1(a). The EDS linescan, as shown in figures1(b) and (c), confirms that the ratio of the composition of the NB arises from the well-defined spatial distribution of
Zn and O elements in the NB structures. The ratio of Zn/O being∼5 indicates the deficiency of oxygen. In addition, the bottom right inset of figure1(a) shows a typical SEM image of ZnO NB field effect transistors (FET) for oxygen sensing.
The room-temperature PL measurement, as shown in figure 2, indicates that the sharp and narrow UV emission located at 383 nm corresponds to the near-bandgap emission of ZnO while strong green luminescence is detected at 479 nm, which is attributed to deep-level emission or trap-state emission. This strong green luminescence refers to the defects induced during the growth, leading to the formation of considerable amounts of trapped states within the bandgap. Direct comparison of the EDS and PL concludes the oxygen-deficient ZnO nanostructures. It is known that unintentionally doped ZnO shows intrinsic n-type conductance with high electron densities because of a deviation from stoichiometry due to the presence of native defects such as oxygen deficiencies [28]. Similar results were also observed for various oxide nanomaterials [14,29]. The presence of ambient oxygen has a considerable influence on the performance of ZnO NB FETs. This is mainly due to conductance changes caused by the formation of an electron depletion region, induced by O2 molecular adsorption near the surface of ZnO NBs. O2molecules absorbed at the surface of metal oxides act as electron acceptors to form O2−, O−or O2−, depending on the ambient temperature [30]. These oxygen chemisorptions deplete the surface electron states, which result in the reduction of the channel conductance. Compared with bulk materials of ZnO, such surface effects are more significant on the conductance of nanostructures since the surface-to-volume ratio of the nanostructures is much larger [18,22,23,31]. The large change in the conductance refers to the high sensitivity. The representative sensing behavior of an unmodified ZnO NB in the dark is shown in figure3. Under the fixed bias at around 1 V, the responses of ZnO NBs to oxygen gas with a concentration of 150 ppm were varied by ambient temperatures in the dark in order to probe their chemical sensing abilities. Here the sensitivity for detecting oxygen gas is defined as −(Goxy− Gvac)/Gvac, where Goxyis the conductance in the presence of oxygen and Gvacis the conductance under vacuum. Obviously, there was no response at any working temperature below 175◦C. The sensitivity on oxygen gas was increased with the working temperature. The sensitivity of unmodified ZnO NBs can be as high as 9.32% at 300◦C in 300 s with the oxygen concentration of 150 ppm due to the enhancement of the surface molecular sorption kinetics on the surface of ZnO NBs at higher temperatures.
ZnO NB devices were coated with ultrathin PP-AN films (∼20 nm) using PECVD, and its effect on the conductance response was observed while detecting oxygen gas in the dark, as shown in figure 4. It was observed that oxygen could be detected at temperatures as low as 150◦C by covering PP-AN on ZnO NBs due to the enhanced sensitivity, which resulted from the large change in the conductance of ZnO NBs. With the aid of a functional polymer coating, the sensitivity for oxygen detection could be enhanced to as high as 18% at 150◦C as compared to the sensitivity of untreated ZnO NBs (0, 3.06, 4.93 and 9.32% at 150, 200, 250 and 300◦C, 2
Figure 1. (a) TEM image of the ZnO NB and the composition distribution of (b) zinc and (c) oxygen obtained from the EDS analysis. The top
right and bottom right insets in (a) are SEM images of the ZnO NB and NB FET, respectively.
Figure 2. The photoluminescence spectrum of ZnO NB.
respectively). Note that all experiments on the gas sensing of PP-AN/ZnO NBs were performed below 150◦C since the thermal degradation of polyacrylonitrile at temperatures above 200◦C has been observed [31,32]. The amplification
of gas sensitivity resulted from the sorption properties of polymers [11]. To confirm this speculation, the electrical measurement for PP-AN films was performed, as shown in figure5. PP-AN showed the absence of photoconduction under UV illumination and the response to ambient oxygen. It is known that PP-AN is an insulating polymer with ultrahigh electrical resistivity. The study on the electron transport of a PP-AN/ZnO NB device has shown that there was no obvious change in the current transported through the ZnO NBs after PP-AN coating [8]. This effect of enhanced oxygen sensing can be attributed to the high specific surface nature of the metal oxide nanostructure conductance responses. PP-AN layers increased the concentration of the adsorption centers and the capacity of adsorption at the surface of ZnO. While the sorbent polymers certainly concentrated the oxygen molecules, it was only oxygen molecules at the ZnO surface that interacted with ZnO to form a low energy configuration and modified the electrical properties of the ZnO NBs severely.
In addition to this sorbent polymer-assisted detection scheme, we have developed a method with higher sensitivities by employing UV light illumination (365 nm) on the devices that detect the oxygen molecules. The light intensity at the sample surface was 8900 mW cm−2. The nanosensors which were illuminated by UV light produced a more rapid and
Nanotechnology 20 (2009) 065503 J H He et al
Figure 3. Gas-sensing properties of the unmodified ZnO NB under
the fixed bias at around 1 V in responding to oxygen of 150 ppm concentration in the dark at a working temperature of (a) 175◦C, (b) 200◦C, (c) 250◦C and (d) 300◦C. The sensitivity for detecting oxygen gas is defined as−(Goxy− Gvac)/Gvac, where Goxyis the
conductance in the presence of oxygen and Gvacis the conductance
under vacuum. The interval between oxygen being on and off is 300 s.
Figure 4. Gas-sensing properties of PP-AN/ZnO NB under the fixed
bias at around 1 V in response to oxygen of 150 ppm concentration in the dark at a working temperature of (a) 125◦C and (b) 150◦C. The sensitivity for detecting oxygen gas is defined as
−(Goxy− Gvac)/Gvac, where Goxyis the conductance in the presence
of oxygen and Gvacis the conductance under vacuum. The interval
between oxygen being on and off is 300 s.
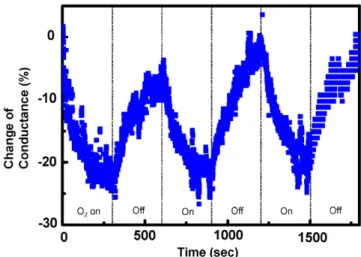
reversible change in conductance (on average 25.3%) at a working temperature of 150◦C in 300 s, as shown in figure6. The noise level is about 3 units. As the maximum level of 27 units is equivalent to 150 ppm, the minimum sensitivity achievable is 16.6 ppm, which is decent for gas sensors, especially combined with a low working temperature. It has been found that using UV light illumination drastically increased the conductance of PP-AN/ZnO NB [8]. The strong photoconducting responses of the PP-AN/ZnO NBs can achieve equally favorable adsorption/desorption behavior by illuminating the devices with UV light of energy higher than the ZnO bandgap. The active desorption process is thus
Figure 5. The measurements of the conductance change with time
for PP-AN films at around 1 V showing no response to the UV illumination and ambient oxygen.
Figure 6. Gas-sensing properties of PP-AN/ZnO NB under the fixed
bias at around 1 V in response to oxygen of 150 ppm at a working temperature of 150◦C under 365 nm UV light illumination. The sensitivity for detecting oxygen gas is defined as
−(Goxy− Gvac)/Gvac, where Goxyis the conductance in the presence
of oxygen and Gvacis the conductance under vacuum. The interval
between oxygen being on and off is 300 s.
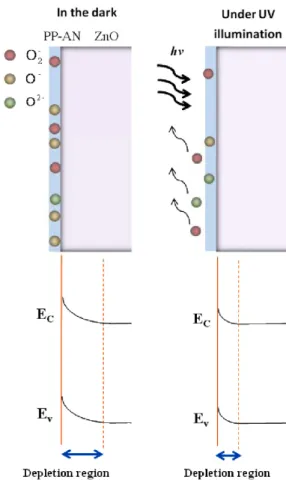
photoinduced molecular desorption. A schematic diagram for the oxygen sensing of a PP-AN/ZnO NB and the related band diagrams in the dark and under UV illumination are shown in figure 7. As an individual PP-AN/ZnO NB under UV illumination is exposed in the oxygen, the increase of the conductance is due both to the generation of a photocurrent, which directly increases the number of free electron–hole pairs, and to the desorption of charging surface species with a concomitant thinning of the electron depletion region near the NB surface by modifying the surface potential. This acts to ‘clean’ the polymer near the surface of the ZnO NBs and eliminate the charged oxygen molecules. Since UV light irradiation on the PP-AN/ZnO, increasing electrons and holes, changes the concentration of the adsorption centers of each given type and the capacity of adsorption on the 4
Figure 7. The schematic longitudinal cross sections of a PP-AN/ZnO
NB and related band diagrams in the dark and under UV illumination. In the illuminated state, photogenerated holes recombine with trapped electrons at the surface, desorbing electron-trapping oxygen molecules, such as O2−, O−and O2−. The
depletion layer thins and the ZnO NB conductivity rises by modifying the surface potential. Ambient oxygen levels are tracked by monitoring changes in conductance in the illuminated state.
surface of ZnO, the gas molecules can be detected at low temperature [16, 33, 34]. While the addition of oxygen is applied on the clean polymer near the surface of the ZnO NBs, the effect of oxygen adsorption in the polymer on thickening the electron depletion region in the ZnO is relatively distinct. Therefore, greater sensitivity of PP-AN/ZnO NB to oxygen molecules under UV light illumination is observed. Work is in progress to better understand the phenomena involved in the surface molecular sorption kinetics and photo-excitation process.
4. Conclusion
PP-AN/ZnO NBs as oxygen nanosensors offer significant advantages over conventional ZnO nanostructure-based gas sensors in terms of sensitivity and working temperature. Moreover, the gas sensor composed of a PP-AN-coated ZnO NB has shown a response enhancement under UV light illumination (from 0% to 25.3%) at 150◦C in comparison with an unmodified ZnO NB in the dark. The lowest concentration value at which a sensor signal can still be distinguished from the noise is as low as 16.6 ppm. This permits its use in
areas where it is not possible to work at higher temperatures since lowering the working temperature of sensors can avoid structural degradation in the sensing layers, causing instability in the response.
Acknowledgments
The research was supported by the National Science Council grant nos. NSC 96-2112-M-002-038-MY3 and NSC 96-2622-M-002-002-CC3.
References
[1] Asuri P, Karajanagi S S, Kane R S and Dordick J S 2007 Small
3 50–3
[2] Chang C Y et al 2006 Appl. Phys. Lett.88 173503
[3] Lee C Y, Haung Y T, Su W F and Lin C F 2006 Appl. Phys.
Lett.89 231116
[4] Konenkamp R, Word R C and Godinez M 2005 Nano Lett.
5 2005–8
[5] Beek W J E, Wienk M M, Kemerink M, Yang X N and Janssen R A J 2005 J. Phys. Chem. B109 9505–16
[6] Huynh W U, Dittmer J J and Alivisatos A P 2002 Science
295 2425–7
[7] Borghetti J, Derycke V, Lenfant S, Chenevier P, Filoramo A, Goffman M, Vuillaume D and Bourgoin J P 2006 Adv.
Mater.18 2535–40
[8] He J H, Lin Y H, McConney M E, Tsukruk V V, Wang Z L and Bao G 2007 J. Appl. Phys.102 084303
[9] Lao C S, Li Y, Wong C P and Wang Z L 2007 Nano Lett.
7 1323–8
[10] Liu X L, Ly J, Han S, Zhang D H, Requicha A, Thompson M E and Zhou C W 2005 Adv. Mater.
17 2727–32
[11] Snow E S, Perkins F K and Robinson J A 2006 Chem. Soc. Rev.
35 790–8
[12] He J H, Zhang Y Y, Liu J, Moore D, Bao G and Wang Z L 2007 J. Phys. Chem. C111 12152–6
[13] Hsin C L, He J H, Lee C Y, Wu W W, Yeh P H, Chen L J and Wang Z L 2007 Nano Lett.7 1799–803
[14] He J H, Wu T H, Hsin C L, Chen L J, Chueh Y L, Chou L J and Wang Z L 2006 Small2 116–20
[15] Kolmakov A, Zhang Y X, Cheng G S and Moskovits M 2003
Adv. Mater.15 997–1000
[16] Law M, Kind H, Messer B, Kim F and Yang P D 2002 Angew.
Chem., Int. Edn41 2405–8
[17] Comini E, Faglia G, Sberveglieri G, Pan Z W and Wang Z L 2002 Appl. Phys. Lett.81 1869–71
[18] Wang Z L 2004 Annu. Rev. Phys. Chem.55 159–96
[19] Pan Z W, Dai Z R and Wang Z L 2001 Science291 1947–9
[20] He H, Hsin C L, Liu J, Chen L J and Wang Z L 2007 Adv.
Mater.19 781–4
[21] He H, Lao C S, Chen L J, Davidovic D and Wang Z L 2005
J. Am. Chem. Soc.127 16376–7
[22] Fan Z Y, Wang D W, Chang P C, Tseng W Y and Lu J G 2004
Appl. Phys. Lett.85 5923–5
[23] Sberveglieri G 1995 Sensors Actuators B23 103–9
[24] Grate J W 2000 Chem. Rev.100 2627–48
[25] Thomas S W and Swager T M 2006 Adv. Mater.18 1047–50
[26] Yasuda H 1985 Plasma Polymerization (New York: Academic) [27] Singamaneni S, LeMieux M C, Jiang H, Bunning T J and
Tsukruk V V 2007 Chem. Mater.19 129–31
[28] ¨Ozg¨ur ¨U, Alivov Y I, Liu C, Teke A, Reshchikov M A, Do˘gan S, Avrutin V, Cho S-J and Morkoc¸ H 2005 J. Appl.
Nanotechnology 20 (2009) 065503 J H He et al
[29] He J H, Wu W W, Lee S W, Chen L J, Chueh Y L and Chou L J 2005 Appl. Phys. Lett.86 263109
[30] Chaabouni F, Abaab M and Rezig B 2004 Sensors Actuators B
100 200–4
[31] LaCombe E M 1957 J. Polym. Sci.24 152–4
[32] Grassie N and Hay J N 1962 J. Polym. Sci.56 189–202
[33] Comini E, Faglia G and Sberveglieri G 2001 Sensors Actuators B78 73–7
[34] Mishra S, Ghanshyam C, Ram N, Bajpai R P and Bedi R K 2004 Sensors Actuators B97 387–90