Introduction
Late embryogenesis abundant (LEA) genes are highly expressed in seeds during late development. In addition, some LEA genes can be induced in vegetative tissues by osmotic stress, including salt treatment and desiccation, as well as with exogenous abscisic acid (ABA) treatment [reviewed in Vol. 19, No. 3, 168-181, September 1, 2008
1-4]. The genes have been studied exten-sively and were isolated from plants, fungi, microbes, and even animals [reviewed in 3-5]. LEA proteins are classified into at least 5 groups on the basis of their con-served sequences [3,4,6] or at least 6 groups according to the “Protein or Oligonucleotide Probability Profile,” which shows over- or under-representation of particular amino
Expression of Glycine max Physiologically mature Genes
in Soybean (Glycine max L.) Tissues
Yuan-Ching Tsai
1, Ming-Tsung Wu
1, Jaw-Shu Hsieh
2, Yue-Ie C. Hsing
1and Ming-Der Shih
1*1. Institute of Plant and Microbial Biology, Academia Sinica, Taipei, 11529, Taiwan
2. Department of Agronomy, National Taiwan University, Taipei, 10617, Taiwan
Late embryogenesis abundant (LEA) genes, in abundance in seeds dur-ing the late stages of development, are associated with desiccation tolerance. In the present work, we characterized the expression patterns of 15 soybean Glycine max physiologically mature (GmPM) genes, including 11 typical and 4 atypical LEA genes, by RT-PCR and in silico analysis. The transcripts of all tested genes were detected in mid-developmental or pod-dried soybean seeds. However, only the expression of GmPM6, a LEA II gene, was sig-nificantly enhanced under high salt and dehydration conditions. In silico study confirmed this observation. Under normal conditions, GmPM4, 6, 17, 25, 30, and 39 were expressed in mature flowers. In addition, GmPM4, 5, 6, 17, 25, and 28 expression was detected in several vegetative tissues, such as nodules, roots, stems, leaves, or germinating cotyledons. Therefore, soy-bean GmPM genes might be involved in a complex regulation pathway for osmotic stress.
Key words: expression patterns, in silico analysis,
late embryogenesis abundant, RT-PCR, soybean
∗ Corresponding authors: Ming-Der Shih; Institute of Plant and Microbial Biology, Academia Sinica,
128 Academia Rd Sec 2, Taipei, Taiwan. Tel: 886-2-2787-1049 FAX: 886-2-2782-7954 <patlabor@gate.sinica.edu.tw>
acids in protein sequences [5,7]. The pri-mary structures and hydropathy studies suggested that the LEA I to IV proteins are hydrophilic, whereas the LEA V proteins are hydrophobic. Structural analysis suggested that hydrophilic LEA proteins are members of “natively unfolded proteins” and change their conformations when internal water molecules are removed [e.g. 8-11]. The 3-D structures of a D-95-type LEA V protein, Arabidopsis AtLEA7-1, revealed an αβ-fold consisting of one α-helix and seven β-strands that form two antiparallel β-sheets [12].
The expression profiles of LEA genes in diverse species were also reported. In gen-eral, most LEA genes, such as rice Rab16A-D [13], barley HVA1 [14], soybean GmPM8 [15] and tomato Le25 [16], are expressed in both reproductive and vegetative organs. However, some, such as wheat Em [17], cot-ton D19 [18], carrot Dc8 [19], and B. napus Lea76 [20], are seed or embryo specific, and others, such as Arabidopsis Cor47 [21] and Lit29 [22], spinach Cap85 [23], and several Craterostigma plantagineum LEA II, III, IV, and V genes [24-26], are vegetative-tissue specific. Detailed analysis revealed that the expression of LEA genes is regulated sig-nificantly by temporal and spatial effects. The mRNA expression of HVA1, a barley LEA III gene, was induced in roots, as well as in coleoptiles and leaves, in ABA- or drought-treated 3-day-old seedlings but was barely detected in leaves and coleoptiles in 7-day-old treated seedlings [14]. The expression patterns of two Arabidopsis Em genes, AtEm1 and AtEm6, showed AtEm1 transcripts first detected about 13-14 days after pollination and mainly accumulated in vasculature and pericycle, whereas those of AtEm6 were detected 3 days later and expressed throughout the embryo [27,28]. Therefore, considering their redundancy and high level of expression, extreme hydro-philic property, protein structure, as well as timing for accumulation, LEA proteins were suggested to be one of the key factors in
the acquisition of desiccation tolerance. For example, the protein accumulation of barley HVA1 conferred water-deficit and salt-stress tolerance in transgenic cereals [29-32].
Orthodox seeds undergo dehydration and maturation during the late developmen-tal stage. Several studies from various spe-cies suggested that the moisture loss during seed maturation is critical for the embryo phase transition [33,34]. Interestingly, arti-ficially drying immature soybean seeds also revealed similar embryo phase transition. This process was termed as precociously maturation or physiology maturation [35]. We isolated 41 cDNA clones from preco-ciously matured soybean seeds and charac-terized several of them [10,15,36-39]. These clones were designated GmPM, for Glycine max physiologically mature. Over half of them belong to LEA genes, including 1 LEA I, 3 LEA II, 6 LEA III, 6 LEA IV, and 5 LEA V and 7 atypical LEA genes. Although the abundance of LEA genes during treatment to induce precocious maturation suggests the relation between LEA genes and water loss and the expression of numerous LEA genes is induced under exogenous ABA or envi-ronmental stress treatment, little information exists on the expression profiles of soybean LEA genes under hormone or water-deficit treatment. In the present study, we character-ized the expression patterns of 15 soybean GmPM genes, including 11 typical and 4 atypical LEA genes, by RT-PCR and in silico analysis. The transcripts of all tested GmPM genes showed high accumulation in mid-developmental or pod-dried seeds.
Materials and Methods
Plant materials
Soybean (Glycine max L. cv. Shi-shi) seeds were kindly provided by the Kaohsiung Agricultural Experimental Station, Pintong, Taiwan. Plants were grown to maturity in a greenhouse or field
environ-ment under normal day-length. Germinating cotyledons were sampled around the single-leaf stage. Nodules, roots, stems, leaves or mature flowers were collected during flowering. Pods were harvested at mid-developmental stage, about 35 days after flowering (DAF), and seeds were induced to undergo precocious maturation by air-drying the intact pods (pod-dried) for 4 days as described previously [10]. Soybean seed-lings with the first compound leaves fully expanded were cultured for stress treatments in half-strength Johonson’s solution [40]. Leaves of control plants or those treated with 100 µM ABA, 50 or 150 mM NaCl, or 2% PEG6000 solution for 1 day, or air-dried for 3 hr at room temperature were harvested. All plant materials were frozen in liquid nitrogen and stored at -80 oC until use.
RNA extraction and analysis
An amount of 500 mg tissue was ground to a fine powder in the presence of liquid nitrogen. Total RNA was extracted by use of
TRIzol (ver. 12, Invitrogen) and chloroform, then precipitated with a 0.5 volume of iso-propanol and sodium chloride. After wash-ing and cleanwash-ing, the RNA was redissolved with double-distilled water. Gene expression analysis involved quantitative RT-PCR. First-strand cDNA synthesis was performed with 0.2 µg total RNA and the Superscript III first-strand cDNA synthesis kit according to the manufacturer's instructions (Invitrogen). Various primers used to detect the endoge-nous LEA genes or elongation factor 1 (EF-1) are listed in Table 1. PCR reaction carried out 28 cycles of denaturation for 30 sec at 95 °C; annealing for 45 sec at 54 °C; and exten-sion for 60 sec at 72 °C.
Sequence analysis
Digital Northern analysis of GmPM genes involved use of the public soybean expressed sequence tag (EST) database downloaded from NCBI by use of BLAST algorithms [41]. The query sequences were for typical LEA proteins, such Table 1. Primers used in RT-PCR analysis of GmPm genes in soybean.
Clone Forward Reverse
GmPM 1 CTGGAACCGCCACGTATTC TTAAGTACCCCCAGTCCCATACC GmPM 2 TGCAAGGAGGAAAATGGAAG GAAGAGAATGTAAACAAGCAAACC GmPM 3 CTTGGCTTTGCTAGTTGGTGC GGCTTGTGCATTGGATCTCC GmPM 4 GAAACCGTTTAAAAACACTATGGG AACACAGCGTTCACTCAAGAG GmPM 5 CCATGATTAGCACCTCAACTCC ACACGTGACCCCAGGTTGTG GmPM 6 TACTATGGAACCAACACCGC GCACAACATCTCTGCCTTTATT GmPM 8 GGTGATGTAGCACAGAATGC TTCTCAAAGCTCAGCATCCC GmPM11 GATGAAAGAGCAAGGCAAGGAG CACATGCTAGTGAAGGGTGTTACAT GmPM12 TCTGAGGATGATGGGCAAGG TGCACAAGATACTCAGCCCATG GmPM16 CAAGTGCTGCCAAGGAACAAG TGGCCAATGGGACAAGTACC GmPM17 GTCGCAGTTGTTGGACAAAGC CCAATGTCCTTTGCCAAGCT GmPM25 GTGGCGTCACAGTCACAGAAAC AAGCAGCAGCTGATTGAGCC GmPM28 ATGGCGAAGAGCAAGGAAGAC TTTGGGGCTATCCAGGAGCT GmPM30 AAGCTGGTCAAACTAAGGGCC CCCTTCACCTTCTCCCCTGT GmPM39 GTAATGTGGGTGTGGAACAGGAC CTGTCCCTGGGCTATTCACATAC
EF1* TGTTGCTGTTAAGGATTTGAAGCG AACAGTTTGACGCATGTCCCTAAC
as GmPM1 (M80666), 2 (M80664), 6 (M94012), 8 (Z22872), 11 (AF004805), 12 (AF004807), 16 (AF004810), 17 (U08108), 25 (AF116754), 28 (AF117724), and 30 (AF117884), and for atypical LEA proteins, such as GmPM3 (L20806), 4 (U59626), 5 (U59425), and 39 (AF169024).
Results
Tissue-specific expression of GmPM
clones
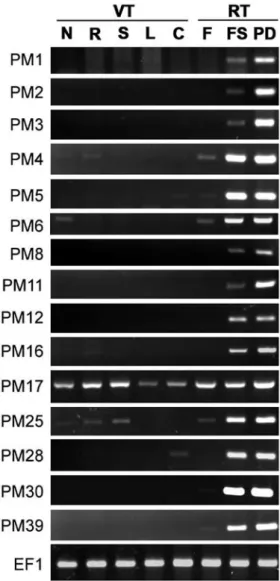
To understand the expression patterns of GmPM genes in different tissues, we isolated total RNA from germinating coty-ledons, leaves, roots, stems, nodules and flowers from mature plants, as well as mid-developmental or pod-dried seeds, under normal conditions for RT-PCR analysis. All GmPM transcripts could be detected in mid-developmental or pod-dried seeds, with fewer transcripts for GmPM1, 2, 3, 8, and 11 in mid-developmental seeds (Fig. 1). Thus, all tested GmPM genes were LEA-A-type genes [42]. Mid-developmental and pod-dried seeds showed a similar expression level of GmPM4, 5, 6, 25, 28, and 30, which also suggested that the expression of these genes started before the seed water content dropped below 68%-79% [34]. In addition, the transcripts of GmPM4, 6, 17, 25, 30, and 39 were slightly accumulated in mature flow-ers. Because pollen grains are able to reduce moisture during maturation as seeds develop, these genes might be expressed in the mature pollen grains. Moreover, the expression of most GmPM genes, except for GmPM17 and GmPM25, was barely detectable in vegeta-tive tissues. GmPM4 and 16 were slightly expressed in roots, among all tissues ana-lyzed, whereas the transcripts of GmPM5 and 28 were detected in germinating cotyledons. In addition, the mRNA level of GmPM6 was slightly accumulated in nodules and germi-nating cotyledons. By contrast, the D-95-type LEA V gene GmPM17 was expressed
con-stitutively in all vegetative tissues, whereas the D-34-type gene GmPM25 was expressed in nodules, roots, or stems.
Fig. 1. Tissue-specific expression of GmPM (PM) genes in different tissues and developmental stages in soybean. The samples included vegetative tissues (VT), such as nodules (N), roots (R), stem (S), leaves (L), germinating cotyledons (C), as well as reproductive tissues (RT), such as mature flowers (F), 35 DAF seeds (FS), and 4-day pod-dried seeds (PD). Transcripts of soybean elongation factor 1 gene (EF1) were used as a control.
ABA or osmotic stress differentially
regulated the expression of GmPM
genes
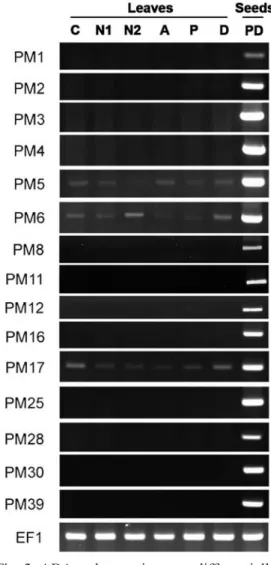
The expression patterns of GmPM genes in response to ABA or osmotic stress treat-ment are illustrated in Figure 2. Although several LEA genes were suggested to be expressed during ABA or osmotic stress, RT-PCR analysis revealed most soybean GmPM genes not expressed under these treatments. Only the transcripts of GmPM5, 6, and 17 were detected under both stresses. The expression level of GmPM5 under ABA or osmotic stress treatment was simi-lar to that of the control, but the level was decreased with NaCl or PEG treatment. Under high salt conditions (150 mM NaCl), the expression of GmPM5 was nearly sup-pressed. The expression of GmPM17 was decreased with ABA, salt, or PEG treat-ment. GmPM6 expression was enhanced by high salt or dehydration; however, the level of GmPM6 was barely detected with ABA or PEG treatment and was slightly reduced with weak salt treatment. Thus, the regula-tion of GmPM6 might be independent of the ABA signaling pathway.
In silico analysis of soybean GmPM
clones
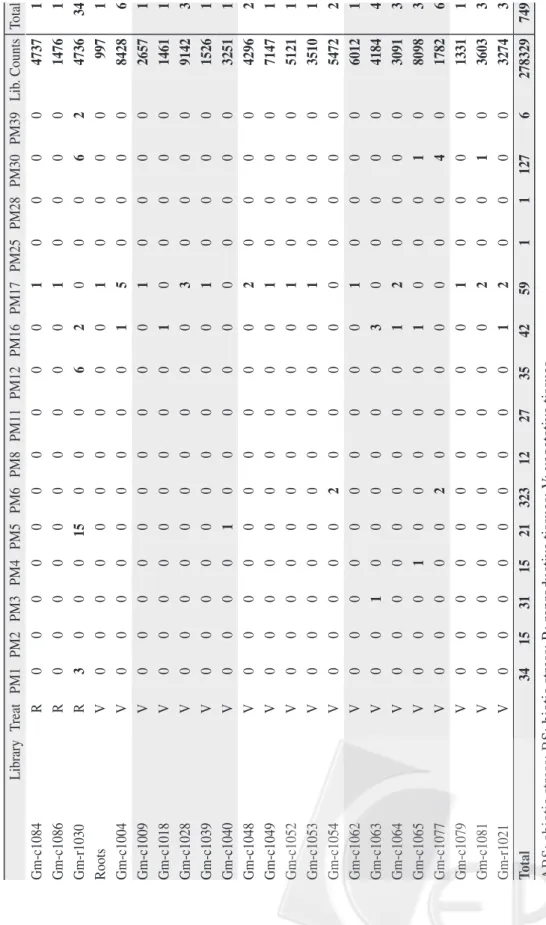
The publicly available EST databases are a useful tool for gene functional analy-sis. At the end of June 2008, the soybean EST database contained 435,347 entries. Hence, we used this database to quantify the expression level of specific genes in various tissues or under various treatments. BLASTN Algorithm was used to search the current soybean EST database, with selected GmPM sequences used as queries (see Materials and Methods). In total, we found 537 GmPM ESTs from 48 librar-ies (Table 2). These included 155 ESTs of 68,153 entries for reproductive tissues such as seeds (including cotyledons, seed coats, and seed pods), somatic embryos, and
flo-ral tissues, as well as another 314 ESTs of 62,088 entries for biotic or abiotic stress-treated tissue. Only 45 ESTs of 165,485 entries were found for seedlings or vegeta-tive tissues (leaves, roots, or stems), and 64 ESTs of 87,947 entries were from mixed libraries. No library was constructed from
Fig. 2. ABA and osmotic stress differentially regulate the expression of GmPM genes. The samples included compound-leaf untreated (C) or treated with 50 mM NaCl (N1), 150 mM NaCl (N2), 100 µM ABA (A), 2% PEG, and drought treatment (D), as well as 4-day pod-dried seeds (PD). Transcripts of soybean EF1 were used as controls.
Table 2. In silico analysis of soybean GmPM clones Library Treat PM1 PM2 PM3 PM4 PM5 PM6 PM8 PM11 PM12 PM16 PM17 PM25 PM28 PM30 PM39 Lib . Counts Total Gm-c1066 ABS 8 0 2 0 0 68 0 0 0 2 0 0 0 5 0 3720 85 Gm-c1068 ABS 13 0 1 1 0 178 0 0 0 2 2 0 0 9 1 5809 207 gmrtDrNS01 ABS 3 0 0 0 0 0 0 0 0 2 2 0 0 4 0 13430 11 Salic ylic Acid ABS 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 29540 1 H. glycines infection BS 0 0 0 0 0 3 0 0 0 0 0 0 0 1 0 359 4 cyst nematode’ s infection BS 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 444 1 cyst nematode’ s infection BS 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 20 1 P. sojae infection BS 0 0 0 0 0 0 0 0 0 1 1 0 0 2 0 3553 4 JCVI-SO Y1 M 0 0 6 2 3 7 4 1 1 2 3 0 0 11 1 22155 41 JCVI-SO Y2 M 0 0 1 0 0 3 0 0 1 0 12 0 0 1 0 21252 18 JCVI-SO Y3 M 0 0 0 0 0 0 0 0 0 1 2 0 0 2 0 17777 5 GmaxSC R 1 1 0 0 0 0 0 0 0 0 0 0 0 3 0 2076 5 Gm-c1007 R 0 0 0 0 0 0 0 0 2 0 0 0 0 4 0 2396 6 Gm-c1010 R 0 0 0 0 0 0 0 0 1 1 0 0 0 0 0 1154 2 Gm-c1011 R 0 0 0 0 0 0 0 0 2 0 0 0 0 0 0 337 2 Gm-c1015 R 0 0 0 0 0 0 0 0 1 0 1 0 0 0 0 5827 2 Gm-c1016 R 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 9146 1 Gm-c1023 R 0 0 1 1 0 2 1 0 0 0 2 0 0 1 0 3689 8 Gm-c1027 R 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 7656 1 Gm-c1029 R 0 0 0 0 0 0 0 0 0 1 0 0 0 2 0 1540 3 Gm-c1036 R 1 0 1 0 0 3 0 0 9 9 0 1 0 5 0 10720 29 Gm-c1051 R 0 0 0 0 0 0 0 0 0 0 3 0 0 0 0 6591 3 Gm-c1055 R 0 0 0 0 2 0 0 0 1 0 0 0 0 0 0 3565 3 Gm-c1071 R 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 4230 1 Gm-c1075 R 0 0 0 0 0 1 0 0 7 4 0 0 0 0 0 4034 12
Library Treat PM1 PM2 PM3 PM4 PM5 PM6 PM8 PM11 PM12 PM16 PM17 PM25 PM28 PM30 PM39 Lib . Counts Total Gm-c1084 R 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 4737 1 Gm-c1086 R 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 1476 1 Gm-r1030 R 3 0 0 0 15 0 0 0 6 2 0 0 0 6 2 4736 34 Roots V 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 997 1 Gm-c1004 V 0 0 0 0 0 0 0 0 0 1 5 0 0 0 0 8428 6 Gm-c1009 V 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 2657 1 Gm-c1018 V 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 1461 1 Gm-c1028 V 0 0 0 0 0 0 0 0 0 0 3 0 0 0 0 9142 3 Gm-c1039 V 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 1526 1 Gm-c1040 V 0 0 0 0 1 0 0 0 0 0 0 0 0 0 0 3251 1 Gm-c1048 V 0 0 0 0 0 0 0 0 0 0 2 0 0 0 0 4296 2 Gm-c1049 V 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 7147 1 Gm-c1052 V 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 5121 1 Gm-c1053 V 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 3510 1 Gm-c1054 V 0 0 0 0 0 2 0 0 0 0 0 0 0 0 0 5472 2 Gm-c1062 V 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 6012 1 Gm-c1063 V 0 0 1 0 0 0 0 0 0 3 0 0 0 0 0 4184 4 Gm-c1064 V 0 0 0 0 0 0 0 0 0 1 2 0 0 0 0 3091 3 Gm-c1065 V 0 0 0 1 0 0 0 0 0 1 0 0 0 1 0 8098 3 Gm-c1077 V 0 0 0 0 0 2 0 0 0 0 0 0 0 4 0 1782 6 Gm-c1079 V 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 1331 1 Gm-c1081 V 0 0 0 0 0 0 0 0 0 0 2 0 0 1 0 3603 3 Gm-r1021 V 0 0 0 0 0 0 0 0 0 1 2 0 0 0 0 3274 3 Total 34 15 31 15 21 323 12 27 35 42 59 1 1 127 6 278329 749
ABS: abiotic stress; BS: biotic stress; R: reproducti
ve tissues; V : v egetati ve tissues. Table 2. In silico analysis of soybean GmPM clones (Continued)
mature or pod-dried seeds in all 146 soy-bean EST libraries. Hence, the entries of GmPM ESTs for reproductive tissues might be low in the present libraries. For example,
GmPM2, 11, and 25 appeared only once in
the EST databases, and no EST was found for GmPM28. However, our RT-PCR results showed high accumulation of the transcripts of these genes in mid-developmental or pod-dried seeds. A total of 59 GmPM17 ESTs appeared in 30 libraries, which suggests that
GmPM17 might be expressed constitutively,
which confirmed our RT-PCR results. Salt- and drought-stressed tissues accounted for 292 ESTs of 9,529 entries, including 246
GmPM6 ESTs. This finding suggested that
GmPM6 genes are highly regulated
dur-ing salt or drought stress in vegetative tis-sues, which confirmed our RT-PCR results. Although several LEA genes were reported to be involved in the osmotic-response path-way, the EST sequences of 8 tested GmPM clones, GmPM2, 4, 5, 8, 11, 12, 25, and 28, were not detected in abiotic stress-treated vegetative tissues.
Discussion
In the present study, we characterized the expression profiles of several soybean GmPM genes, including 11 typical and 4 atypical LEA genes, by RT-PCR and in
silico analysis in different tissues or under
various stress conditions. All GmPM genes were expressed in seeds 35 DAF, which suggests that they are LEA-A-type genes. In addition, the expression of all genes was induced in response to seed moisture loss, so they respond to dehydration. The results of RT-PCR or in silico analysis suggest that most GmPM genes are regulated by spatial effects.
GmPM11 is similar to Em6-type LEA
I by having only one 20-mer motif [43]. Previous analysis suggested that LEA I genes, such as radish p8B6 [44], wheat Em [45], cotton D-19 [18], barley B19 [46,47],
carrot Emb-1 [48], Arabidopsis AtEm1 and
AtEm6 [27,43,49], Avena fatua AF14 [50],
and soybean Sle1 [51], were seed or embryo specific. Our RT-PCR and in silico results confirmed these observations. In addi-tion, Arabidopsis AtEm1 transcripts were detected about 13-14 days after pollination and AtEm6 transcripts 3 days later, which indicates that the AtEm1 gene belongs to
LEA-A-type genes, whereas AtEm6 is an
LEA type, and GmPM11 performs a LEA-A-type temporal regulation. Thus, the number of 20-mer motifs might not be related to the regulation of the Em gene.
Two soybean LEA II genes, GmPM6 (Y2K type) and GmPM12 (Y3SK type), were
shown to be LEA-A type in mid-develop-mental seeds. However, in vegetative tissues, these two genes had different expression patterns. Both RT-PCR and in silico analy-sis indicated that GmPM6 was expressed in vegetative tissues and mature flowers and the expression was induced in response to high salt or dehydration. By contrast, GmPM12 is seed or embryo specific, and transcripts did not accumulate under ABA or stress treatment. Previous studies had suggested that dehydrins in the same group might or might not give similar organ-, development-, or stress-specific expression patterns. For instance, 5 Arabidopsis dehydrin genes --
Cor47 (SK3 type), Lti29 (SK2 type), Erd14
(SK2 type), Lti30 (K6 type), and Rab18
(Y2SK2 type) -- show differential expres-sion patterns in response to low temperature, salinity, and ABA treatment in root, stem and leaf tissues. The accumulation of Erd14 and Lti30 mRNA is constitutive, but Lti30 is induced in response to low temperature or salinity in tested tissues. Cor47 and Lti29 are mainly expressed in roots and stems and slightly expressed in leaves under low temperature. Rab18 mRNA accumulates only in ABA- and salinity-treated tissues. As is common, the accumulation of dehydrin genes is higher in roots and stems than in leaves [22,52,53]. Four members of the rice
Rab family (Rab21, rab16B to D), which are arrayed in tandem on chromosome 11 and have similar sequences (YSK2 type), are expressed in ABA-treated seedlings, and only Rab16D is undetected in mature seeds [13,54]. Thus, the expression patterns of dehydrins reveal complex regulation pathways in seeds or under environmental stresses.
Three soybean LEA III genes, with their deduced protein sequences containing dif-ferent numbers and consensus sequences of 11-mer repeats, were used to examine expression profiles. The deduced protein sequence of GmPM2 genes contains 19 11-mer repeats and one additional 36-mer motif. RT-PCR and in silico results sug-gested that GmPM2 is seed or embryo spe-cific, although the expression of its wheat ortholog MA1949 is induced by water stress in seedlings [55]. The deduced pro-tein sequence of GmPM8 shows 32 11-mer repeats and a signal peptide in the N ter-minal. Our previous northern blot analysis revealed that the transcripts of the GmPM8 homolog GmPM10 accumulate in callus or seedlings under drought or chilling treat-ment, as well as in seeds [15]. In the present study, however, the transcripts of GmPM8 were detected only in mid-developmental seeds. GmPM30 is related to D-7-type LEA genes, with five 11-mer repeats. The tran-scripts of GmPM30 were detected in mid-developmental seeds. In silico results sug-gested that GmPM30 might be expressed under abiotic stress. Considering the low level of EST entries for this gene -- 5, 9, and 4 ESTs of 3,720; 5,809; and 13,430 entries, respectively -- GmPM30 transcripts pos-sibly were not detected by RT-PCR. Animal orthologs of GmPM30 were suggested to be induced in response to anhydrobiotic stress [e.g. 56-59]. In conclusion, although numbers of LEA III genes are responsive to various environmental stresses, the tested soybean LEA III genes are mainly expressed in seeds 35 DAF.
We used three different LEA IV genes --
GmPM1, GmPM16 and GmPM28 --
accord-ing to their conserved N-terminal domains to examine their expression profiles. RT-PCR results showed these genes highly expressed in pod-dried seeds and their expression not induced in response to exogenous ABA or osmotic stress treatment, although in
silico analysis showed small amounts of
GmPM1 or GmPM16 ESTs. LEA IV genes
have been isolated from several plant spe-cies, including cotton, cultivar or wild-type tomato, sunflower, soybean, Lemna gibba, Arabidopsis, Phaseolus vulgaris, white spruce, birch, and C. plantagineum, as well as genome sequences from various plant species [see reviewed in 1,3,4]. Unlike the protein sequences of LEA I to III genes, the deduced protein sequences of LEA IV genes lack consensus motifs or sequences. In gen-eral, the N-terminal domains contain several conserved small and charged amino acid residues, whereas the variable C-terminal regions include a large number of non-polar amino acid residues. In most studies, LEA IV genes were expressed in both mature seeds and stress-treated vegetative tissues [e.g. 16,25,60-62]. Arabidopsis PAP51, however, is seed specific [49].
As typically hydrophobic LEA genes, D - 9 5 - t y p e G m P M 1 7 a n d D - 3 4 - t y p e
GmPM25 displayed expression patterns
dif-ferent from those of typically hydrophilic LEA genes. The transcripts of GmPM17 were detected in all tested tissues, although the accumulation in leaves or germinating cotyledons was lower than that in other tis-sues. In silico analysis also confirmed the observation. However, the expression of
GmPM17 was decreased under exogenous
ABA or osmotic stress treatment in vegeta-tive tissue. The transcripts of GmPM25 accumulate in roots, stems, flowers, or seeds. No GmPM25 transcripts were detected in leaves under normal or stress-treated condi-tions. We found only one GmPM25 hit in the whole EST database (435,347 entries).
This finding might be explained by ran-dom selection for each cDNA library or the tissue to construct the library. Previous analysis showed several LEA V genes, such as cotton D-34 and D-95, carrot ECP31, or Arabidopsis PAP140 and AtRAB28, expres-sed in seeds or induced in young embryos by dehydration [18,49,63,64]. The expression of other LEA V genes, such as C. Plantagineum
pcC27-45 and pcC16-81, maize RAB28,
tomato ER5, and hot pepper CaLEA6, was induced or enhanced by salt or dehydration in vegetative tissues [24,26,65-68]. Soybean
GmPM17 and GmPM25 and Arabidopsis
Atecp31 [64] are examples of LEA V genes
expressed both in seeds and vegetative tis-sues.
Similar to the protein sequences of LEA V genes, the deduced sequences of 4 tested atypical LEA genes are hydrophobic. The
GmPM3 homolog, wheat AWPM-19, was
suggested to be involved in freezing toler-ance [69]. The deduced proteins of GmPM3 and AWPM-19 contain a conserved domain that may act as transmembrane domains. In our study, the transcripts of GmPM3 were detected only in mid-developmental or pod-dried seeds and were absent under exogenous ABA or osmotic stress treat-ment. GmPM4 protein and its homolog pea SBP65 are biotinylated proteins and mainly accumulate in mature seeds [37,70]. Our RT-PCR analysis revealed the transcripts of GmPM4 in nodules, roots, and mature flowers under normal conditions and not in seedlings under exogenous ABA or osmotic stress treatment. The product of GmPM5 was annotated as a 7S seed globulin precur-sor, with its transcripts slightly accumulating in germinating cotyledons or leaves under normal conditions and detected in seedlings under exogenous ABA or osmotic stress treatment. Severe salt stress might suppress the expression of GmPM5. RT-PCR and in
silico results revealed GmPM39 expressed
in seeds or mature flowers under normal conditions and not ABA- or stress-treated
conditions. However, the GmPM39 homolog spinach CAP160 showed the response under chilling or severe water stress [71]. Hence, these two genes might be involved in diverse regulation pathways.
Acknowledgements
We express our deep thanks to Ms. Laura Heraty for editing services. This research was supported by National Science Council and Academia Sinica, Taiwan, to YIC Hsing.
References
[1] Dure L, Crouch M, Harada J, Ho THD, Mundy J, Quatrano R, Thomas T, Sung ZR: Common amino acid sequence domains among the LEA proteins of higher plants. Plant Molecular Biology 1989; 12, 475-486. [2] Ingram J, Bartels D: The molecular basis of dehydration tolerance in plants. Annual Review of Plant Physiology and Plant Molecular Biology 1996; 47, 377-403. [3] Cuming AC: LEA proteins. In Seed
Proteins Edited by Shewry PR, Casey R. Kluwer Academic Publishers, Dordrecht, Netherlands. 1999; 753-780.
[4] Shih MD, Hoekstra, FA, Hsing YIC: Late embryogenesis abundant proteins. Advance in Botanical Research 2008; in press [5] Tunnacliffe A Wise MJ: The
continu-ing conundrum of the LEA proteins. Naturwissenschaften (2007); 94, 791-812. [6] Dure III L: Structural motifs in Lea proteins.
In Plant responses to cellular dehydration during environmental stress Edited by Close TJ, and Bray EA. American Society of Plant Physiologyogists Series. USA. 1993; 91-103
[7] Wise MJ: LEAping to conclusions: A com-putational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinformatics 2003; 4, 52-70. [8] Wolkers WF, McCready S, Brandt WF,
Lindsey GG, Hoekstra FA: Isolation and characterization of a D-7 LEA protein from pollen that stabilizes glasses in vitro. Biochimica et Biophysica Acta 2001; 1544, 196-206.
[9] Goyal K, Tisi L, Basran A, Browne J, Burnell A, Zurdo J, Tunnacliffe A:
Transition from natively unfolded to folded state induced by desiccation in an anhydrobiotic nematode protein. Journal of Biological Chemistry 2003; 278, 12977-12984.
[10] Shih MD, Lin SC, Hsieh JS, Tsou CH, Chow TY, Lin TP, Hsing YIC: Gene cloning and characterization of a soybean (Glycine
max L.) LEA protein, GmPM16. Plant Molecular Biology 2004; 56, 689-703. [11] B o u d e t J , B u i t i n k J , H o e k s t r a FA ,
Rogniaux H, Larre C, Satour P, Leprince O: Comparative analysis of the heat stable pro-teome of radicles of Medicago truncatula seeds during germination identifies late embryogenesis abundant proteins associated with desiccation tolerance. Plant Physiology 2006;140, 1418-1436.
[12] Singh S, Cornilescu CC, Tyler RC, Cornilescu G, Tonelli M, Lee MS, Markley JL: Solution structure of a late embryo-genesis abundant protein (LEA14) from
Arabidopsis thaliana, a cellular stress-related protein. Protein Science 2005; 14, 2601-2609.
[13] Mundy J, Chua NH: Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO Journal 1988; 7, 2279-2286.
[14] Hong B, Barg R, Ho THD: Developmental and organ-specific expression of an ABA- and stress-induced protein in barley. Plant Molecular Biology 1992; 18, 663-674. [15] Hsing YIC, Chen ZY, Shih MD, Hsieh JS,
Chow TY: Unusual sequences of group 3 LEA mRNA inducible by maturation or drying in soybean seeds. Plant Molecular Biology 1995; 29, 863-868.
[16] Cohen A, Bray EA: Nucleotide sequence of an ABA-induced tomato gene that is expressed in wilted vegetative organs and developing seeds. Plant Molecular Biology 1992; 18, 411-413.
[17] Morris PC, Kumar A, Bowles DJ, Cuming AC: Osmotic stress and abscisic acid in-duce expression of the wheat Em genes. European Journal of Biochemistry 1990; 190, 625-630.
[18] Hughes DW, Galau GA: Developmental and environmental induction of Lea and LeaA mRNAs and the postabscission program during embryo culture. Plant Cell 1991; 3, 605-618.
[19] Choi JH, Liu LS, Borkird C, Sung ZR: Cloning of genes developmentally regulated
during plant embryogenesis. Proceedings of the National Academy of Sciences of the United States of America 1987; 84, 1906-1910.
[20] Harada JJ, DeLisle AJ, Baden CS, Crouch ML: Unusual sequence of an abscisic acid-inducible mRNA which accumulates late in Brassica napus seed development. Plant Molecular Biology 1989; 12, 395-401. [21] Gilmour SJ, Artus NN, Thomashow MF:
cDNA sequence analysis and expression of two cold-regulated genes of Arabidopsis thaliana. Plant Molecular Biology 1992; 18, 13-21.
[22] Nylander M, Svensson J, Palva ET, Welin BV: Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana. Plant Molecular Biology 2001; 45, 263-279.
[23] Neven LG, Haskell DW, Hofig A, Li QB, Guy CL: Characterization of a spinach gene responsive to low temperature and water stress. Plant Molecular Biology 1993; 21, 291-305.
[24] Piatkowski D, Schneider H, Salamini F, Bartels D: Characterization of five ab-scisic acid-responsive cDNA clones iso-lated from the desiccation-tolerance plant
Craterostigma plantagineum and their re-lationship to other water-stress genes. Plant Physiology 1990; 94, 1682-1688.
[25] Ditzer A., Kirch HH, Nair A, and Bartels D: Molecular characterization of two alanine-rich Lea genes abundantly expressed in the resurrection plant C. plantagineum in re-sponse to osmotic stress and ABA. Journal of Plant Physiology 2001; 158, 623-633. [26] Smith-Espinoza CJ, Richter A, Salamini F,
Bartels D: Dissecting the response to dehy-dration and salt (NaCl) in the resurrection plant Craterostigma plantagineum. Plant, Cell & Environment 2003; 26, 1307-1315. [27] Bies N, Aspart L, Carles C, Gallois P,
Delseny M: Accumulation and degradation of Em proteins in Arabidopsis thaliana: Evidence for post-transcriptional controls. Journal of Experimental Botany 1998; 49, 1925-1933.
[28] Vicient CM, Gruber V, Delseny M: The Arabidopsis AtEm1 promoter is active in
Brassica napus L. and is temporally and spatially regulated. Journal of Experimental Botany 2001; 52, 1587-1591.
[29] Xu D, Duan X, Wang B, Hong B, Ho THD, Wu R: Expression of a late embryogenesis
abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiology 1996; 110, 249-257.
[30] Sivamani E, Bahieldinl A, Wraith JM, Al Niemi T, Dyer WE, Ho THD, Qu R: Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively express-ing the barley HVA1 gene. Protein Science 2000; 155, 1-9.
[31] Maqbool B, Zhong H, El Maghraby Y, Ahmad A, Chai B, Wang W, Sabzikar R, Sticklen B: Competence of oat (Avena
sati-va L.) shoot apical meristems for integrative transformation, inherited expression and os-motic tolerance of transgenic lines contain-ing hva1. Theoretical and Applied Genetics 2002; 105, 201-208.
[32] Babu RC, Zhang J, Blum A, Ho THD, Wu R, Nguyen HT: HVA1, a LEA gene from barley confers dehydration tolerance in transgenic rice (Oryza sativa L.) via cell membrane protection. Protein Science 2004; 166, 855-862
[33] Armstrong C, Black M, Chapman JM, Norman HA, Angold R: The induction of sensitivity to gibberellin in aleurone tissue of developing wheat grains. Planta 1982; 154: 573-577.
[34] Rosenberg LA, Rinne RW: Moisture loss as a prerequisite for seedling growth in soybean seeds (Glycine max L. Merr.). Journal of Experimental Botany 1986; 37, 1663-1674.
[35] Rosenberg LA, Rinne RW: Protein synthesis during rehydration, germination and seed-ling growth of naturally and precociously matured soybean seeds (Glycine max). Annals of Botany 1989; 64, 77-86
[36] Hsing YIC, Wu S: Cloning and character-ization of cDNA clones encoding soybean seed maturation polypeptides. Botanical Bulletin of Academia Sinica 1992; 33, 191-199
[37] Hsing YIC, Tsou CH, Hsu TF, Chen ZY, Hsieh KL, Hsieh JS, Chow TY: Tissue- and stage-specific expression of a soybean (Glycine max L.) seed-maturation, biotinyl-ated protein. Plant Molecular Biology 1998; 38: 491-490
[38] Lee PF, Hsing YIC, Chow TY: Promoter activity of a soybean gene encoding a seed maturation protein, GmPM9. Botanical Bulletin of Academia Sinica 2000; 41,
175-182
[39] Shih MD, Hsieh JS, Hsing YIC: Regulation of the Soybean GmPM9 Promoter in Callus Tissue. Journal of Genetics and Molecular Biology 2001; 12, 125-137.
[40] Hewitt EJ: Sand and water culture method used in study of plant nutrition. Technical C o m m u n i c a t i o n N o . 2 2 ( R e v i s e d ) , Commonwealth Bureau of Horticulture and Plantation Crops, East Mailing Maidstone, Kent, England. 1966.
[41] Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ: Gapped BLAST and PSI-BLAST: a new generation of protein database search pro-grams. Nucleic Acids Research 1997; 25, 3389-3402.
[42] Hughes DW, Galau GA: Temporally modu-lar gene expression during cotyledon de-velopment. Genes & Development 1989; 3, 358-369.
[43] Gaubier P, Raynal M, Hull G, Huestis GM, Grellet F, Arenas C, Pages M, Delseny M: Two different Em-like genes are expressed in Arabidopsis thaliana seeds during matu-ration. Molecular & General Genetics 1993; 238, 409-418.
[44] Raynal M, Depigny D, Cooke R, Delseny M: Characterization of a radish nuclear gene expressed during late seed maturation. Plant Physiology 1989; 91, 829-836.
[45] Morris PC, Kumar A, Bowles DJ, Cuming AC: Osmotic stress and abscisic acid induce expression of the wheat Em genes. European Journal of Biochemistry 1990; 190, 625-630 [46] Espelund M, Saeboe-Larssen S, Hughes
DW, Galau GA, Larsen F, Jakobsen KS: Late embryogenesis-abundant genes encod-ing proteins with different numbers of hy-drophilic repeats are regulated differentially by abscisic acid and osmotic stress. Plant Journal 1992; 2, 241-252
[47] Espelund M, De Bebout JA, Outlaw Jr. WH, Jakobsen KS: Environmental and hormonal regulation of barley late-embryogenesis-abundant (Lea) mRNAs is via different signal transduction pathways. Plant, Cell & Environment 1995; 18, 943-949.
[48] Wurtele ES, Wang H, Durgerian S, Nikolau BJ, Ulrich TH: Characterization of a gene that is expressed early in somatic embryo-genesis of Daucus carota. Plant Physiology 1993; 102, 303-312.
[49] Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J:
Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous ab-scisic acid. Plant Cell 1994; 6, 1567-1582. [50] Li B, Foley ME: Cloning and
characteriza-tion of differentially expressed genes in im-bibed dormant and afterripened Avena fatua embryos. Plant Molecular Biology 1995; 29, 823-831.
[51] Calvo ES, Wurtle ES, Shoemaker RC: Cloning, mapping, and analyses of expres-sion of the Em-like gene family in soybean [Glycine max (L) Merr]. Theoretical and Applied Genetics 1997; 94, 957-967 [52] Kiyosue T, Yamaguchi-Shinozaki K,
Shinozaki K: Characterization of two cD-NAs (ERD10 and ERD14) corresponding to genes that respond rapidly to dehydration stress in Arabidopsis thaliana. Plant & Cell Physiology 1994; 35, 225-231
[53] Welin BV, Olson A, Nylander M, Palva ET: Characterization and differential expression of dhn/lea/rab-like genes during cold ac-climation and drought stress in Arabidopsis
thaliana. Plant Molecular Biology 1994; 26, 131-144.
[54] Yamaguchi-Shinozaki K, Mundy J, Chua NH: Four tightly linked rab genes are differ-entially expressed in rice. Plant Molecular Biology 1990; 14, 29-39.
[55] Curry J, Walker-Simmons MK: Unusual sequence of group 3 LEA (II) mRNA induc-ible by dehydration stress in wheat. Plant Molecular Biology 1993; 21, 907-912. [56] Browne J, Tunnacliffe A, Burnell A:
Anhydrobiosis: plant desiccation gene found in a nematode. Nature 2002; 416, 38. [57] Tunnacliffe A, Lapinski J, Mcgee B: A
putative LEA protein, but no trehalose, is present in anhydrobiotic bdelloid rotifers. Hydrobiologia 2005; 546, 315-321.
[58] Kikawada T, Nakahara Y, Kanamori Y, Iwata K, Watanabe M, McGee B, Tunnacliffe A, Okuda T: Dehydration-induced expression of LEA proteins in an anhydrobiotic chironomid. Biochemical and Biophysical Research Communications 2006; 348, 56-61.
[59] Hand SC, Jones D, Menze MA, Witt TL: Life without water: expression of plant
LEA genes by an anhydrobiotic arthropod. Journal of Experimental Zoology - Part A: Ecological Genetics and Physiology 2006; 307, 62-66.
[60] Kahn TL, Fender SE, Bray EA, O'Connell
MA: Characterization of expression of drought- and abscisic acid-regulated to-mato genes in the drought-resistant species
Lycopersicon pennellii. Plant Physiology 1993; 103, 597-605
[61] C o l m e n e r o - F l o r e s J M , C a m p o s F, G a r c i a r r u b i o A , C ova r r u b i a s A A : . Characterization of Phaseolus vulgaris cDNA clones responsive to water deficit: identification of a novel late embryogen-esis abundant-like protein. Plant Molecular Biology 1997; 35, 393-405.
[62] Colmenero-Flores JM, Moreno LP, Smith CE, Covarrubias AA: Pvlea-18, a member of a new late-embryogenesis-abundant pro-tein family that accumulates during water stress and in the growing regions of well-irrigated bean seedlings. Plant Physiology 1999; 120, 93-104.
[63] Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K, Higashi K, Satoh S, Kamada H, Harada H: Isolation and characteriza-tion of a cDNA that encodes ECP31, an embryogenic-cell protein from carrot. Plant Molecular Biology 1992; 19, 239-249 [64] Arenas-Mena C, Raynal M, Borrell A,
Varoquaux F, Cutanda MC, Stacy RA, Pages M, Delseny M, Culianez-Macia FA: Expression and cellular localization of
Atrab28 during Arabidopsis embryogenesis. Plant Molecular Biology 1999; 40, 355-363 [65] Michel D, Furini A, Salamini F, Bartels D:
Structure and regulation of an ABA- and desiccation-responsive gene from the resur-rection plant Craterostigma plantagineum. Plant Molecular Biology 1994; 24, 549-560. [66] Niogret MF, Culianez-Macia FA, Goday A,
Mar AM, Pages M: Expression and cellular localization of rab28 mRNA and Rab28 protein during maize embryogenesis. Plant Journal 1996; 9, 549-557
[67] Zegzouti H, Jones B, Marty C, Lelievre JM, Latche A, Pech JC, Bouzayen M: ER5, a tomato cDNA encoding an ethylene-respon-sive LEA-like protein: characterization and expression in response to drought, ABA and wounding. Plant Molecular Biology 1997; 35, 847-854.
[68] Kim HS, Lee JH, Kim JJ, Kim CH, Jun SS, Hong YN: Molecular and functional characterization of CaLEA6, the gene for a hydrophobic LEA protein from Capsicum
annuum. Gene 2005; 344, 115-123.
[69] Koike M, Takezawa D, Arakawa K, Yoshida S: Accumulation of 19-kDa plasma
mem-brane polypeptide during induction of freezing tolerance in wheat suspension-cultured cells by abscisic acid. Plant & Cell Physiology 1997; 38, 707-716.
[70] Duval M, Job C, Alban C, Douce R, Job D: Developmental patterns of free and protein-bound biotin during maturation and germi-nation of seeds of Pisum sativum:
character-ization of a novel seed-specific biotinylated protein. Biochemistry Journal 1994; 299, 141-150.
[71] Kaye C, Neven L, Hofig A, Li QB, Haskell D, Guy C: Characterization of a gene for spinach CAP160 and expression of two spinach cold-acclimation proteins in tobac-co. Plant Physiology 1998; 116, 1367-1377.