行政院國家科學委員會專題研究計畫 期中進度報告
奈米金屬及金屬化合物之合成(1/3)
計畫類別: 整合型計畫
計畫編號: NSC91-2113-M-009-020-
執行期間: 91 年 08 月 01 日至 92 年 07 月 31 日
執行單位: 國立交通大學應用化學系
計畫主持人: 裘性天
報告類型: 精簡報告
報告附件: 出席國際會議研究心得報告及發表論文
處理方式: 本計畫涉及專利或其他智慧財產權,1 年後可公開查詢
中 華 民 國 92 年 5 月 26 日
2
Two communications have been written and submitted for publication.
1.
Attachment 1, submitted to J. Am. Chem. Soc., in revision.
In-Situ Generation of Silica Shell Layer - Key Factor to Simple High
Yield Synthesis of Silver Nanowires
2.
Attachment 2, submitted to Chem. Comm.
New nanotube synthesis strategy - application of sodium nanotubes
formed inside anodic aluminium oxide as a reactive template
See attached files.
In-Situ Generation of Permethylsiloxane Polymer Soft Template - Key
Factor to Simple High Yield Synthesis of
Cable-Like
Silver Nanowires
Chih-Hao Hsia,† Ming-Yu Yen,†Chi-Young Lee,‡and Hsin-Tien Chiu*,†
Department of Applied Chemistry, National Chiao Tung University ,Hsinchu, Taiwan, 30050, R.O.C,
Chi-Young Leeand Materials Science Center, Nati onal Tsing Hua University Hsinchu, Taiwan, 30043, R.O.C
3
Synthesis of silver nan owires is an area un der inten sive investigation.1 ManyMany m eth o ds h av e b een e xp lo r ed , frequently, including hard and soft templates are needed to assisted shape formation of these nanowires.and electrochemical reduction.ref 1[1-3] Previously, we have reported that addition of polydimethylsiloxane (PDMS) to a new solvent-free vapor-solid reaction growth (VSRG) method process assisted to prepare
cable-like Cu nanowires formation significantlyin good yield by reacting polydim eth ylsiloxan e (PDMS) coated C uCl w ith (Me3Si)4Si in sealed tubes.[4] Also, we have demonstrated the importance of polymeric shell in controlling the electron beam induced growth of Cu nanowires.3 The reaction is a vapor-solid reaction growth (VSRG) and a plausible mechanism has been proposed to elucidate the growth process. Here, we wish to report a simple high yield synthesis of Ag nanowires. Unlike other literature processes,4 the polymer soft template found in this observation is generated in situ in the reaction.We are curious whether employing the VSRG strategy to synthesize other metal nanowires is possible. After some exploration, we wish to report another VSRG example, a A simple high yield synthesis of cable-like Ag nanowires via a parallel process which is much simpler than the processes reported before .3
Reacting AgNO3 and (Me3Si)4Si in a sealed tube under low
pressure a t 4 0 0 K (Caution: The reaction generates gaseous byproducts. The tube should have enough volume to allow their expansion so that the pressure will not build up excessively inside. The gas escaping the tube turned brown in air.).ref45 After workup, it was found that AgNO3 was converted nearly quantitative into
metallic silver, as determined by X-ray diffraction (XRD). The XRD peaks were indexed to a face-centered cubic (fcc) material. The lattice constant a, calculated from the diffraction pattern, was 0.4090 nm, close to the reported value of Ag, a = 0.4086 nm.[5]56 Fig. 1a shows a scanning electron microscopic (SEM) image of the product. It contains bundles of nanowires with an average length of ca. 10 µm. An enlarged image (Fig. 1b) shows that the
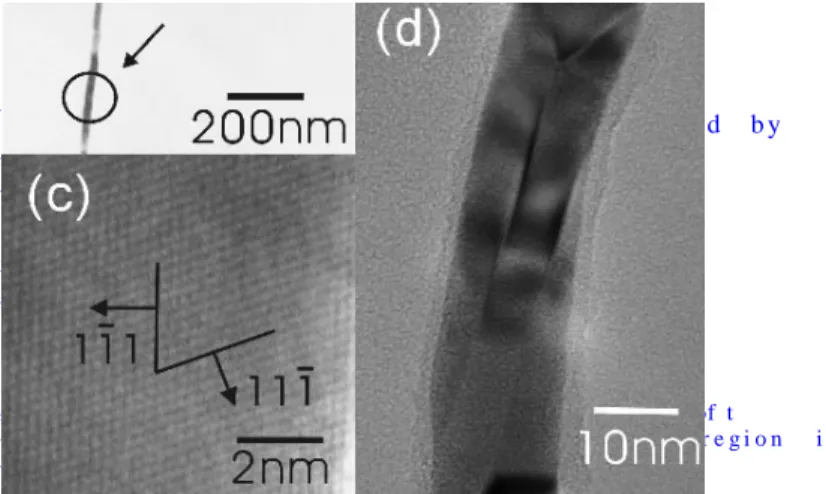
nanowires have a mean diameter of 25 ± 5 nm. It is estimated that the density of our nanowires is smaller than which synthesized by template strategies, such as AAO and calix[4]hydroquinone nanotubes.3 An energy dispersive spectrum (EDS), shown in Fig. 1cC, suggests that in addition to sample holdertapeAg, the sample contains Si, O an d C also. A typical transmission electron microscopic (TEM) image of a nanowire is shown in Fig. 2a.
While the overall wire structure is shown in Fig. 2B, indicates
s h o w n iF ni g . 2 B , i n d i c a t te hs a t t h e m a r k e d a r e a i s w i t h i tn h e d o m a i n o f a s i n g l e c r y s t a l . F r o m t h e d o t p a t t e r n , t h e l a t t i c e p a r a m e t e a r i s e s t i m a t e d t o b e c r y s t a l . F r o m t h e d o t p a t t e r n , t h e l a t t i c e p a r aa m e t e r i s e s t i m a ___________________________t e d
_________________________polycr ys t allin e, se le c te d are a electron diffraction (SAED) pattern, shown in Fig. 2b, indicates that the marked area is within the domain of a single crystal. From the dot pattern, the lattice
†
National Chiao Tung University
‡
4
that the marked area is within the domain of a single crystal. From the dot pattern, the lattice parameter a is estimated to be crystal. From the dot pattern, the lattice parameter a is estimated to be 0.42 nm, closed to the reported value of Ag.[5]56 A high resolution TEM (HRTEM) image of the area is shown in Fig. 2c. The directions of two plans (1,-1,1) and (1,1,-1) are identified, showing that the included angle of 70.1° is close to the theoretical value of 70.5°. The {111} d-spacing was measured to be 0.24 nm, close to the value estimated from the literature data, 0.233 nm. As shown in Fig. 2d, a layer of amorphous material, with a thickness of 1 - 3 nm encapsulating the wire, is observed. Based on the EDS data, the layer is tentatively identified to be a permethylsiloxane polymer (PMSP), formed as a high molecular weight byproduct formed in situfrom the reaction.
†
Department of Applied Chemistry
‡
Materials Science Center
This layer, probably acting acted as a soft template, and assisted thenanowire growth via VSRG via the VSRG pathway proposed before.[5]5.
majorthesilicaO-Si-O1255 cm-1 and 1100?1070 cm-1Si-(CH3)2
820? and 480? cm-1. In addition to the absorptions of silica, signals assignable to –OSiMe groups
a-silicaPMSQat 844 cm-1, CH
3 deformation at 1384 cm-1, Si-O
bending at 805 cm-1, and Si-O rocking at 443 cm-1. In addition to the absorptions of silica, signals assignable to –OSiMe groups solution,solution,7,a siloxane that contains Si-O-Si cross-linking structure7The key factor to this simple process is the in situ formation of the PMSP layer. The material is formulated based on the following information . From prelim inary analysis of th e volatile byproducts by gas chromatography – mass spectrometry (GC-MS) and infrared spectroscopy (IR), (Me3Si)2O, (Me3SiO)4Si
and other permethylsiloxane oligomers were found. Detection of these lower molecular weight products suggests that higher molecular weight portion might also be formed in the reaction. IR of th e bu lk solid product confirmed the presence of peaks assignable to PMSP. Based on the TEM, EDS and IR data, we conclude that the Ag nanowire is enclosed in a PMPS shell. The role of (Me3Si)4Si in the reaction is important. It acts as th e
reductant. The atom Si bonded with four tr.imethylsilyl groups is important, because it will form Si-O-Si cross-linking structures which make siloxane inflexible. We have tried the reaction of AgNO3 an d (Me2Si)6, a redu ctant w ith a sim ilar chemical
reactivity,2 but the yield of silver nanowires is lower
than the reaction of AgNO3 and (Me3Si)4Si. In preliminary
an alysis, th e sh ell of reaction of AgNO3 a n d ( M e2Si)6 i s
polydimethylsiloxane (PDMS) , a flexible structure.
, the oxygen scavenger capable of removing oxygen atoms from the nitrate group, as well as the source of PMSP. In addition to the siloxanes, N2O was detected. When the sealed reaction tube s were
op en ed an d ex pos ed to a ir , a br ow n vapor w as f orm e d immediately. This observation suggests that NO, which turned
into NO2, was in the byproducts.7 (Revised to here)
Other mechanisms could not rationalize the growth properly. In a typical Vapor-Liquid-Solid (VLS) process, the mechanism used most frequently to rationalize the growth of many nanowires,[6]68 components of the nanowires are evaporated and nucleated under the assistan ce of a nano-sized liquid phase catalyst at high temperatures. In this work, no catalysts were added to assist the growth. In addition, at the employed reaction temperature of 400 K, the solid phase reactant AgNO3 (m.p. = 485 K) neither melted
nor evaporated. Therefore, the whole process differs from the VLS condition considerably. Also, the process could neither be a physical vapor deposition (PVD) nor a chemical vapor deposition (CVD). It could not be a simple thermal decomposition of AgNO3
either because the reaction temperature was much below the temperature of decomposition of AgNO3, 713 K. The discussion
above has ruled out many unlikely growth mechanisms. In contrast,
many similarities exist bet wee n this study a nd our previous preparat ion of cable-like Cu nanowires.[4] In both reactions, (Me3Si)4Si was employed to react with solid metal compounds under overall comparable reaction conditions.
The only significant difference is that the cable sheath material, PDMS in the Cu case, was not added in this study. Instead, the polymeric layer enclosing the Ag nanowires was a reaction byproduct generated in situ. From preliminary analysis of the volatile byproducts by GC-MS and infrared spectroscopy, (Me3Si)2O , (M e3SiO)4S i an d o th er
permethylsiloxane oligomers were found. Detection of these lower molecular weight byproducts supports the formulation of the polymeric shell outside the Ag nanowires to be PMSP. The role of (Me3Si)4Si in the reaction is important. It acts as the
reductant, the oxygen scavenger capable of removing oxygen atoms from the nitrate group, as well as the source of PMSP. In addition to the siloxanes, N2O was detected. When the sealed
reaction tubes were opened and exposed to air, a brown vapor was formed immediately. This observation suggests that NO, which turned into NO2, was in the byproducts.[7]7
In conclusion, we have discovered a remarkably simple on -step solvent-free method to syn th esize bundles of cable-like Ag nanowires in high yield. The reaction is another example of VSRG of nanowires. Exploration of other possibilities is in progress.
5
ACKNOWLEDGMENT T h is w o r k w a s s u p p o r te d b y NSC-91-2113-M-009-020 of the National Science Council of the Republic of China.
Supporting Information Available: More SEM and TEM images. Experimental details. GC-MS and IR of the byproducts. This material is available free of charge via the Internet at http://pubs.acs.org.
Figure 1. a) SEM image of silver nanowires. b) E nlarged view of the
ma r k e d r e gi o n ( a ) . i n c ) E D S o f t h e m a r k e d r e g i o n i n (a).
Figure 2. a) TEM image of a silver nanowire. b) SAED of the marked area in (a). c) HRTEM image of the marked area in (a). d) TEM image of a silver nanowire, showing the presence of a polymeric shell.
REFERENCES [1] X ia, Y . ; Yang, P.; Sun, Y . ; W u , Y . ; M a y e r s , B . ; Gates, B.; Yin, Y. ; K im , F . ; Ya n, H . Adv. Mater. 2003, 15, 353. (a) U garte, D.; Châtelain, A . ; Heer, W . A . , Nature 1996, 274, 1897. (b) Huang, M. H.; Choudrey, A.; Yang, P. Chem. Comm. 2000, 1063. (c) Murphy, C. J.; Jana, N. R. Adv. Mater. 2002, 14, 80. (d) Hong B. H.; Bae, S. C.; Lee, C.-W.; Jeong, S.; Kim, K. S. Science 2001, 294, 348. (e) Sun, Y.; Xia, Y. Adv. Mater. 2002, 14, 833. (f) Yin, Y.; Lu, Y.; Sun, Y.; Xia, Y. Nano. Lett. 2002, 2, 427. (f) Choi, J.; Sauer, G.; Nielsch, K.; Wehrspohn, R. B.; Gösele, U. Chem. Mater.2003, 15, 776 Yen, M.-Y. ; Chiu, C.-W. ; Chiu, H.-T.; Lee, C.-Y.; Chen, F.-R.; Kai, J.-J.
manuscript submitted for publication(a) Ugarte, D.; Châtelain, A. ; Heer, W. A., Nature 1996, 274, 1897. (b) Huang, M. H.; Choudrey, A.; Yang, P. Chem. Comm. 2000, 1063. (c)
[2] a) Murphy, C. J.; Jana, N. R. Adv. Mater. 2002, 14, 80. b)Murphy, C. J.; Jana, N. R. Adv. Mater. 2002, 14, 80. (d) Hong B. H.; Bae, S. C.; Lee, C.-W.; Jeong, S.; Kim, K. S. Science 2001, 294, 348. (ec) Sun, Y.; Xia, Y. Adv. Mater. 2002, 14, 833. (fd) Yin, Y.; Lu, Y.; Sun, Y.; Xia, Y. Nano. Lett. 2002, 2, 427. (f)
[3] Choi, J.; Sauer, G.; Nielsch, K.; Wehrspohn, R. B.; Gösele, U. Chem. Mater.2003, 15, 776.
(a) Ugarte, D.; Châtelain, A.; Heer, W. A., NatureScience 1996, 274, 1897. (b)
Huang, M . H .; Choudrey, A.; Yang, P. Chem. Comm. 2000, 1063. (c) Murphy, C. J.; Jana, N. R. Adv. Mater. 2002, 14, 80. (d) Hong B. H.; Bae, S. C.; Lee, C.-W.; Jeong, S.; Kim, K. S. Science 2001, 294, 348. (e) Sun, Y.; Xia, Y. Adv. Mater. 2002, 14, 833. (f) Yin, Y.; Lu, Y.; Sun, Y.; Xia, Y.
Nano. Lett. 2002, 2, 427. (fg) Choi, J.; Sauer, G.; Nielsch, K.; Wehrspohn, R. B.; Gösele, U. Chem. Mater. 2003, 15, 776
(5) [8]9[4] Yen, M.-Y.; Chiu, C.-W.; Hsia, C.-H.; Chen, F.-R.; Kai, J.-J.; Lee,
C.-Y.;Chiu, H.-T. Adv. Mater. 2003, 15, 235.
56[5] J o in t C o mm it t ee f o r P o wd er D if f r a ctio n ( J C P D S ) F ile N o . 04-0783.International Center for Diffraction Data, 1982.
(7) Chemistry of the Elements; Greenwood, N. N., Earnshaw, A.; Pergamon, 1984; Ch. 11, p. 511.
68[6] Hu, J.; Odom, T. W.; Lieber, C. M. Acc. Chem. Res. 1999, 32, 435.
[7](7) Chemistry of the Elements; Greenwood, N. N., Earnshaw, A.;
Pergamon, 1984; Ch. 11, p. 511.
6
ExperimentalSynthesis of Silver Nanowires. Manipulation of chemicals was performed under dry and oxygen-free environment. Crystals of AgNO3 (0.10 g, 0.59 mmol, Fisher Scientific) and (Me3Si)4Si (0.10 g, 0.31 mmol) [8] were
manually pulverized together into powders in an agate mortar. The powders were collected and sealed into a Pyrex tube under vacuum (Caution: The reaction generates gaseous byproducts. The tube should have enough volume to allow for their expansion.). In a tube furnace, the sealed tube was ramped to 400K in 10 min and held at the temperature for 2 h. After the tube was opened (Caution: Vapor phase byproducts produced in the reaction may cause a pressure build up in the tube. The byproducts escaped from the tube turned brown when they encountered air, indicating the formation of NO2.), the samples were washed by THF and dried at 400 K to remove reaction
byproducts. The solid products on carbon tapes were coated with a thin layer of gold (~5 nm) and characterized by SEM (JEOL JSM-6330F at 15 kV). Also, the samples on carbon film coated copper grid were investigated by TEM (JEOL JEM-2010 at 200 kV) and HRTEM (Philips TECNAI 20 at 200 kV).
[8] Gilman, H.; Smith, C. L. J. Am. Chem. Soc., 1964, 86, 1454.
Authors are required to submit a graphic entry for the Table of Contents (TOC) that, in conjunction with the manuscript title, should give the reader a representative idea of one of the following: A key structure, reaction, equation, concept, or theorem, etc., that is discussed in the manuscript. The TOC grap hic should be no wider than 4.72 in. (12 cm) and no taller than 1.81 in.
(4.6 cm).
Insert Table of Contents artwork here
ABSTRACT FOR WEB PUBLICATION (Word Style “BD_Abstract”). Authors are required to submit a concise, self-contained, one-paragraph abstract for Web publication.