Abstract.
It is reported that Houttuynia cordata Thunb. (HCT), a traditional Chinese herbal medicine, has many biological properties such as antiviral, antibacterial and antileukemic activities. However, the molecular mechanisms of cytotoxicity and apoptosis in human primary colorectal cancer cells are not clear. In this study, whether HCT induced cytotoxicity in primary colorectal cancer cells obtained from three patients was investigated. The results indicated that HCT inhibited growth of cancer cells in a dose-dependent manner. After treatment with HCT (250 μg/ml) for 24 h, cells exhibited chromatin condensation (an apoptotic characteristic). HCT increased reactive oxygen species (ROS) production and decreased the mitochondrial membrane potential (ΔΨm) in examined cells. Mitochondria-dependent apoptotic signaling pathway was shown to be involved as determined by increase in the levels of cytochrome c, Apaf-1, and caspase-3 and -9.The decrease in the level of ΔΨm was associated with an increase in the BAX/BCL-2 ratio which led to activation of caspase-9 and -3. Based on our results, HCT induced apoptotic cell death in human primary colorectal cancer cells through a mitochondria-dependent signaling pathway. Colorectal cancer (CRC) is one of the most prevalent types of cancer worldwide and a common cause of morbidity and mortality in humans (1). According to the reports of the Department of Health, Taiwan (ROC) in 2009, colorectal cancer is the third leading cause of cancer-related death in Taiwan, accounting for 11.4% of all cancer deaths. In clinical practice, surgery, radiotherapy and chemotherapy are main strategies for treating colon cancer patients. Significant improvements in patient survival rates have been achieved in recent years due to successful treatment (2). However, most patients still die of their disease. Approximately 20% of patients have distant metastatic disease at the time of presentation and are rarely cured (3). The median overall survival duration for patients with metastatic disease is currently about 20 months (4). Chemotherapy resistance in cancer is one of the most serious obstacles. Therefore, in this study, we focus on identifying a new agent to treat colorectal cancer.
Apoptosis is the process of programmed cell death via a highly regulated mechanism. There are three major signaling pathways leading to apoptosis. Firstly, the death receptor pathway is triggered by the binding of extrinsic signals to surface receptors including CD95/Fas, tumor necrosis factor (TNF), and death receptors. This ligand receptor interaction initiates death signals from the extracellular micro-environment *Both Authors contributed equally to this work.
Correspondence to: Jing-Gung Chung, Department of Biological Science and Technology, China Medical University, No 91, Hsueh-Shih Road, Taichung 404, Taiwan, R.O.C. Tel: +886 422053366 ext. 2161, Fax: +886 422053764, e-mail: jgchung@mail.cmu.edu.tw and Jai-Sing Yang, Department of Pharmacology, School of Medicine, China Medical University, No 91, Hsueh-Shih Road, Taichung 404, Taiwan, R.O.C. Tel: +886 422052121 ext. 7730, Fax: +886 422053764, e-mail: jaising@mail.cmu.edu.tw
Key Words: Houttuynia cordata Thunb. (HCT), chinese herb, apoptosis, human primary colorectal cancer cells.
Houttuynia cordata Thunb Extract Inhibits Cell Growth and
Induces Apoptosis in Human Primary Colorectal Cancer Cells
KUANG-CHI LAI1,2,YU-JEN CHIU2, YIH-JING TANG3,4, KUEI-LI LIN5, JO-HUA CHIANG6, YI-LIN JIANG7, HSIU-FANG JEN8, YUEH-HSIUNG KUO9,10, SAKAE AGAMAYA10,11,
JING-GUNG CHUNG12,13*and JAI-SING YANG7,10*
Departments of 1Surgery, and 8Nursing, China Medical University Beigang Hospital, Yunlin 651, Taiwan, R.O.C.;
2School of Medicine, Departments of 7Pharmacology, 12Biological Science and Technology, 9Graduate Institute of Chinese Pharmaceutical Sciences,
10Tsuzuhi Institute for Traditional Medicine, China Medical University, Taichung 404, Taiwan, R.O.C.; 3Department of Family Medicine, Taichung Veterans General Hospital, Taichung 406, Taiwan, R.O.C.;
4School of Medicine, Chung-Shan Medical University, Taichung 402, Taiwan, R.O.C.; 5Department of Radiation Oncology, Chi-Mei Medical Center, Tainan 710, Taiwan, R.O.C.; 6Department of Life Sciences, National Chung Hsing University, Taichung 402, Taiwan, R.O.C.;
11Nihon Pharmaceutical University, Ina, Saitama 362-0806, Japan;
to the cytoplasm, then inducing apoptosis (5). Secondly the mitochondrial pathway is triggered by various stimuli including DNA damage, cellular distress, hypoxia, cytotoxic agent damage inside the cell and increased intracellular calcium concentration. This pathway is involved in mitochondrial function and BCL-2 family. BCL-2 exerts anti-apoptotic effects, but BAX induces pro-apoptotic responses. When an excess of pro-apoptotic over anti-apoptotic signals occurs, it initiates mitochondrial dysfunction and leads to apoptosis (6). To date, induction of apoptosis in cancer cells has been considered as a possible strategy in cancer treatment (7).
Hottuynia cordata Thunb. (HCT), a traditional Chinese medicine, is a perennial herb that is native to Japan, Korea, southern China and Southeast Asia. It has been reported that Houttuynia cordata has many biological properties such as anti-allergy (8), antioxidant (9), antiviral (10) and antibacterial activities (11). Furthermore, Chang et al. demonstrated that HCT has antileukemic activities in various leukemia cell lines including L1210, U937, K562 and P3HR1 (12). However, the molecular mechanisms of its exerting cytotoxicity in cancer cells are not well understood. Our previous study also showed that HCT induced apoptosis through a mitochondria-dependent pathway in human colorectal adenocarcinoma HT-29 cells (13), but there are no reports showing the effects of HCT on human primary colorectal cancer cells. In this study, we evaluated the possible molecular signaling of HCT-induced apoptosis in human primary colorectal cancer cells from three patients in vitro.
Materials and Methods
Preparation of HCT. HCT 50% ethanol extracts (yield: 6.73% of dry wt.) were obtained by 48 h maceration of plant material at room temperature. The ethanol extract was filtered through a 0.45 μm filter (Osmonics, Minnetonka,
MN, USA), lyophilized, and kept at 4˚C. The dried extract was resolublized in distilled water before use.
Chemicals and reagents. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), propidium iodide (PI) and 4’,6-diamidino-2-phenylindole (DAPI) were purchased Table I. Patient information.
Patient 1 Patient 2 Patient 3
TNM T3N1M0 T3N2M0 T3N1M1
Location Cecum Sigmoid Sigmoid
Familial Non-contributory Non-contributory Non-contributory history
Histology Adenocarcinoma, Adenocarcinoma, Adenocarcinoma, moderately moderately moderately differentiated differentiated differentiated
Pre-op None None None
chemotherapy
Pre-op None None None
radiotherapy
Pre-op CEA 5.90 14.1 3.03
Patient background and diagnosis were determined by the histopathologist. CEA: Carcinoembryonic antigen.
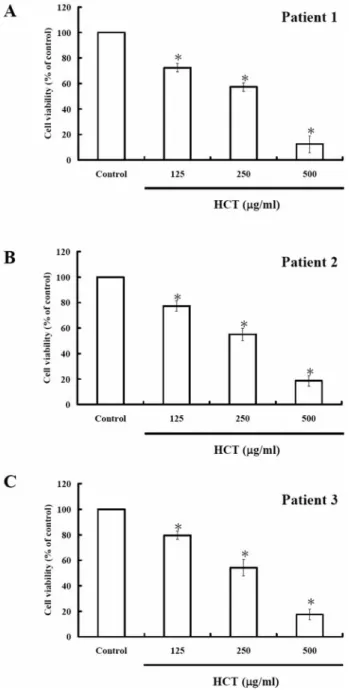
Figure 1. Effects of HCT on cell viability in human primary colorectal cancer cells. After treatment with different concentrations of HCT for 24 h, the cell viability of three human primary colorectal cancer cells significantly decreased. Patient 1, IC50= 289.62±10.36 μg/ml (A)
patient 2, IC50=321.09±15.34 μg/ml (B) and patient 3, IC50=
296.41±9.68 μg/ml (C). Data from three independent experiments are presented (*p< 0.05 as compared with control treatments).
from Sigma-Aldrich Corp. (St. Louis, MO, USA). RPMI-1640, fetal bovine serum (FBS), trypsin-EDTA, and penicillin/streptomycin were obtained from GIBCO BRL/Invitrogen Corp. (Grand Island, NY, USA). Proteinase K was purchased from Roche Diagnostics (Gmbh, Mannheim, Germany). 2,7-Dichlorodihydrofluorescein
diacetate (DCFH-DA) and 3,3’-dihexyloxacarbocyanine iodide (DiOC6) were purchased from Molecular Probes (Invitrogen, Eugene, OR, USA). The Bio-100™ DNA Ladder marker was obtained from PROtech Technology Enterprise Co. (Taipei, Taiwan). All other chemicals used were of analytical grade.
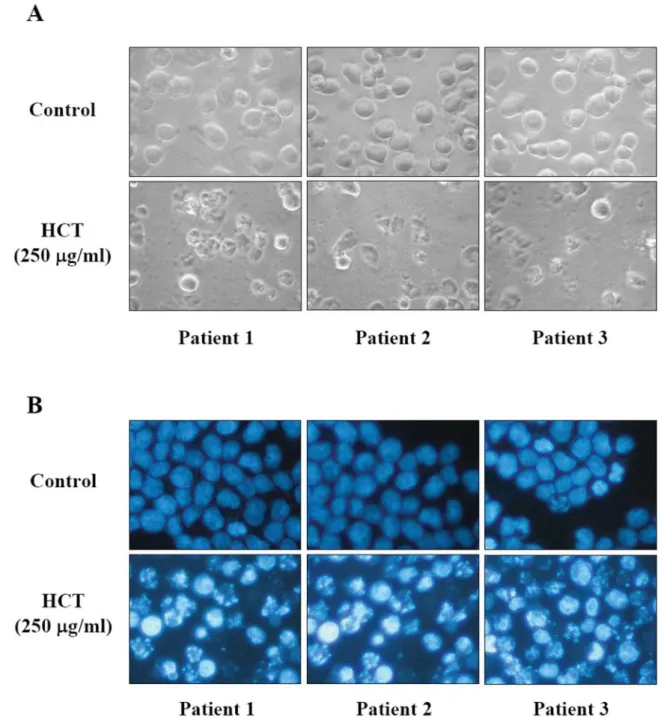
Figure 2. Effects of HCT on cell morphological changes and DNA condensation in human primary colorectal cancer cells. After incubation with HCT (250 μg/ml) for 24 h, cells exhibited nuclear shrinkage and chromatin condensation, whereas those of the control group were well spread with flattened morphology (A). DNA condensation was determined by DAPI staining (B). Cells were examined and photographed under fluorescence microscopy (×200) as described in the Materials and Methods.
Human primary colorectal cancer cells isolation. Colorectal carcinoma specimens from three patients were obtained around 2008 to 2009 from the Department of Surgery, China Medical University Hospital, Taichung, Taiwan after approval of the study by the hospital’s Ethical Committee, and with written and informed consent from patients (IRB NO: DMR-96-IRB-72) (Table I). Specimens were dissected into 1 mm3 pieces, immersed in a ten-fold volume of 0.25% trypsin solution (Sigma-Aldrich Corp), kept at 4˚C overnight and then incubated at 37˚C for 1 h. After stopping trypsin activity with FBS, the solution containing released cells was centrifuged at 150×g for 5 min. The precipitated cells were re-suspended with culture medium, and seeded into a 10 cm culture dish. The remaining undigested tissue was immersed in collagenase solution (500 Units/ml in RPMI-1640 medium with 10% serum) (Sigma-Aldrich Corp.) and incubated at 37˚C for 1 h. The cells released were collected, centrifuged, re-suspended with culture medium,
and seeded into a culture flask. The culture medium was RPMI-1640 medium supplemented with 10% FBS. After primary cultures became confluent, cells were detached by trypsin (0.25%)–ethylenediaminetetraacetic acid (EDTA) (0.02%) solution (Sigma-Aldrich Corp.), counted, centrifuged, re-suspended and seeded into new culture flasks (14).
Cell viability assay. Human primary colorectal cancer cells were plated onto 96-well plates at a density of 2×104 cells/well and exposure to 0, 125, 250 or 500 μg/ml of HCT for 24 h. MTT was then added to each well and plates were then incubated for an additional 4 h in the dark at 37˚C. The medium was then aspirated from the wells and the blue formazon product was dissolved in 100 μl of DMSO. The plates were analyzed at OD 570 nm using a spectrophotometric plate reader (Bio-Rad, Tokyo, Japan). Each data point was replicated in triplicate. Percentage of Figure 3. Effects of HCT on the levels of reactive oxygen species (ROS)
production and mitochondria membrane potential (ΔΨm) in human primary colorectal cancer cells. Three human primary colorectal cancer cells were treated with 250 μg/ml of HCT for 0, 6 and 12 h, and the data from flow cytometric analysis showed that ROS production increased (A) and these was loss of ΔΨm (B). Data from three independent experiments are presented (*p<0.05 as compared with control treatments).
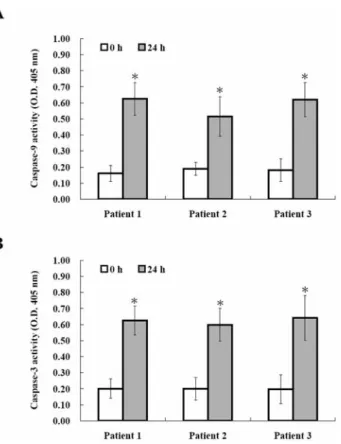
Figure 4. Effects of HCT on the caspase-9 and caspase-3 activity in human primary colorectal cancer cells. After incubation with HCT (250 μg/ml) for 24 h, 50 μg protein were incubated with caspase-3- and -9-specific substrates (Ac-DEVD-pNA and Ac-IETD-pNA) with reaction buffer in a 96-well plate at 37˚C for 1 h. The release of pNA was measured at 405 nm by a spectrophotometer. Caspase-9 (A) and caspase-3 (B) activities are significantly increased. Data from three independent experiments were presented (*p<0.05 as compared with control treatments).
cell viability was calculated as (OD of HCT-treated sample/OD of none treated sample) ×100% (15-16). DAPI staining. Approximately 2×105cells/well were treated with HCT (250 μg/ml) for 24 h, and were then fixed in 4% paraformaldehyde for 30 min. Cells were added 0.1% Triton-X 100 for 10 min and then incubated with 1 μg/ml of DAPI staining solution for 30 min in the dark. Apoptotic cells were observed through fluorescence microscopy (Zeiss, Oberköchen, Germany) as previously described (17). Reactive oxygen species (ROS) production assay. ROS were measured after staining human primary colorectal cancer cells with DCFH-DA. About 2×105cells/well was treated with 250 μg/ml of HCT for 24 h, and then cells were collected and washed with PBS. One ml of PBS containing 20 μM H2DCF-DA was added, and the cells were incubated for 30 min at 37˚C. The fluorescence emission from DCF was analyzed by flow cytometry (FACSCalibur; Becton-Dickinson, San Jose, CA, USA) as previously described (18). Mitochondrial membrane potential (ΔΨm) determination. Changes of ΔΨm were monitored after staining with DiOC6. About 2×105cells/well were treated with 250 μg/ml of HCT for 24 h, cells were collected, trypsinized and washed in PBS, then they were stained with DiOC6(50 nmole/l) for 30 min at 37˚C. The percentage of green fluorescence was estimated by flow cytometry as previously described (19). Western blotting analysis. About 1×106cells/well was treated with 250 μg/ml of HCT for 24 h, and then cells were collected for evaluating the apoptosis associated protein levels. Assay kits for total proteins (Calbiochem/EMD Biosciences Inc., San Diego, CA, USA) were used to assess the release of cytochrome c, Apaf-1, caspase-9, caspase-3, BCL-2 and BAX in cytosol and the purity of the fractions estimated. Fifty μg of proteins were resolved on SDS-PAGE and transferred onto a polyvinylidene fluoride membrane (PVDF; Millipore, Billerica, MA, USA). After blocking with 5% non-fat milk, the blots were incubated with an appropriate dilution of specific monoclonal antibodies for cytochrome c, Apaf-1, caspase-9, caspase-3, BCL-2 and BAX (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 12 h. Blots were washed three times and then incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Inc.). The specific proteins were detected by using enhanced chemiluminescence kits (Amersham ECL kits, Piscataway, NJ, USA) as previously described (20-21).
Caspase activity assay. Approximately 5×105cells/well were treated with 250 μg/ml of HCT for 24 h, after which human primary colorectal cancer cells were collected in lysis buffer
(50 mM Tris-HCl, 1 mM EDTA, 10 mM EGTA, 10 mM digitonin and 2 mM DTT) on ice for 10 min. The lysates were centrifuged at 15,000×g at 4˚C for 10 min. Cell lysates (50 μg) were incubated with caspase-3- and -9-specific substrates AC-ASP-GLU-VAL-ASP-p-nitroanilide DEVD-pNA); AC-ILE-GLU-THR-ASP-nitroanilide; (Ac-IETD-pNA) with reaction buffer in a 96-well plate at 37˚C for 1 h. The release of pNA was measured at 405 nm by a spectrophotometer as previously described (22).
Statistical analysis. Data are presented as the mean±standard deviation (SD) for the indicated number of separate experiments. Statistical analyses of data were carried out with one-way ANOVA followed by Student’s t-test between HCT-treated and control groups, and p<0.05 was considered significant.
Results
Effects of HCT on cell viability in human primary colorectal cancer cells. Cells were treated with HCT at 0, 125, 250 and 500 μg/ml for 24 h. The viabilities were measured by MTT method. As shown in Figure 1, the cell viability significantly decreased in HCT-treated human primary colorectal cancer cells. The concentration required to inhibit growth by 50% (IC50) of the three patients’ primary cells were 289.62, Figure 5. Effects of HCT on apoptosis-associated protein levels in human primary colorectal cancer cells. Cells were treated with 250 μg/ml of HCT for 24 h, and whole cell lysates were prepared and subjected to Western blotting. The resulting blots were probed for cytochrome c, Apaf-1, caspase-9, caspase-3, BCL-2 and BAX. β-Actin served as the loading control.
321.09 and 296.41 μg/ml, respectively. The data indicated that HCT reduced the proportion of viable cells in a dose-dependent manner.
Effects of HCT on cell morphological changes and apoptosis in human primary colorectal cancer cells. To investigate the occurrence of morphological changes and chromatin condensation in HCT-treated human primary colorectal cancer cells, we assessed the nuclear morphological changes by phase-contrast microscopy and DAPI staining. As shown in Figure 2A and B, after incubation with HCT (250 μg/ml) for 24 h, cells exhibited nuclear shrinkage and chromatin condensation, whereas the untreated group were well spread with flattened morphology. The results demonstrated that HCT induced morphological changes and chromatin condensation in human primary colorectal cancer cells. Effects of HCT on the levels of ROS and ΔΨm in human primary colorectal cancer cells. To elucidate whether HCT could cause cell apoptosis through induction of mitochondrial dysfunction in human primary colorectal cancer cells, we measured the intracellular ROS levels and the change of ΔΨm. As shown in Figure 3A and B, cells were treated with 250 μg/ml of HCT for 0, 6 or 12 h and the data showed a significant increase in the level of ROS and a decrease of ΔΨm in a time-dependent manner. The results suggest that mitochondria dysfunction may be involved in HCT-induced apoptosis, and ROS generation is also involved.
Effects of HCT on the caspase-9 and caspase-3 activity in human primary colorectal cancer cells. In order to confirm that HCT-induced apoptosis was mediated by
caspase-dependent pathway, we investigated the caspase-9 and -3 activities by fluorogenic enzymatic assay. As shown in Figure 4A and B, both caspase-9 and caspase-3 activities significantly increased after incubation with 250 μg/ml of HCT for 24 h. The results suggest caspase-dependent signaling pathway is involved in HCT-induced apoptosis of human primary colorectal cancer cells.
Effects of HCT on levels of apoptosis-associated proteins in human primary colorectal cancer cells. To elucidate the possible molecular mechanisms of HCT-induced apoptosis, we investigated the protein levels of cytochrome c, Apaf-1, caspase-9, caspase-3, BCL-2 and BAX by Western blotting analysis. The levels of cytochrome c, Apaf-1, caspase-9 and caspase-3 significantly increased after HCT 24-h treatment (Figure 5A). Furthermore, the pro-apoptotic protein level of BAX was up-regulated, whereas the anti-apoptotic protein level of BCL-2 was down-regulated. The results suggest that the HCT-induced apoptotic response is mediated by the mitochondria-dependent apoptotic pathway.
Discussion
HCT has been used as a traditional Chinese herbal medicine in eastern and southern Asia for a long time. The biological activities of HCT include antioxidant, anti-bacterial, anti-viral effects and inhibition of anaphylactic reaction and mast cell activation (23). In addition, Chen et al. reported the anti-mutagenic effects of HCT under oxidized frying oil feeding-induced oxidative stress in Sprague-Dawley rats (24). Chang et al. demonstrated that HCT inhibited the growth of five leukemia cell lines (L1210, U937, K562, Raji and P3HR1, IC50 was between 478 μg/ml to 662 μg/ml) but is well tolerated by healthy human cells (IC50>1000 μg/ml).(12) Although HCT has been reported to be active against tumors, it anti-cancer effect is not very clear. Our previous study reported that HCT extract induced apoptosis through a mitochondria-dependent pathway in human colon adenocarcinoma HT-29 cells, and the IC50in HT-29 cells was 435 μg/ml. In this study, we firstly investigated the anticancer effects of HCT and the associated molecular mechanisms in human primary colorectal cancer cells in vitro.
Cells undergoing apoptosis exhibited cytoplasmic blebbing, irregularity in shape apoptotic bodies, nuclear condensation and DNA fragmentation. DNA fragmentation into well defined fragments is linked to the activation of endonucleases, leading to apoptosis (25). In Figure 2, the results showed the morphological change and strand breaks of human primary colorectal cancer cells that were detected by DAPI staining. In the HCT-treated group, cells were detached from the surface and contained some debris, whereas cells of the control group were well spread with flattened morphology. For further investigations of the molecular mechanism Figure 6. A proposed model illustrates the molecular mechanism and
the overall possible signaling pathways of HCT-induced apoptosis in human primary colorectal cancer cells.
involved in apoptosis was caused by HCT. We measured the intracellular ROS level and change of ΔΨm by flow cytometry and investigated the apoptotic-related protein expression by Western blotting analysis. It was reported that the exogenous and endogenous ROS, such as hydrogen peroxide, cause apoptosis through mitochondrial permeability transition (26). In Figure 3, our results indicated that HCT induced ROS production and depolarization of ΔΨm. BCL-2 family members were reported in the regulation of mitochondria-mediated apoptotic pathways, including pro-apoptotic proteins (BAX, BAD and BAK) and anti-pro-apoptotic proteins (BCL-2 and BCL-XL).(27) When an excess of pro-apoptotic over anti-pro-apoptotic signal exists, it initiates mitochondrial outer membrane permeabilization (6). Figure 5 indicates that HCT promoted the pro-apoptotic protein level of BAX and inhibited the anti-apoptotic protein level of BCL-2, which led to a change of the ratio of BAX/BCL-BCL-2, resulting in loss of ΔΨm. Additionally, the results also showed that HCT induced up-regulation of cytochrome c, Apaf-1, caspase-9, caspase-3. Chalah et al. and Guicciardi et al. reported that cytochrome c, Apaf-1 and pro-caspase-9 are released from the mitochondrial membrane into the cytosol on the loss of ΔΨm. Subsequently, cytosolic cytochrome c binds to Apaf-1, ATP, and pro-caspase-9, creating a protein complex (apoptosome) which then activates caspase-3 and leads to apoptosis (28-29). These results indicated that the HCT-induced apoptotic response of human primary colorectal cancer cells was mediated via a mitochondria-dependent pathway. Furthermore, as shown in Figure 4, we investigated the caspase-9 and -3 activities by fluorogenic enzymatic assay, and it was shown that both caspase-9 and caspase-3 activities were significantly increased. Activation of caspases is the major mechanism that promotes apoptotic cell death in response to death-inducing signals from cell surface receptors and mitochondrial stress (30).
In conclusion, our results once again confirmed the molecular mechanism in HCT-treated HT-29 cells and human primary colorectal cancer cells. We demonstrated that HCT induced cytotoxicity in human primary colorectal cancer cells by the induction of apoptosis through the mitochondria-dependent pathway. The proposed signaling pathways are shown in Figure 6. This is in agreement with our previous study, the IC50 of three human primary colorectal cancer cells was lower than that for HT-29 cells (three human primary colorectal cancer cells: 289.62, 321.09 and 296.41 μg/ml, HT-29 cells: 435 μg/ml). Human primary colorectal cancer cells seem more sensitive to HCT treatment, and the reason for this is currently under investigation. Taken together, these findings provide important possible molecular mechanisms for the activity of HCT towards primary colorectal cancer cells and confirm that HCT may be useful in anticolon cancer therapy in the future.
Acknowledgements
This work was supported by the National Science Council of the Republic of China (97-2320-B-039-004-MY3) and China Medical University Beigang Hospital (CMUBH R980007), Yunlin, Taiwan R.O.C.
References
1 Smith AJ, Driman DK, Spithoff K, Hunter A, McLeod RS, Simunovic M and Langer B: Guideline for optimization of colorectal cancer surgery and pathology. J Surg Oncol 101: 5-12, 2010.
2 Half E and Arber N: Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother 10: 211-219, 2009.
3 Jemal A, Siegel R, Ward E, Hao Y, Xu J and Thun MJ: Cancer statistics, 2009. CA Cancer J Clin 59: 225-249, 2009.
4 Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R and Kabbinavar F: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335-2342, 2004. 5 Burz C, Berindan-Neagoe I, Balacescu O and Irimie A: Apoptosis in cancer: key molecular signaling pathways and therapy targets. Acta Oncol 48: 811-821, 2009.
6 Brenner D and Mak TW: Mitochondrial cell death effectors. Curr Opin Cell Biol 21: 871-877, 2009.
7 Sanmartin C, Plano D and Palop JA: Selenium compounds and apoptotic modulation: a new perspective in cancer therapy. Mini Rev Med Chem 8: 1020-1031, 2008.
8 Han EH, Park JH, Kim JY and Jeong HG: Houttuynia cordata water extract suppresses anaphylactic reaction and IgE-mediated allergic response by inhibiting multiple steps of FcεRI signaling in mast cells. Food Chem Toxicol 47: 1659-1666, 2009. 9 Kusirisin W, Srichairatanakool S, Lerttrakarnnon P, Lailerd N,
Suttajit M, Jaikang C and Chaiyasut C: Antioxidative activity, polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Med Chem 5: 139-147, 2009.
10 Hayashi K, Kamiya M and Hayashi T: Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus, and HIV. Planta Med 61: 237-241, 1995. 11 Lu H, Wu X, Liang Y and Zhang J: Variation in chemical composition and antibacterial activities of essential oils from two species of Houttuynia Thunb. Chem Pharm Bull (Tokyo) 54: 936-940, 2006.
12 Chang JS, Chiang LC, Chen CC, Liu LT, Wang KC and Lin CC: Antileukemic activity of Bidens pilosa L. var. minor (Blume) Sherff and Houttuynia cordata Thunb. Am J Chin Med 29: 303-312, 2001. 13 Tang YJ, Yang JS, Lin CF, Shyu WC, Tsuzuki M, Lu CC, Chen YF and Lai KC: Houttuynia cordata Thunb. extract induces apoptosis through mitochondrial-dependent pathway in HT-29 human colon adenocarcinoma cells. Oncol Rep 22: 1051-1056, 2009.
14 Failli A, Consolini R, Legitimo A, Spisni R, Castagna M, Romanini A, Crimaldi G and Miccoli P: The challenge of culturing human colorectal tumor cells: establishment of a cell culture model by the comparison of different methodological approaches. Tumori 95: 343-347, 2009.
15 Hsu SC, Yang JS, Kuo CL, Lo C, Lin JP, Hsia TC, Lin JJ, Lai KC, Kuo HM, Huang LJ, Kuo SC, Wood WG and Chung JG: Novel quinolone CHM-1 induces apoptosis and inhibits metastasis in a human osterogenic sarcoma cell line. J Orthop Res 27: 1637-1644, 2009.
16 Lim CB, Ky N, Ng HM, Hamza MS and Zhao Y: Curcuma wenyujin extract induces apoptosis and inhibits proliferation of human cervical cancer cells in vitro and in vivo. Integr Cancer Ther 9: 36-49, 2010.
17 Ho YT, Lu CC, Yang JS, Chiang JH, Li TC, Ip SW, Hsia TC, Liao CL, Lin JG, Wood WG and Chung JG: Berberine induced apoptosis via promoting the expression of caspase-8, -9 and -3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer Res 29: 4063-4070, 2009.
18 Lu HF, Hsueh SC, Ho YT, Kao MC, Yang JS, Chiu TH, Huamg SY, Lin CC and Chung JG: ROS mediates baicalin-induced apoptosis in human promyelocytic leukemia HL-60 cells through the expression of the GADD153 and mitochondrial-dependent pathway. Anticancer Res 27: 117-125, 2007.
19 Ip SW, Liao SS, Lin SY, Lin JP, Yang JS, Lin ML, Chen GW, Lu HF, Lin MW, Han SM and Chung JG: The role of mitochondria in bee venom-induced apoptosis in human breast cancer MCF7 cells. In Vivo 22: 237-245, 2008.
20 Yeh CC, Kuo HM, Li TM, Lin JP, Yu FS, Lu HF, Chung JG and Yang JS: Shikonin-induced apoptosis involves caspase-3 activity in a human bladder cancer cell line (T24). In Vivo 21: 1011-1019, 2007.
21 Wu SJ and Ng LT: Tocotrienols inhibited growth and induced apoptosis in human HeLa cells through the cell cycle signaling pathway. Integr Cancer Ther 9: 66-72, 2010.
22 Yang JS, Hour MJ, Kuo SC, Huang LJ and Lee MR: Selective induction of G2/M arrest and apoptosis in HL-60 by a potent anticancer agent, HMJ-38. Anticancer Res 24: 1769-1778, 2004.
23 Chou SC, Su CR, Ku YC and Wu TS: The constituents and their bioactivities of Houttuynia cordata. Chem Pharm Bull (Tokyo) 57: 1227-1230, 2009.
24 Chen YY, Liu JF, Chen CM, Chao PY and Chang TJ: A study of the antioxidative and antimutagenic effects of Houttuynia cordata Thunb. using an oxidized frying oil-fed model. J Nutr Sci Vitaminol (Tokyo) 49: 327-333, 2003.
25 Darzynkiewicz Z, Galkowski D and Zhao H: Analysis of apoptosis by cytometry using TUNEL assay. Methods 44: 250-254, 2008.
26 Poyton RO, Ball KA and Castello PR: Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab 20: 332-340, 2009.
27 Johansson AC, Appelqvist H, Nilsson C, Kagedal K, Roberg K and Ollinger K: Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis 15: 527-540, 2010. 28 Chalah A and Khosravi-Far R: The mitochondrial death pathway.
Adv Exp Med Biol 615: 25-45, 2008.
29 Guicciardi ME and Gores GJ: Cell stress gives a red light to the mitochondrial cell death pathway. Sci Signal 1: pe9, 2008. 30 Richardson H and Kumar S: Death to flies: Drosophila as a
model system to study programmed cell death. J Immunol Methods 265: 21-38, 2002.
Received April 22, 2010 Revised July 1, 2010 Accepted Julr 6, 2010