This content has been downloaded from IOPscience. Please scroll down to see the full text.
Download details:
IP Address: 140.113.38.11
This content was downloaded on 28/04/2014 at 19:52
Please note that terms and conditions apply.
Preparation of high-Tc superconducting Bi-Pb-Sr-Ca-Cu-O ceramics by the method of thermal
decomposition of an ethylene diamine tetra-acetate colloidal suspension
View the table of contents for this issue, or go to the journal homepage for more 1990 Supercond. Sci. Technol. 3 134
(http://iopscience.iop.org/0953-2048/3/3/006)
Supercond. Sci. Technol. 3 (1990) 134-137. Printed in the UK
Preparation of
h
ig h-T, superconducting
Bi-Pb-sr-Ca-Cu-0 ceramics by
the method of thermal decomposition of
an ethylene diamine tetra-acetate
colloidal suspension
G
C Tu,F
H Chen andH
S Kooinstitute of Materials Science and Engineering, National Chiao-Tung University, Hsinchu 30049, Taiwan, Republic of China
Received 3 November 1989
Abstract. High-T, superconducting Bi-Pb-Sr-Ca-Cu oxide with T, (zero) of 108 K was obtained by ultrastructure processing via the EDTA route, using nitrates as the starting materials. Excellent sinterability of precursor induced by the EDTA
processing permits a relatively short time to achieve the desirable Bi-based superconducting compound compared with that obtained by the traditional solid-state reaction method. Fourier transform-infrared spectroscopy, thermal analysis, x-ray diffraction analysis and energy-dispersive x-ray fluorescence analysis were employed in this study to elucidate the reaction mechanisms and/or identify the phase transformations.
1. introduction
Since the discovery of two distinct phases with super- conducting transition temperatures of 80 K and 110 K [I] in the Bi-Sr-Ca-Cu-0 system, an intense amount of research activity has been targeted towards enhanc- ing production of or singling out the 110 K phase. Takano et a1 [Z] first proposed that a proper partial replacement of Pb for Bi atoms was effective for increas- ing the zero-transition temperature in parallel with an enrichment of the 110 K phase. To date, the conven- tional solid-state reaction method was generally employed to prepare the Bi-PbSr-Ca-Cu-0 super- conducting compound using the corresponding oxides or carbonates as the starting materials. However, the required sintering time in such a process is too long for practical fabrication of this high-T, superconducting material [3, 41 because of the sluggish formation kinetics of the 110 K phase. Moreover, incomplete mixing of the many constituents of the starting materials in the solid-state reaction process would cer- tainly result in inhomogeneous superconducting pro- ducts. In this paper, an ethylene diamine tetra-acetate (EDTA) route was utilised to prepare the Bi-based super- conductor. The process was carried out in the liquid state, using EDTA anions to chelate the various metallic cations in ethylene glycol and ammonium hydroxide
solution, forming a colloidal suspension with
submicron-size particles. Like other liquid chemical methods, such as the processes employing oxalates [S,
61, citrates [7] and acetates [8] as the starting
materials, the EDTA method produces an excellently homogeneous and readily sinterable superconducting precursor; therefore, less sintering time is required in the present EDTA method than in the traditional solid- state reaction method.
2. Experiments
The high-T, superconducting ceramics, with nominal composition of Bi,.,Pb,.,SrCaCu,O, and made in the EDTA route, were synthesised as follows : the high-purity nitrate salts, i.e. Bi(NO,),
. 5H,O,
Pb(NO,), Sr(N03),,
Ca(NO,),
.
4H,O and Cu(N03), 3H,O wereemployed as the starting materials. The stoichiometric amounts of each nitrate salt with the cation ratio of Bi : Pb : Sr : Ca : Cu = 0.8 : 0.2 : 1 : 1 : 2 were blended with ethylene glycol, EDTA acid and ammonium hydrox- ide in a proper volume percentage, and stirred for 1 h to form a colloidal suspension. Then, the as-formed colloid was dried at 150
"C
for about one day; this converts the colloid into a powdery precursor. Subsequently, the pre- cursor was calcined at 800 "C for 6 h followed by grind- ing, screening and pelletising into a bulk of 10 mmBi-Pb-Sr-Ca-CU-0 preparation via the EDTA route
diameter and 2 mm thickness. The pellets were sintered at 840°C for various lengths of time in an air atmo- sphere and then cooled inside a furnace at 1
"C
min-' from 840°C to 750°C and then at 10°C min-' from 750°C to room temperature.Fourier transform-infrared (FTIR) spectroscopy was used to study the reaction mechanisms for formation of a gel-like precursor through the EDTA route. Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) at a heating rate of 10°C min-' under static air conditions were employed to recognise the pyrolysis reaction and phase transformation of precursor after firing. The electrical resistance temperature behaviours of the sintered compact were determined by the four- probe method. The phases of the pellets were identified by x-ray .diffraction analysis (XRD) using a Cu Kcc source. The approximate elemental composition of the sintered compact was analysed by energy-dispersive x-ray fluorescence analysis (EDAX).
3. Results and discussion
Figure 1 shows the FTIR spectra of the EDTA chelated precursor. The broad absorption peaks between 520 and 800 cm-l may be associated with the stretching of the C-0-M, M - 0 and 0 - M - 0 bonds. The sharp peak at 1392
cm-'
corresponds to the stretching of the C - 0 bond in the EDTA chelated metals. Moreover, peaks at 1600, 1632 and 1744 cm-' are caused by the vibration of the C=O bond in the EDTA chelated metals. Broad absorption peaks observed in the range between 3000 and 3600 c m - I are associated with the stretching vibration of the intermolecular hydrogen bond,0-H.
These results suggest that the complexing of EDTA ions with metal ions, the polymerising of ethyl- ene glycol with metals [6] and the hydrogen-bonded combining of EDTA chelated metals with ethylene glycol are possibly occumng in such a route.displayed in figure 2. The broad peak in the curve of DTA between 70 and 19O"C, corresponding to the slow
The curves Of DTA and TGA Of EDTA precursor are
c
3600 2800 2000 1200 LOO
Wavenumber icm-')
Figure 1. The FTIR spectra of as-dried EDTA chelated precursor.
2 0 1 , " " " " 1 ' " " 1
0 250 500 750 1000
Temperature PCl
Figure 2. DTA (---) and TGA (-) curves Of the as-dried
precursor with a heating rate of 10°C min-'.
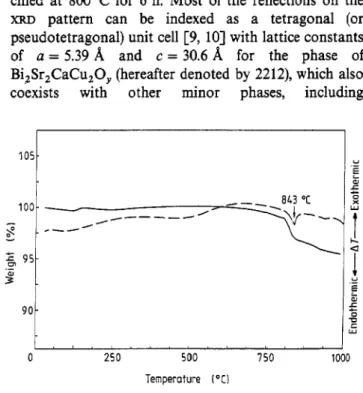
weight loss, is indicative of dehydration of the precur- sor. The exothermic peak at about 240°C appeared simultaneously with the first stage of sharp weight loss around this temperature, and is induced by the carbon- isation of the precursor. On the other hand, a sharper and higher exothermic peak was observed at about 480 "C which is associated with oxidation of the precur- sor, and this reaction was the cause of the appearance of the second stage of sharp weight loss in the TGA curve cursor after calcining at 800°C for 6 h are shown in figure 3. It is obvious that the organic moieties of the EDTA compound were completely removed after calcin- ing at 800°C for 6 h, while an endothermic peak at 843 "C resulting from the partial melting of Bi-based compound was observed in the DTA curve, and this was related to a 2.5 wt% loss at this temperature in the TGA curve.
Figure 4 shows the XRD pattern of precursor cal- cined at 800°C for 6 h. Most of the reflections on the
XRD pattern can be indexed as a tetragonal (or pseudotetragonal) unit cell [9, 103 with lattice constants of a = 5.39 8, and c = 30.6 8, for the phase of Bi,Sr,CaCu,O,, (hereafter denoted by 2212), which also
coexists with other minor phases, including
around 480
"c.
The curves Of DTA and TGA Of EDTA pre-0 250 500 7 50 1000
Temperature ("Cl
Figure 3. DTA (---) and TGA (-) curves of the powdery precursor after calcining at 800°C for 6 h.
0 C Tu eta/ 10- 8- - . C
=
6- 8 -2 4- B . .- VI 2 - c 2 U 2 ' E if
g
a .c C L 6 Yi
0 10 2 0 30 4 2 9 (deglFigure 4. XRD pattern of precursor after calcining at 800 "C for 6 h. 0,2212 phase;
e,
2223 phase; A, 2201 phase.Bi,Sr,Ca,Cu,O, (2223) at 28 = 33.7" with lattice con- stants of a = 5.39
A
and c = 37.1&
and Bi,Sr,CuO, (2201) at 28 = 21.7" and 29.8" with lattice constants of a = 5.39A
and c = 24.4A.
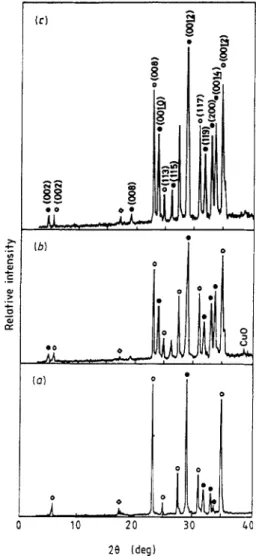
Moreover, traces of non- superconducting phases were also detected in the sample, consisting of Ca,Pb0,(28 = 17.6"), Ca,CuO, (28 = 36.31") and the unreacted CuO (28 = 38.8").XRD patterns of pellets sintered at 840 "C for various times are shown in Figure 5. It is apparent that the sample sintered at 840°C for 24 h led to a further for- mation of the 2223 phase, the disappearance of non- superconducting phases and the sharpening of reflections, which is presumably related to the elimi- nation or decrease of plane defects induced by incom- plete crystallisation treatment. As the sintering time was prolonged to 51 h, the characteristic (002) peak of the 2223 phase was detectable. The volume fraction ( V ) of 2223 phase was about 0.43, estimated roughly by the expression Y = Il/(Zl + I ,
+
13) where I , , I , , I , are the individual intensities of the (002) reflection for the 2223, 2212 and 2201 phases Ell]. Moreover, the enhancement to the growth of the 2223 phase can be found in the sample sintered at 840 "C for 76 h, with the volume fraction of the 2223 phase being increased to about 0.51. Figure 6 shows the electrical resistance/ temperature behaviours mentioned above of the three kinds of sample. The zero-resistance temperature is increased by prolonging the sintering time and the room-temperature resistance of samples decreases as sintering time increases. It should be noted that the sintering time required for obtaining desirable amount of the 2223 phase and high zero-resistance temperature in the EDTA process is much less than that required in the conventional solid-state reaction method, which is about ten days [12] and was due to the sluggish forma-i
I. 0 0 L 10 2 0 3 0 4 28 Ideg)Figure 5. XRD patterns of pellets sintered at 840°C for (a)
24 h, (b) 51 h and (c) 76 h. 0,2212 phase;
e,
2223 phase;0 , Ca,PbO,
.
tion kinetics of the 2223 phase. Therefore, the EDTA process is more efficient for preparing the high-T, super- conducting Bi-based ceramics. Figure 7(u) shows a scan- ning electron micrograph of the fractured surface for the sample sintered at 840°C for 76 h. The micrograph dis- plays clearly that the sample comprises mainly plate- like crystals, which were described as a sequence of 2212 and 2223 layers [13]. These plate-like crystals generally
Temperature I K )
Figure 6. Electrical resistance/temperature behaviours for the pellets sintered at 840°C for 24 h, 51 h and 76 h.
Bi-PbSr-Ca-Cu-0 preparation via the EDTA route
4. Conclusion
High-T, superconducting ceramics with nominal com- position of Bi,,,Pb,,2SrCaCu20, were successfully pre- pared by the method of thermal decomposition of an EDTA colloidal suspension. A high percentage of the 2223 superconducting phase and a zero-resistance tem- perature of 108 K were obtained by such a chemical approach. Obviously, the required sintering time through EDTA route for achieving the desirable super- conductivity in the Bi-Pb-Sr-Ca-Cu-0 system is much shorter than that required through the conventional solid-state reaction route. Therefore, like other chemical methods such as those employing oxalates, citrates and acetates as the starting materials, the EDTA process has been shown to be an efficient method of fabricating high-
r,
superconducting ceramics.Acknowledgment
The authors would like to express their gratitude to Mr
Y S Fran for his valuable suggestions in the analysis of infrared spectra.
References
[I] Maeda H, Tanaka Y, Fukutoimi M and Asano T 1988 [2] Takano M, Takada J, Oda K, Kitaguchi H, lkeda Y,
Japan. J . A p p l . Phys. 27 L209
Tomii Y and Mazaki H 1988 J a p a n . J . A p p l . Phys. 27 L1041
[3] Jin R Y, Shi F. Ran Q Z, Shi Z H and Zhou S 2 1989
Physica C 158 225
[4] Kim C J, Rhee C K, Lee H G, Lee C T, Kang S J-L and Won D Y 1989 J a p a n . J. A p p l . Phys. 28 L45 [5] Chen F H, Koo H S , Tseng T Y, Liu R Sand Wu P T
1989 M a t e r . Ltvr. 8 228
[6] Chen F H, Koo H S and Tseng T Y J . M a t e r . Sci. in the
press
[TJ
Wang N H, Wang C M, Kao H C I, Ling D C, Ku H C and Lii K H 1989 J a p a n . J. Appl. Phys. 23 1505 [8] Tanaka K, Nozue A and Kamiya K 1989 J a p a n . J . A p p l .Phys. 28 L934
[9] Takayama-Muromachi E, Uchida Y, Ono A, lzumi F, Onoda M, Matsui Y, Kosuda K, Takekawa S and Kato K 1988 J a p a n . J. A p p l . Phys. 27 L365 [lo] Tarascon J M, Mckinnon W R, Barbonx P, Hwang D
M, Bagley B G, Greene L H, Hull G W. LePage Y,
Stoffel N and Giround M 1988 Phy. Reo. B 38 8885
and Kumajima H 1989 J a p a n . J . A p p l . Phys. 28
LI 171
[12] Rhee C K, Kim C J, Lee H G, Kuk 1 H, Lee J M, Chang I S, Rim C S, Han P S, Pyun S I and Won D Y 1989
Japan. J . A p p l . Phys. 23 L1137
[I31 Herkert W, Neumuller H W and Wilhelm M 1989 Solid
S t a t e C o m m u n . 69 183
[I41 Kijima N, Endo H, Tsuchiya J, Sumiyama A, Mizunmo M and Oguri Y 1988 J a p a n . J . Appl. Phys. 27 L1852
Flgure 7. (e) Scanning electron micrograph of fractured surface of the sample sintered at 840°C for 76 h and (b) the typical elemental composition of the plate-like crystals determined by EDAX.
[I 1) Oota A, Ohba K, Ishida A, Kirihigashi A, Iwasaki K
grow in random directions and form a porous structure, which leads to a low critical current density for Bi- based superconductor. A typical elemental composition of the plate-like crystals analysed by EDAX is shown in figure 7(h). This result is similar to those reported earlier by Takano et a1 [2] and Kijima et a1 [14].