Chemical Constituents and Anti-platelet Aggregation
Activity from the Root of Peucedanum formosanum
Yu-Chang Chen1,2, Peng-Yin Chen1, Chin-Chung Wu1, ian-lih tsai1 and ih-sheng Chen1*1. Graduate Institute of Natural Products, College of Pharmacy, Kaohsiung Medical University, Kaohsiung City 807, Taiwan (R.O.C.) 2. School of Chinese Medicine Resources, College of Pharmacy, China Medical University, Taichung City 404, Taiwan (R.O.C.)
(Received: July 13, 2007; Accepted: October 8, 2007) aBstRaCt
Analysis of the root extract of Peucedanum formosanum (Taiwan Qian-Hu) led to the isolation of 32 known compounds. The structures of these isolates were determined by spectral data. Some of them displayed strong anti-platelet aggregation activities. Analysis showed that most of the constituents found in P. formosanum were the same as those found in P. praeruptorum (Bai-Hua Qian-Hu), in that many isolates of both plants’ roots belong to seselin-type dihydropyranocoumarins and psoralen-type furanocou-marins.
Key words: Peucedanum formosanum, Umbelliferae, root, Qian-Hu, furanocoumarin, dihydropyranocoumarin, anti-platelet aggregation
intROduCtiOn
Peucedanum formosanum Hay. (Umbelliferae) is
an endemic perennial herb in Taiwan and distributed at
medium to high altitudes through the island(1). Its root
has been used as folk medicine to treat coughs, fever, headache and excessive sputum caused by colds. In this regard it resembles the traditional Chinese medicine Qian-Hu, which is derived from the roots of P.
praerup-torum (Bai-Hua Qian-Hu) and Angelica decursiva (P. decursivum, Porphyroscias decursiva; Zi-Hua Qian-Hu).
Two new compounds, peuformosin and (+)-anomalin, have been isolated by means of ether extraction from the
root of P. formosanum(2,3). The methanolic extract of the
root exerted anti-platelet aggregation activity in prelimi-nary screening and subsequent investigation, which led to the isolation of 32 known compounds. In this paper, we report the isolation, the anti-platelet aggregation activities of these isolates, and the comparisons of the constituents of several Peucedanum species used in folk or traditional Chinese medicine.
MateRials and MethOds
I. General
All melting points were determined on a YANACO
micro-melting point apparatus and were uncorrected. 1
H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra were
taken on a Varian Unity plus-400 and Varian Mercury plus-400. Chemical shifts were given in δ with TMS as an internal standard. EI-mass spectra were performed on a VG Biotech Quattro 5002 using a direct inlet system. HR-mass spectra were recorded on a JEOL JMX-HX 110 spectrometer. UV spectra were determined on a Hita-chi U-2000 double beam spectrophotometer in methanol (MeOH) solution. IR spectra were recorded on a Perkin Elmer system 2000 FT-IR (KBr or neat) spectrophotom-eter. Optical rotation was measured on a JASCO P-1020 polarimeter. Column chromatography (CC) was carried out on silica gel (Merck, 70-230 and 230-400 mesh) or Sephadex LH-20 gel (Pharmacia, Fine Chemicals AB, Uppsala). Prep. TLC was run on silica gel plates (Merck, 60 F-254). The HPLC system is consisted of a Hitachi L-7100 pump, a Biscoff RI detector and a silica gel column
(LiChroCART® 250-10, Merck).
II. Plant
The roots of P. formosanum were collected from Wutai, Pingtung County, Taiwan, in Aug. 2003 and iden-tified by Dr. Ih-Sheng Chen, College of Pharmacy, Kaoh-siung Medical University. A voucher specimen (Chen 6145) was deposited in the Herbarium of the School of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan, R.O.C.
* Author for correspondence. Tel: +886-7-3121101 ext. 2191; Fax: +886-7-3210683; E-mail: m635013@kmu.edu.tw
III. Extraction and Isolation
Dried root (3.4 kg) of P. formosanum was sliced and extracted four times with cold MeOH. The precipi-tate filtered from the concentrated MeOH solution was washed by ethyl acetate (EtOAc), then recrystallized from EtOH to obtain D-mannitol (1, 80 g). The filtrate was removed from the solvent in vacuum and partitioned
into CHCl3 soluble fraction (180 g), n-BuOH soluble
frac-tion (40 g), and H2O soluble fraction (360 g). The CHCl3
-soluble fraction (180 g) was chromatographed over silica
gel (2.0 kg), eluted with CHCl3 and gradually enriched
with MeOH to give 12 fractions (frs. 1–12).
Fr. 3 (18.5 g; CHCl3–MeOH, 49:1) was washed by
n-hexane to get the crystalline mass (11.3 g) which was
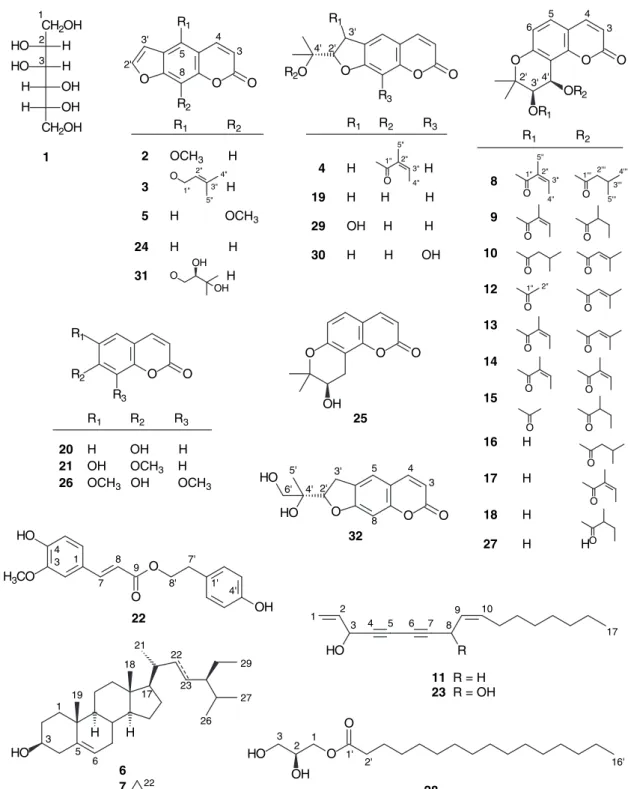
silica gel CC (330 g) eluted with n-hexane/acetone (20:1) with gradually increasing polarity to afford bergapten (2, 33.5 mg), isoimperatorin (3, 12.2 mg), (–)-deltoin (4, 23.5 mg), and xanthotoxin (5, 10.4 mg), a mixture (75.8 mg) of β-sitosterol (6) and stigmasterol (7), a mixture (23.5 mg) of (+)-praeruptorin E (8) and (+)-hyuganin A (9), and (–)-cis-3’-isovaleryl-4’-senecioylkhellactone (10, 4.5 mg). The washings (4.5 g) of fr. 3 were silica gel CC (135 g) and eluted with n-hexane–acetone (5:1) to produce panaxynol (11, 156 mg).
Fr. 4 (40.1 g; CHCl3/MeOH, 97:3) was washed with
n-hexane to obtain the crystalline mass (15.2 g). Part
(1.0 g) of the crystalline mass was subjected to prepara-tive HPLC (n-hexane/EtOAc, 4:1, flow rate = 3 mL/min) to produce (–)-isosamidin (12, 18.5 mg), (+)-peuformosin (13, 141 mg), (+)-anomalin (14, 86.2 mg), and (+)-cis-3’-acetoxy-4’-(2-methylbutyroyloxy)-3’,4’-dihydroseselin (15, 7.8 mg). The washings (20 g) of fr. 4 were silica gel CC (600 g) eluted with n-hexane/EtOAc (10:1), with grad-ually increasing polarity to obtain a mixture (3.4 mg) of
cis-3’-hydroxy-4’-isovaleryloxy-3’,4’-dihydroseselin (16)
and laserpitin (17), (–)-cis-3’-hydroxy-4’-(2-methylbuty-ryloxy)-3’,4’-dihydroseselin (18, 6.8 mg), and (+)-marme-sin (19, 8.9 mg).
Part (5.0 g) of fr. 5 (33.4 g; CHCl3/MeOH, 24:1) was
silica gel CC (150 g) eluted with n-hexane/CH2Cl2 (5:1),
with gradually increasing polarity with CH2Cl2/EtOAc
(10:1) to produce umbelliferone (20, 4.6 mg), isoscopo-letin (21, 1.6 mg), p-hydroxyphenethyl ferulate (22, 8.9 mg), falcarindiol (23, 32.6 mg), and psoralen (24, 1.5 mg).
Fr. 7 (3.4 g; CHCl3/MeOH, 96:4) was silica gel CC (150
g) eluted with CHCl3/MeOH (10:1), with gradually
increas-ing polarity to afford (+)-lomatin (25, 3.2 mg), isofraxidin (26, 1.8 mg), and (–)-cis-khellactone (27, 3.8 mg).
Fr. 8 (2.6 g; CHCl3/MeOH, 9:1) was
chromato-graphed on Sephadex LH-20 eluted with MeOH to obtain 1-O-hexadecanoyl glycerol (28, 2.5 mg), (+)-3’-hydroxy-marmesin (29, 4.7 mg) and (+)-rutaretin (30, 28.3 mg).
Fr. 9 (0.8 g; CHCl3/MeOH, 4:1) was
chromato-graphed on Sephadex LH-20 eluted with MeOH to afford (+)-oxypeucedanin hydrate (31, 1.7 mg) and (+)-dorsteni-ol (32, 1.8 mg).
IV. Isolates
D-Mannitol (1): colorless needles, m.p. 166–168°C
(EtOH), [α]24
D : +45.6° (c 1.55, pyridine).
Bergapten (2): colorless needles, m.p. 192–194°C
(Et2O), EI-MS m/z (%): 216 ([M]+, 100), 201 (31), 173 (57),
145 (20). IR ν KBrmax cm-1: 1731(C=O). UV λ MeOHmax nm (log ε):
222 (4.58), 249 (4.46), 259 (4.42), 267 (4.46), 310 (4.38). 1H-NMR (CDCl 3, 200 MHz): δ 4.26 (3H, s, OMe-5), 6.26 (1H, d, J = 9.8 Hz, H-3), 7.01 (1H, br d, J = 2.4 Hz, H-3’), 7.12 (1H, br s, H-8), 7.59 (1H, d, J = 2.4 Hz, H-2’), 8.14 (1H, d, J = 9.8 Hz, H-4).
Isoimperatorin (3): light yellow needles, m.p. 108–
110°C (Et2O), EI-MS m/z (%): 270 ([M]+, 0.2), 202 (100),
174 (19), 69 (70). IR ν KBrmax cm-1: 1728 (C=O). UV λ MeOHmax nm
(log ε): 222 (4.73), 250 (4.63), 259 (4.58), 267 (4.57), 308 (4.53). 1H-NMR (CDCl 3, 200 MHz): δ 1.70 (3H, s, H-4”), 1.80 (3H, s, H-5”), 4.92 (2H, d, J = 7.4 Hz, H-1”), 5.53 (1H, br t, J = 7.4 Hz, H-2”), 6.27 (1H, d, J = 10.0 Hz, H-3), 6.95 (1H, br d, J = 2.2 Hz, H-3’), 7.15 (1H, br s, H-8), 7.59 (1H, d, J= 2.2 Hz, H-2’), 8.16 (1H, d, J = 10.0 Hz, H-4).
(–)-Deltoin (4): colorless prisms, m.p. 86–88°C
(n-hexane), [α]25
D : –70.4° (c 0.08, CHCl3), EI-MS m/z (%):
328 ([M]+, 0.1), 228 (28), 214 (15), 213 (100), 187 (13), 83
(43). IR ν KBrmax cm-1: 1708 (C=O). UV λ MeOHmax nm (log ε): 222
(4.78), 258 (4.15), 333 (4.17). 1H-NMR (CDCl3, 200 MHz): δ 1.60 (3H, s, Me-4’), 1.62 (3H, s, Me-4’), 1.67 (3H, s, H-5”), 1.88 (3H, br d, J = 6.0 Hz, H-4”), 3.26 (2H, d, J = 8.4 Hz, H-3’), 5.06 (1H, t, J = 8.4 Hz, H-2’), 5.98 (1H, br q, J = 6.0 Hz, H-3”), 6.21 (1H, d, J = 9.4 Hz, H-3), 6.73 (1H, s, H-8), 7.21 (1H, br s, H-5), 7.59 (1H, d, J = 9.4 Hz, H-4).
Xanthotoxin (5): colorless needles, m.p. 144–146°C
(MeOH), EI-MS m/z (%): 216 ([M]+, 100), 201 (23), 173
(46), 145 (21), 89 (26). IR ν KBrmax cm-1: 1713 (C=O). UV λ MeOHmax
nm (log ε): 218 (4.54), 248 (4.52), 300 (4.24). 1H-NMR
(CDCl3, 200 MHz): δ 4.30 (3H, s, OMe-8), 6.38 (1H, d,
J = 9.6 Hz, H-3), 6.82 (1H, d, J = 2.2 Hz, H-3’), 7.36 (1H, s, H-5), 7.69 (1H, d, J = 2.2 Hz, H-2’). 7.77 (1H, d, J = 9.6
Hz, H-4).
β-Sitosterol (6) & stigmasterol (7): colorless needles,
m.p. 142–144°C (MeOH), [α]25D : –54.3° (c 0.08, CHCl3).
(+)-Praeruptorin E (8) & (+)-hyuganin A (9):
color-less prisms, m.p. 127–130°C (n-hexane), [α]25
D : +36.18° (c
2.78, CHCl3), GC-EI-MS m/z (%): 8: 428 ([M]+, 0.2), 328
(9), 313 (2), 244 (24), 229 (100), 85 (1.8), 83 (1.6); 9: 428
([M]+, 0.2), 328 (10), 313 (12), 244 (12), 229 (100), 85 (3),
83 (6). IR ν KBrmax cm-1: 1735 (C=O). UV λ MeOHmax nm (log ε):
220 (4.94), 245 (4.44), 255 sh (4.31), 300 sh (4.67), 321 (4.85). 1H-NMR (CDCl 3, 200 MHz): 8: δ 0.94 (3H, d, J = 6.2 Hz, H-4’’’), 0.95 (3H, d, J = 6.6 Hz, H-5’’’), 1.44 (3H, s, Me-2’), 1.47 (3H, s, Me-2’), 1.88 (3H, br q, J = 1.2 Hz, H-5”), 1.96 (3H, br dq, J = 7.2, 1.2 Hz, H-4”), 2.0–2.4 (3H, m, H-2’’’, 3’’’), 5.37 (1H, d, J = 5.0 Hz, H-3’), 6.12 (1H, br q, J = 7.2 Hz, H-3”), 6.21 (1H, d, J = 9.4 Hz, H-3), 6.61 (1H, d, J = 5.0 Hz, 4’), 6.80 (1H, d, J = 8.6 Hz, H-6), 7.35 (1H, d, J = 8.6 Hz, H-5), 7.60 (1H, d, J = 9.4 Hz, H-4); 9: δ 0.89 (3H, t, J = 7.4 Hz, H-4’’’), 1.17 (3H, d, J =
6.8 Hz, H-5’’’), 1.44 (3H, s, Me-2’), 1.47 (3H, s, Me-2’), 1.56–1.80 (2H, m, H-2’’’), 5.37 (1H, d, J = 5.0 Hz, H-3’), 6.21 (1H, d, J = 9.4 Hz, 3), 6.59 (1H, d, J = 5.0 Hz, H-4’), 6.80 (1H, d, J = 8.6 Hz, H-6), 7.35 (1H, d, J = 8.6 Hz, H-5), 7.60 (1H, d, J = 9.4 Hz, H-4). (–)-cis-3’-Isovaleryl-4’-senecioylkhellactone (10): colorless oil, [α]25 D : –13.3° (c 0.08, CHCl3), EI-MS m/z (%): 428 ([M]+, 0.1), 326 (5), 311 (10), 261 (5), 244 (8), 229 (39), 231(4.4), 189 (3), 83 (100). IR ν KBrmax cm-1: 1731
(C=O). UV λ MeOHmax nm (log ε): 217 (4.82), 255 sh (4.09), 300
sh (4.32), 323 (3.50). 1H-NMR (CDCl3, 200 MHz): δ 0.95 (3H, d, J = 6.2 Hz, H-4”), 0.97 (3H, d, J = 6.2 Hz, H-5”), 1.41 (3H, s, Me-2’), 1.45 (3H, s, Me-2’), 1.89 (3H, s, H-4’’’), 2.23 (3H, s, H-5’’’), 1.90–2.20 (3H, m, H-2”, 3”), 5.33 (1H, d, J = 4.8 Hz, H-3’), 5.63 (1H, br s, H-2’’’), 6.21 (1H, d, J = 9.6 Hz, H-3), 6.59 (1H, d, J = 4.8 Hz, H-4’), 6.79 (1H, d, J = 8.8 Hz, 6), 7.33 (1H, d, J = 8.8 Hz, H-5), 7.58 (1H, d, J = 9.6 Hz, H-4). 13C-NMR (CDCl 3, 100 MHz): δ 20.4 (C-5’’’), 22.4 (C-4”, 5”), 22.5 (Me-2’), 25.3 (Me-2’, C-3”), 27.5(C-4’’’), 43.1 (C-2”), 59.6 (C-4’), 70.2 (C-3’), 77.5 (C-2’), 107.6 (C-8), 112.5 (C-4a), 113.3 (C-3), 114.3 (C-6), 115.1 (C-2’’’), 129.0 (C-5), 143.1 (C-4), 154.0 8a), 156.7 7), 158.2 3’’’), 159.9 2), 165.1 (C-1”), 171.9 (C-1’’’).
Panaxynol (11): colorless oil, [α]25D : –20.8° (c 0.23,
CHCl3), EI-MS m/z (%): 244 ([M]+, 3), 243 (9), 202 (13),
159 (47), 145 (35), 141 (42), 131 (53), 129 (65), 128 (50),
117 (68), 115 (94), 91 (100). IR ν neatmax cm-1: 3421 (OH), 2233
(C≡C).
(–)-Isosamidin (12): colorless needles, m.p. 120–
122°C (n-hexane), [α]25
D : –71.6° (c 0.08, CHCl3),
EI-MS m/z (%): 386 ([M]+, 0.3), 355 (7), 326 (4), 311 (10),
244 (7), 229 (38), 83 (100). IR ν KBrmax cm-1: 1741 (C=O).
UV λ MeOHmax nm (log ε): 222 (4.93), 255 (4.36), 300 sh (4.63),
323 (4.81). 1H-NMR (CDCl 3, 400 MHz): δ 1.42 (3H, s, Me-2’), 1.46 (3H, s, Me-2’), 1.89 (3H, d, J = 1.2 Hz, H-4’’’), 2.09 (3H, s, H-2”), 2.23 (3H, d, J = 1.2 Hz, H-5’’’), 5.30 (1H, d, J = 4.8 Hz, H-3’), 5.64 (1H, q, J = 1.2 Hz, H-2’’’), 6.22 (1H, d, J = 9.6 Hz, H-3), 6.58 (1H, d, J = 4.8 Hz, H-4’), 6.79 (1H, d, J = 8.8 Hz, H-6), 7.34 (1H, d, J = 8.8 Hz, H-5), 7.58 (1H, d, J = 9.6 Hz, H-4).
(+)-Peuformosin (13): colorless needles, m.p. 155–
156°C (n-hexane), [α]25
D : +48.1° (c 0.43, CHCl3), EI-MS
m/z (%): 426 ([M]+, 0.1), 326 (4), 311 (7), 244 (4), 229
(22), 213 (3), 189 (1), 83(100). IR ν KBrmax cm-1: 1747 1725
(C=O). UV λ MeOHmax nm (log ε): 218 (4.88), 255 sh (4.41), 300
sh (4.64), 323 (4.48). 1H-NMR (CDCl 3, 400 MHz): δ 1.38 (3H, s, Me-2’), 1.43 (3H, s, Me-2’), 1.80 (3H, br s, H-5”), 1.81 (3H, s, H-4’’’), 1.92 (3H, d, J = 7.0 Hz, H-4”), 2.12 (3H, s, H-5’’’), 5.32 (1H, d, J = 5.0 Hz, H-3’), 5.56 (1H, br s, H-2’’’), 6.05 (1H, br q, J = 7.0 Hz, H-3”), 6.13 (1H, d, J = 9.5 Hz, H-3), 6.58 (1H, d, J = 5.0 Hz, H-4’), 6.75 (1H, d, J = 8.5 Hz, H-6), 7.31 (1H, d, J = 8.5 Hz, H-5), 7.55 (1H, d, J = 9.5 Hz, H-4). 13C-NMR (CDCl3, 100 MHz): δ 15.6 (C-4”), 20.2 (C-5”), 20.2 (C-5’’’), 22.2 (Me-2’), 25.5 (Me-2’), 27.3 (C-4’’’), 59.3 (C-4’), 70.3 (C-3’), 77.3 (C-2’), 107.4 (C-8), 112.3 (C-4a), 113.0 (C-3), 114.2 (C-6), 115.0 2’’’), 127.0 2”), 129.1 5), 139.3 3”), 143.1 (C-4), 153.8 (C-8a), 156.6 (C-7), 157.7 (C-3’’’), 159.7 (C-2), 164.8 (C-1’’’), 166.1 (C-1”).
(+)-Anomalin (14): colorless needles, m.p. 171–173°C
(n-hexane), [α]25
D : +32.4° (c 0.2, CHCl3), EI-MS m/z (%):
426 ([M]+, 0.1), 327 (37), 311 (53), 229 (100). IR ν KBrmax cm-1:
1731 (C=O), 1604. UV λ MeOHmax nm (log ε): 209 (4.81), 217
(4.80), 255 (4.36), 300 sh (4.45), 323 (4.47). 1H-NMR (CDCl3, 400 MHz): δ 1.45 (3H, s, 2’), 1.49 (3H, s, Me-2’), 1.82 (3H, br s, H-5”), 1.85 (3H, br s, H-5’’’), 1.92–1.98 (6H, m, H-4”, 4’’’), 5.45 (1H, d, J = 5.0 Hz, H-3’), 6.01 (1H, br q, J = 7.2 Hz, H-3”), 6.02 (1H, br q, J = 7.2 Hz, H-3’’’), 6.22 (1H, d, J = 9.4 Hz, H-3), 6.70 (1H, d, J = 5.0, H-4’), 6.81 (1H, d, J = 8.8 Hz, H-6), 7.34 (1H, d, J = 8.8 Hz, H-5), 7.59 (1H, d, J = 9.4 Hz, H-4). (+)-cis-3’-Acetoxy-
4’-(2-methylbutyroyloxy)-3’,4’-dihydroseselin (15): colorless oil, [α]25
D : +12.6° (c
0.39, CHCl3), EI-MS m/z (%): 388 ([M]+, 0.2), 328 (2),
327 (7), 312 (7), 261 (7), 244 (11), 230 (15), 229 (100).
IR ν neatmax cm-1: 1743 (C=O), 1608. UV λ MeOHmax nm (log ε): 208
(4.76), 215 sh (4.65), 245 (4.12), 255 (4.05), 300 sh (4.43), 323 (4.62). 1H-NMR (CDCl 3, 400 MHz): δ 0.94 (3H, t, J = 7.6 Hz, H-4’’’), 1.21 (3H, d, J = 7.2 Hz, H-5’’’), 1.42 (3H, s, Me-2’), 1.45 (3H, s, Me-2’), 1.48 (1H, m, H-3’’’), 1.72 (1H, m, H-3’’’), 2.10 (3H, s, H-2”), 2.41 (1H, m, H-2’’’), 5.30 (1H, d, J = 5.0 Hz, 3’), 6.22 (1H, d, J = 9.4 Hz, H-3), 6.52 (1H, d, J = 5.0 Hz, H-4’), 6.79 (1H, d, J = 8.6 Hz, H-6), 7.36 (1H, d, J = 8.6 Hz, H-5), 7.59 (1H, d, J = 9.4 Hz, H-4). 13C-NMR (CDCl 3, 100 MHz): δ 11.6 (C-4’’’), 16.6 (C-5’’’), 20.7 (C-2”), 21.9 (Me-2’), 25.5 (Me-2’), 26.6 (C-3’’’), 41.3 (C-2’’’), 60.3 (C-4’), 70.7 (C-3’), 77.3 (C-2’), 107.4 (C-8), 112.3 (C-4a), 113.3 (C-3), 114.3 (C-6), 129.3 (C-5), 143.2 (C-4), 154.0 (C-8a), 156.5 (C-7), 159.7 (C-2), 169.8 (C-1”), 175.6 (C-1’’’). cis-3’-Hydroxy-4’-isovaleryloxy-3’,4’-dihydroseselin
(16) & laserpitin (17): colorless oil, [α]25
D : –107.5°(c 0.22,
CHCl3), GC-EI-MS m/z (%): 16: 346 ([M]+, 8), 328 (8),
312 (9), 244 (50), 229 (100); 17: 344 ([M]+, 8), 326 (8), 310
(22), 244 (50), 229 (100). IR ν neatmax cm-1: 3473 (OH), 1729
(C=O), 1606. UV λ MeOHmax nm (log ε): 205 (4.05), 215 sh
(3.80), 324 (3.56). 1H-NMR (CDCl3, 200 MHz): 16: δ 0.97 (3H, d, J = 6.2 Hz, H-4”), 1.01 (3H, d, J = 6.2 Hz, H-5”), 1.41 (3H, s, Me-2’), 1.45 (3H, s, Me-2’), 2.10–2.40 (3H, m, H-2”, 3”), 2.88 (1H, br s, OH-3’), 4.03 (1H, d, J = 5.0 Hz, H-3’), 6.23 (1H, d, J = 9.6 Hz, H-3), 6.42 (1H, d, J = 5.0 Hz, H-4’), 6.80 (1H, d, J = 8.8 Hz, H-6), 7.35 (1H, d, J = 8.8 Hz, H-5), 7.60 (1H, d, J = 9.6 Hz, H-4); 17: δ 1.41 (3H, s, Me-2’), 1.45 (3H, s, Me-2’), 1.88 (3H, br q, J = 1.2 Hz, H-5”), 1.96 (3H, br dq, J = 7.2, 1.2 Hz, H-4”), 2.88 (1H, br s, OH-3’), 4.08 (1H, d, J = 5.0 Hz, H-3’), 6.12 (1H, br q, J = 7.2 Hz, H-3”), 6.23 (1H, d, J = 9.6 Hz, H-3), 6.49 (1H, d, J = 5.0 Hz, H-4’), 6.80 (1H, d, J = 8.8 Hz, H-6), 7.35 (1H, d, J = 8.8 Hz, H-5), 7.60 (1H, d, J = 9.6 Hz, H-4).
(–)-cis-3’-Hydroxy-4’-(2-methylbutyryloxy)-3’,4’-dihydroseselin (18): colorless oil, [α]25D : –80.5° (c 0.34,
CHCl3), EI-MS m/z (%): 346 ([M]+, 4), 328 (6), 313 (7),
1607. UV λ MeOHmax nm (log ε): 206 (4.48), 215 sh (4.16), 246 (3.56), 256 (3.50), 325 (4.12). 1H-NMR (CDCl 3, 200 MHz): δ 0.94 (3H, t, J = 7.4 Hz, H-4”), 1.24 (3H, d, J = 6.8 Hz, H-5”), 1.42 (3H, s, Me-2’), 1.49 (3H, s, Me-2’), 1.50 (1H, m, 3”), 1.75 (1H, m, 3”), 2.49 (1H, m, H-2”), 2.87 (1H, br s, OH-3’), 4.05 (1H, d, J = 5.2 Hz, H-3’), 6.24 (1H, d, J = 9.6 Hz, 3), 6.39 (1H, d, J = 5.2 Hz, H-4’), 6.79 (1H, d, J = 8.8 Hz, H-6), 7.36 (1H, d, J = 8.8 Hz, H-5), 7.61 (1H, d, J = 9.6 Hz, H-4).
(+)-Marmesin (19): colorless prisms, m.p. 187–188°C
(n-hexane/CHCl3), [α]25D : +26.2° (c 1.1, CHCl3), EI-MS
m/z (%): 246 ([M]+, 36), 213 (31), 188 (65), 187 (100), 160
(26), 131 (16). IR ν KBrmax cm-1: 3447 (OH), 1703 (C=O), 1625,
1566. UV λ MeOHmax nm (log ε): 207 (4.50), 225 (4.24), 248
(3.81), 258 (3.73), 335 (4.45). 1H-NMR (CDCl 3, 200 MHz): δ 1.23 (3H, s, Me-4’), 1.37 (3H, s, Me-4’), 1.80 (1H, br s, OH-4’), 3.22 (2H, d, J = 8.4 Hz, H-3’), 4.73 (1H, t, J = 8.4 Hz, H-2’), 6.20 (1H, d, J = 9.6 Hz, H-3), 6.74 (1H, s, H-8), 7.21 (1H, s, H-5), 7.59 (1H, d, J = 9.6 Hz, H-4).
Umbelliferone (20): yellow prisms, m.p. 224–226°C
(Et2O/acetone), EI-MS m/z (%): 162 ([M]+, 100), 135 (55),
134 (63), 106 (13), 105 (16), 78 (29). IR ν KBrmax cm-1: 3161
(OH), 1709 (C=O). UV λ MeOHmax nm (log ε): 207 (4.27), 325
(4.31). UV λ MeOH+KOHmax nm (log ε): 210 (4.39), 230 (4.09),
371 (4.40). 1H-NMR (acetone-d6, 200 MHz): δ 6.16 (1H,
d, J = 9.4 Hz, H-3), 6.74 (1H, d, J = 2.2 Hz, H-8), 6.83
(1H, dd, J = 8.8, 2.2 Hz, 6), 7.50 (1H, d, J = 8.8 Hz, H-5), 7.85 (1H, d, J = 9.4 Hz, H-4).
Isoscopoletin (21): yellow prisms, m.p. 184–186°C
(Et2O/acetone), EI-MS m/z (%): 192 ([M]+, 100), 188
(25), 177 (42), 164 (34), 149 (43), 121 (23), 79 (7), 69
(12). IR ν KBrmax cm-1: 3442 (OH), 1707 (C=O), 1609, 1567,
1293. UV λ MeOHmax nm (log ε): 207 (4.02), 227 (3.75), 255 sh
(3.23), 293 (3.30), 338 (3.56). UV λ MeOH+KOHmax nm (log ε):
208 (4.09), 244 (3.61), 278 (3.20), 391 (3.66). 1H-NMR
(CDCl3, 200 MHz): δ 3.95 (3H, s, OMe-7), 6.14 (1H, br s,
OH-6, D2O exchangeable), 6.26 (1H, d, J = 9.6 Hz, H-3),
6.85 (1H, s, H-8), 6.92 (1H, s, H-5), 7.59 (1H, d, J = 9.6 Hz, H-4).
p-Hydroxyphenethyl ferulate (22): colorless oil,
EI-MS m/z (%): 314 ([M]+, 0.5), 194 (100), 177 (12), 145
(18), 120 (36). IR ν KBrmax cm-1: 3380 (OH), 1693 (C=O), 1595,
1515. UV λ MeOHmax nm (log ε): 220 (4.66), 300 sh (4.51), 325
(4.67). UV λ MeOH+KOHmax nm (log ε): 210 (4.62), 225 (4.58),
311 (4.20), 378 (4.74). 1H-NMR (CDCl
3, 400 MHz): δ 2.94
(2H, t, J = 7.2 Hz, H-7’), 3.93 (3H, s, OMe-3), 4.37 (2H, t,
J = 7.2 Hz, H-8’), 4.95 (1H, br s, OH-4, D2O
exchange-able), 5.87 (1H, s, OH-4’, D2O exchangeable), 6.27 (1H,
d, J = 16.0 Hz, H-8), 6.78 (2H, d, J = 8.8 Hz, H-3’, 5’), 6.91 (1H, d, J = 8.0 Hz, 6), 7.01 (1H, d, J = 2.0 Hz, H-2), 7.06 (1H, dd, J = 8.0, 2.0 Hz, H-5), 7.12 (2H, d, J = 8.8 Hz, H-2’, 6’), 7.59 (1H, d, J = 16.0 Hz, H-7). 13C-NMR (CDCl3, 100 MHz): δ 167.3 9), 154.3 4’), 147.9 (C-4), 146.7 (C-3), 144.9 (C-7), 130.0 (C-2’, 6’), 129.9 (C-1’), 127.0 (C-1), 123.1 (C-6), 115.3 (C-3’, 5’, 8), 114.6 (C-5), 109.3 (C-2), 65.1 (C-1”), 55.9(OMe-3), 34.3 (C-2”).
Falcarindiol (23): colorless oil, [α]25
D : +95.2° (c
0.12, CHCl3), EI-MS m/z (%): 260 ([M]+, 4), 242 (6),
229 (35), 157 (27), 129 (87), 128 (100), 115 (53), 91 (57).
IR ν neatmax cm-1: 3380 (OH), 2231, 2146 (C≡C). 1H-NMR
(CDCl3, 400 MHz): δ 0.88 (3H, t, J = 6.8 Hz, H-17), 1.25–1.40 (10H, br s, 12–16), 2.11 (2H, q, J = 7.2 Hz, H-11), 4.93 (1H, br d, J = 5.2 Hz, H-3), 5.20 (1H, d, J = 8.2 Hz, H-8), 5.26 (1H, d, J = 10.4 Hz, Ha-1), 5.47 (1H, d, J = 16.8 Hz, Hb-1), 5.51 (1H, dd, J = 10.0, 8.2 Hz, H-9), 5.60 (1H, dt, J = 10.0, 7.2 Hz, H-10), 5.93 (1H, ddd, J = 16.8, 10.4, 5.2 Hz, H-2). 13C-NMR (CDCl3, 100 MHz): δ 14.1 (C-17), 22.6 (C-16), 27.7 (C-11), 29.0 (C-12), 29.1 (C-13), 29.2 (C-14), 31.7 (C-15), 58.6 (C-8), 63.4 (C-3), 68.6 (C-6), 70.2 (C-5), 78.2 (C-4), 79.8 (C-7), 117.3 (C-1), 127.6 (C-9), 134.7 (C-10), 135.8 (C-2).
Psoralen (24): yellow needles, m.p. 161–163°C
(Et2O), EI-MS m/z (%): 186 ([M]+, 100), 158 (64), 130
(20), 102 (25). IR ν KBrmax cm-1: 1724 (C=O). UV λ MeOHmax nm
(log ε): 210 (4.18), 240 sh (4.29), 246 (4.31), 290 (3.94), 328 (3.72).
(+)-Lomatin (25): colorless needles, m.p. 156-158°C
(n-hexane), [α]25
D : +45.3° (c 0.13, CHCl3), EI-MS m/z (%):
246 ([M]+, 38), 213 (16), 188 (11), 177 (17), 176 (100), 147
(13), 91 (8). IR ν KBrmax cm-1: 3440 (OH), 1712 (C=O), 1603.
UV λ MeOHmax nm (log ε): 209 (4.57), 215 sh (4.16), 247 (3.83),
257 (3.80), 327 (4.49). 1H-NMR (CDCl3, 200 MHz): δ 1.35 (3H, s, Me-2’), 1.42 (3H, s, Me-2’), 2.97 (1H, dd, J = 17.4, 5.2 Hz, H-4’), 3.16 (1H, dd, J = 17.4, 5.2 Hz, H-4’), 3.93 (1H, t, J = 5.2 Hz, 3’), 6.24 (1H, d, J = 9.6 Hz, H-3), 6.79 (1H, d, J = 8.4 Hz, H-6), 7.26 (1H, d, J = 8.4 Hz, H-5), 7.64 (1H, d, J = 9.6 Hz, H-4).
Isofraxidin (26): yellow needles, m.p. 144–146°C
(n-hexane), EI-MS m/z (%): 222 ([M]+, 100), 207 (29), 194
(35), 179 (29), 167 (27), 161 (20), 149 (49). IR ν KBrmax cm-1:
3442 (OH), 1703 (C=O), 1264. UV λ MeOHmax nm (log ε): 209
(4.30), 220 sh (4.08), 339 (3.75). UV λ MeOH+KOHmax nm (log
ε): 215 (4.27), 398 (3.97). 1H-NMR (CDCl
3, 200 MHz): δ
3.95 (3H, s, OMe-6), 4.10 (3H, s, OMe-8), 6.13 (1H, br s,
OH-7, D2O exchangeable), 6.29 (1H, d, J = 9.6 Hz, H-3),
6.66 (1H, s, H-5), 7.60 (1H, d, J = 9.6 Hz, H-4).
(–)-cis-Khellactone (27): colorless prisms, m.p.
145–147°C (Et2O), [α]25D : –17.4° (c 0.18, CHCl3), EI-MS
m/z (%): 262 ([M]+, 15), 192 (13), 191 (100), 162 (16), 134
(16), 107 (14). IR ν KBrmax cm-1: 3418 (OH), 1712 (C=O), 1603.
UV λ MeOHmax nm (log ε): 219 (4.67), 245 sh (4.12), 257 (4.04),
300 sh (4.46), 326 (4.70). 1H-NMR (CDCl 3, 400 MHz): δ 1.39 (3H, s, Me-2’), 1.44 (3H, s, Me-2’), 3.23 (1H, br s, OH, D2O exchangeable), 3.84 (1H, d, J = 4.8 Hz, H-3’), 4.19 (1H, br s, OH, D2O exchangeable), 5.18 (1H, d, J = 4.8 Hz, H-4’), 6.23 (1H, d, J = 9.6 Hz, H-3), 6.77 (1H, d, J = 8.6 Hz, H-6), 7.30 (1H, d, J = 8.6 Hz, H-5), 7.64 (1H, d, J = 9.6 Hz, H-4).
1-O-Hexadecanoyl glycerol (28): amorphous solid,
m.p. 66–68°C (CHCl3), [α]25D : –12.4° (c 0.01, CHCl3),
FAB-MS m/z (%): 353 ([M+Na]+, 19), 313 (12), 239 (14),
IR ν KBrmax cm-1: 3415 (OH), 1730 (C=O). 1H-NMR (CDCl3,
(24H, br s, H-4’–15’), 1.62 (2H, m, H-3’), 2.35 (2H, t, J = 7.6 Hz, H-2’), 3.59 (1H, dd, J = 11.4, 5.6 Hz, H-3), 3.70 (1H, dd, J = 11.4, 4.0 Hz, H-3), 3.93 (1H, m, H-2), 4.13 (1H, dd, J = 11.6, 4.8 Hz, H-1), 4.22 (1H, dd, J = 11.6, 4.0 Hz, H-1).
(+)-3’-Hydroxymarmesin (29): colorless prisms,
m.p. 120–122°C (Et2O), [α]25D : +36.5° (c 0.17, CHCl3),
EI-MS m/z (%): 262 ([M]+, 18), 186 (100), 158 (36), 131
(4), 102 (6). IR ν KBrmax cm-1: 3394 (OH), 1725 (C=O), 1628.
UV λ MeOHmax nm (log ε): 205 (4.01), 222 (3.60), 257 sh (2.81),
327 (3.67). 1H-NMR (CDCl 3, 200 MHz): δ 1.55 (3H, s, H-5’), 1.60 (3H, s, H-6’), 4.36 (1H, d, J = 6.2 Hz, H-2’), 5.37 (1H, br d, J = 6.2 Hz, H-3’), 6.25 (1H, d, J = 9.6 Hz, H-3), 6.82 (1H, br s, H-8), 7.51 (1H, s, H-5), 7.65 (1H, d, J = 9.6 Hz, H-4). 13C-NMR (CDCl3, 50 MHz): δ 25.3 (C-6’), 28.5 (C-5’), 71.8 (C-3’), 73.0 (C-4’), 90.5 (C-2’), 99.0 (C-8), 100.5 (C-4a), 112.9 (C-3), 124.8 (C-5), 128.1 (C-6), 143.6 (C-4), 152.2 (C-7), 156.7 (C-8a), 162.3 (C-2). CH2OH HO H HO H OH H H OH CH2OH 1 O O O R1 R2 R1 R2 2 OCH3 H 3 H 5 H OCH3 24 H H 31 H O O R1 R3 O R2O R1 R2 R3 4 H H 19 H H H 29 OH H H 30 H H OH O O OH OH O O O O OR1 OR2 O O R1 R2 R3 O O O HO HO O O O OH R1 R2 8 9 10 12 13 14 15 16 H 17 H 18 H 27 H H O O O O O O O O O O O O O O O 25 32 R1 R2 R3 20 H OH H 21 OH OCH3 H 26 OCH3 OH OCH3 O O HO H3CO OH 22 HO R 11 R = H 23 R = OH HO O OH O 28 H H HO 6 7 22 O O 3 4 5 8 3' 2' 5 4 3 6 2' 3' 4' 2' 3' 4' 5' 6' 8 5 4 3 4' 3' 2' 1 2 3 1' ' 6 1 ' 2 1 2 3 4 5 6 7 8 9 10 17 1 3 4 7 8 9 1' 4' 7' 8' 1 2 3 1 3 6 19 18 5 17 22 23 21 29 26 27 1" 2" 5" 3" 4" 1" 2" 5" 3" 4" 1" 2" 3" 4" 5" 1''' 2''' 3''' 4''' 5''' 1" 2"
(+)-Rutaretin (30): yellow prisms, m.p. 179–181°C
(n-hexane/acetone), [α]25
D : +42.2° (c 0.09, CHCl3),
EI-MS m/z (%): 262 ([M]+, 44), 244 (5), 229 (23), 204 (62),
203 (100), 191 (26), 176 (28), 147 (16), 91 (10). IR ν KBrmax
cm-1: 3420 (OH), 1700 (C=O), 1618, 1586. UV λ MeOHmax nm
(log ε): 213 (4.68), 240 sh (4.13), 266 (3.54), 332 (3.99).
UV λ MeOH+KOHmax nm (log ε): 219 (4.65), 282 (4.29), 339
(4.23). 1NMR (acetone-d6, 200 MHz): δ 1.23 (3H, s, H-5’), 1.29 (3H, s, H-6’), 3.24 (1H, ddd, J = 15.7, 9.2, 1.0 Hz, H-3’), 3.31 (1H, ddd, J = 15.7, 8.0, 1.0 Hz, H-3’), 3.84 (1H, br s, OH-4’, D2O exchangeable), 4.78 (1H, dd, J = 9.2, 8.0 Hz, H-2’), 6.13 (1H, d, J = 9.6 Hz, H-3), 6.97 (1H, t, J = 1.0 Hz, H-5), 7.82 (1H, d, J = 9.6 Hz, H-4), 8.60 (1H, br
s, OH-8, D2O exchangeable). 13C-NMR (acetone-d6, 50
MHz): δ 161.4 (C-2), 26.5 (C-5’), 31.8 (C-3’), 72,5 (C-4’), 92.8 (C-2’), 112.9 (C-3), 114.8 (C-4a), 115.6 (C-5), 127.1 (C-6), 130.1 (C-8), 145.2 (C-8a), 146.1 (C-4), 152.5 (C-7).
(+)-Oxypeucedanin hydrate (31): colorless needles,
m.p. 132–134°C (Et2O), [α]25D : +26.6° (c 0.09, CHCl3),
EI-MS m/z (%): 304 ([M]+, 23), 259 (30), 202 (91), 167 (100),
147 (29), 137 (47), 59 (45). IR ν KBrmax cm-1: 3146 (OH), 1726
(C=O). UV λ MeOHmax nm (log ε): 220 (4.71), 249 (4.50), 265
sh (4.42), 312 (4.40). 1H-NMR (CDCl 3, 400 MHz): δ 1.31 (3H, s, H-5”), 1.36 (3H, s, H-4”), 3.88 (1H, d, J = 8.0 Hz, H-2”), 4.45 (1H, dd, J = 10.0, 8.0 Hz, H-1”), 4.53 (1H, dd, J = 10.0, 3.2 Hz, H-1”), 6.32 (1H, d, J = 9.6 Hz, H-3), 6.99 (1H, dd, J = 2.4, 1.2 Hz, 3’) , 7.20 (1H, d, J =1.2 Hz, H-8), 7.61 (1H, d, J = 2.4 Hz, H-2’), 8.18 (1H, dd, J = 9.6 Hz, H-4).
(+)-Dorsteniol (32): colorless prisms, m.p. 135–
137°C, [α]25
D : +36.8° (c 0.09, CHCl3), EI-MS m/z (%): 262
([M]+, 39), 213(35), 188 (73), 187 (100), 160 (20), 131 (24),
IR ν KBrmax cm-1 3415 (OH), 1713 (C=O), 1624. UV λ MeOHmax nm
(log ε): 210 (4.66), 225 (4.61), 250 sh (4.20), 259 (4.16), 300 sh (4.31), 333 (4.62). 1H-NMR (CDCl 3, 400 MHz): δ 1.19 (3H, s, H-5’), 3.21 (1H, dd, J = 16.0, 9.6 Hz, H-3’), 3.34 (1H, dd, J = 16.0, 7.6 Hz, H-3’), 3.48 (1H, d, J =11.0 Hz, H-6’), 3.53 (1H, d, J = 11.0 Hz, H-6’), 4.97 (1H, dd, J = 9.6, 7.6 Hz, H-2’), 6.14 (1H, d, J = 9.5 Hz, H-3), 6.64 (1H, s, H-8), 7.43 (1H, s, H-5), 7.86 (1H, d, J = 9.5 Hz, H-4).
V. Anti-platelet Aggregation Test
Blood was collected from the marginal vein of a rabbit, anticoagulated with EDTA (6 mM) and centri-fuged for 10 min at 90 × g at room temperature to obtain platelet-rich plasma (PRP). Platelet suspension was prepared from this EDTA-anticoagulated PRP according
to the washing procedures described previously(4).
Plate-let numbers were counted by a Coulter counter (Model
ZM) and adjusted to 3 × 108 platelets/mL. The platelet
pellets were then suspended in Tyrode’s solution of the following composition (mM): NaCl (136.8), KCl (2.8),
NaHCO3 (11.9), MgCl2 (2.1), NaH2PO4 (0.33), CaCl2 (1.0)
and glucose (11.2), containing bovine serum albumin (0.35%). Platelet aggregation was measured by
turbid-metric method described by O’Brien(5). The absorbance
of the platelet suspension was taken as 0% aggrega-tion, and that of Tyrode’s solution as 100% aggregation. Aggregation was measured by an aggregometer (Chrono-Log Co., Havertown, PA) with consistent stirring at 1200 rpm. All tested compounds were dissolved in dimethyl sulfoxide (DMSO). To eliminate the effect of the solvent on the aggregation, the final concentration of DMSO was fixed at 0.5%, which did not affect the measured aggrega-tion. Aspirin was used as a positive control. Data were analyzed using Student’s t test.
Results and disCussiOn
All of the isolates, including D-mannitol (1)(6),
bergapten (2)(7), isoimperatorin (3)(7), (–)-deltoin (4)(7),
xanthotoxin (5)(7), β-sitosterol (6)(8), stigmasterol (7)(8),
(+)-praeruptorin E (8)(9), (+)-hyuganin A (9)(10),
(–)-cis-3’-isovaleryl-4’-senecioylkhellactone (10)(11), panaxynol (11)(12), (–)-isosamidin (12)(9), (+)-peuformosin (13)(3), (+)-anomalin (14)(9), (+)-cis-3’-acetoxy-4’-(2-methylbu-tyroyloxy)-3’,4’-dihydroseselin (15)(10), cis-3’-hydroxy-4’-isovaleryloxy-3’,4’-dihydroseselin (16)(9), laserpitin (17)(9),
(–)-cis-3’-hydroxy-4’-(2-methylbutyryloxy)-3’,4’-dihydroseselin (18)(9), (+)-marmesin (19)(7),
umbellifer-one (20)(13), isoscopoletin (21)(14), p-hydroxyphenethyl
ferulate (22)(15), falcarindiol (23)(12), psoralen (24)(7),
(+)-lomatin (25)(16), isofraxidin (26)(17),
(–)-cis-khel-lactone (27)(13), 1-O-hexadecanoyl glycerol (28)(18),
(+)-3’-hydroxymarmesin (29)(19), (+)-rutaretin (30)(20),
(+)-oxypeucedanin hydrate (31)(7), (+)-dorsteniol (32)(21) were
readily identified by comparison of physical and
spectro-scopic data (UV, IR, 1H-NMR, [α]
D, and mass
spectrom-etry data) with values found in the literature. Among these compounds, 11, 15, 16, 18, 22, 25, 26, 28, 29, and
32 were firstly isolated from this genus.
The constituents of three Hu, Bai-Hua
Qian-Hu (P. praeruptorum)(13,22-34), Taiwan Qian-Hu (P.
formosanum), and Zi-Hua Qian-Hu (A. decursiva = P. decursivum)(35-47), are compared in Table 1,
show-ing that seselin-type dihydropyranocoumarins (8–10,
12–18, 25, 27) and psoralen-type furanocoumarins (2–5, 19, 24, 28–32) are two major groups of constituents in P. formosanum. These two types of coumarins are also
the major compounds in P. praeruptorum. The major constituents in Angelica decursiva are xanthyletin-type dihydropyranocoumarins and psoralen-type furano-coumarins; the former has not been found in P.
formo-sanum and few have been found in P. praeruptorum. P. japonicum(2,7,11,48-60), another species of Peucedanum in
Taiwan(1), also has seselin-type dihydropyranocoumarins
and psoralen-type furanocoumarins as its major constitu-ents, and these may be used as a key to the chemotax-onomy of Peucedanum and Angelica.
Some of the isolates in P. formosanum have been reported to exert strong anti-platelet aggregation
table 1. The constituents of Bai-Hua Qian-Hu (Peucedanum
pra-eruptorum), Taiwan Qian-Hu (P. formosanum), and Zi-Hua Qian-Hu (Angelica decursiva = P. decursivum)
Compound Qian-HuBai-Hua Qian-HuTaiwan Qian-HuZi-Hua
O O simple coumarin isofraxidin (26) – + – isoscopoletin (21) + + – peucedanol + – – scopoletin + – + scopolin + – – umbelliferone (20) + + + furanocoumarin O O O psoralen type bergapten (2) – + + decurside I – – + imperatorin – – + isoimperatorin (3) – + – 5,8-dimethoxypsoralen + – – (+)-oxypeucedanin hydrate (31) – + – psoralen (24) + + – xanthotoxin (5) – + – O O O dihydropsoralen type decuroside V – – + decuroside VI – – + decuroside VII – – + (–)-deltoin (4) – + – (+)-dorsteniol (32) – + – 1-O-hexadecanoyl glycerol (28) – + – (+)-3’-hydroxymarmesin (29) – + – isorutarin + – – (+)-marmesin (19) – + – nodakenetin – – + nodakenin + – + praeroside + – – (+)-rutaretin (30) – + – rutarin + – – O O O angelicin type angelicin + – –
Compound Bai-Hua Qian-Hu Qian-HuTaiwan Qian-HuZi-Hua
O O O dihydroangelicin type apterin + – – columbianadin – – + pyranocoumarin O O O dihydroxanthyletin type 3’(S)-acetoxy-4’(R)-isovaleryloxy-3’,4’-dihydroxanthyletin – – + 3’(S)-acetoxy-4’(R)-angeloyloxy-3’,4’-dihydroxanthyletin – – + AD-I (3’(S)-angeloyloxy-4’(R,S)-isovaleryloxy-3’,4’- dihydroxanthyletin) – – + andelin (AD-II, 3’(S)- angeloyloxy-4’(R)-senecioyloxy-3’,4’- dihydroxanthyletin) – – + decursidin – – + decursin – – + decursinol + – + decursitin B – – + decursitin C – – + decursitin D – – + decursitin F – – + Pd-C-I (3’(S)-senecioyloxy- 4’(R)-hydroxy-3’,4’-dihydroxanthyletin) + – + Pd-C-II (3’(S)-hydroxy- 4’(R)-senecioyloxy-3’,4’-dihydroxanthyletin) – – + Pd-C-III (3’(S)-angeloyloxy- 4’(R)-acetoxy-3’,4’-dihydroxanthyletin) – – + qainhucoumarin F + – – O O O dihydroseselin type (+)-cis-3’-acetoxy-4’-(2- methylbutyroyloxy)-3’,4’-dihydroseselin (15) – + – (+)-anomalin (14, Pd-II) + + – cis-3’,4’-disenecioyl-3’,4’-dihydro-seselin + – – table 1. continued
(+)-anomalin (14)(62), psoralen (24)(7) and
(–)-cis-khellac-tone (27)(62). The anti-platelet aggregation effects of the
other isolates are shown in Table 2. p-Hydroxyphenethyl ferulate (22) at 5 µg/mL showed nearly complete inhibi-tion of platelet aggregainhibi-tion induced by arachidonic acid (AA). (–)-Isosamidin (12), (+)-peuformosin (13), (+)-cis-3’-acetoxy-4’-(2-methylbutyroyloxy)-3’,4’-dihydroseselin (15),
p-hydroxy-phenethyl ferulate (22) at 100 µg/mL showed
complete or near complete inhibition of platelet aggregation induced by collagen. (–)-cis-3’-Isovaleryl-4’-senecioylkhel-lactone (10), (+)-cis-3’-acetoxy-4’-(2-methylbutyroyloxy)-3’,4’-dihydroseselin (15) and p-hydroxyphenethyl feru-late (22) at 100 µg/mL abolished pferu-latelet-activating factor (PAF)-induced platelet aggregation. Of these compounds,
p-hydroxyphenethyl ferulate (22) showed the strongest
antiplatelet aggregation activities, with IC50 values of 5.1,
10.5 and 99.4 µM for platelet aggregation induced by AA, collagen and PAF, respectively.
aCKnOWledgeMents
This research was financially supported by the National Science Council of the Republic of China.
ReFeRenCes
1. Kao, M. T. 1993. Umbelliferae. In “Flora of Taiwan”. 2nd. ed. Vol. III. pp. 1010-1045. Editorial Committee of the Flora of Taiwan. Taipei, Taiwan.
2. Hata, K., Koawa, M., Ikeshiro, Y. and Yen, K. Y. 1968. New coumarins isolated from the root of Peucedanum
formosanum and Peucedanum japonicum. Yakugaku
Zasshi 88: 513-520.
3. Hata, K., Kozawa, M. and Yen, K. Y. 1966. Constitu-tion of peuformosin, a new coumarin isolated from the root of Peucedanum formosanum. Chem. Pharm. Bull. 14: 442-446.
4. Teng, C. M., Chen, W. Y., Ko, W. C. and Ouyang, C. 1987. Antiplatelet effect of butylidenephthalide. Biochim. Biophys. Acta 924: 375-382.
5. O’Brien, J. R. 1962. Platelet aggregation II. Some results from a new method of study. J. Clin. Path. 15: 452-455.
6. Pomilio, A. B., Gonzalez, M. D. and Eceizabarrena, C. C. 1996. 7,8-Dihydroajugasterone C, norhygrine and other constituents of Nierembergia hippomanica. Phy-tochemistry 41: 1393-1398.
7. Chen, I. S., Chang, C. T., Sheen, W. S., Teng, C. M., Tsai, I. L., Duh, C. Y. and Ko, F. N. 1996. Coumarins and antiplatelet aggregation constituents from Formosan Peucedanum japonicum. Phytochemstry 41: 525-530.
8. Kojima, H., Sato, N., Hatano, A. and Ogura, H. 1990. Sterol glucosides from Prunella vulgaris. Phytochemis-try 29: 2351-2355.
Compound Qian-HuBai-Hua Qian-HuTaiwan Qian-HuZi-Hua cis-3’-hydroxy-4’-isovaleryloxy-3’,4’-dihydroseselin (16) – + – (–)-cis-3’-hydroxy-4’-(2-methyl- butyryloxy)-3’,4’-dihydrosese-lin (18) – + – (+)-hyuganin A (9) – + – (–)-isosamidin (12) – + – (–)-cis-3’-isovaleryl-4’-senecioyl-khellactone (10) – + – (–)-cis-khellactone (27) – + – trans-khellactone + – – laserpitin (17) – + – (+)-lomatin (25) – + – Pd-Ib + – + peucedanocoumarin I + – – peucedanocoumarin II + – – peucedanocoumarin III + – – (+)-peuformosin (13) – + – praeroside II + – – praeroside III + – – praeroside IV + – – praeroside V + – – praeruptorin A + – – (+)-praeruptorin E (8) + + – qainhucoumarin A + – – qainhucoumarin B + – – qainhucoumarin C + – – qainhucoumarin D + – – qainhucoumarin E + – – qainhucoumarin H + – – samidin + – – chromone skimmin + – – steroid stigmasterol (7) + + – β-sitosterol (6) + + + β-sitosterol-β-D-glucoside + – + saccharide galactitol + – – D-mannitol (1) + + – polyacetylene falcarindiol (23) – + – panaxynol (11) – + – benzenoid decursidate – – + p-hydroxyphenethyl ferulate (22) – + – table 1. continued
9. Swager, T. M. and Cardellina II, J. H. 1985. Coumarins from Musineon divaricatum. Phytochemistry 24: 805-813.
10. Matsuda, H., Murakami, T., Nishida, N., Kageura, T. and Yoshikawa, M. 2000. Medicinal foodstuff. XX. table 2. Inhibitory effectsa of compounds on the aggregation of washed rabbit platelets induced by thrombin (Thr), arachidonic acid (AA),
collagen (Col) and platelet-activating factor (PAF)
Conc. Aggregation (%)
Compound (µg/mL) AA (100 µM) Col (10 µg/mL) Thr (0.1 U/mL) PAF (2 ng/mL)
control 84.2 ± 1.0 (3) 84.8 ± 2.3 (3) 89.3 ± 1.6 (3) 87.8 ± 0.5 (3) (–)-cis-3’-isovaleryl-4’-senecioylkhel-lactone (10) 100 91.4 ± 3.2 (3) 32.8 ± 15.0 (3) d 86.3 ± 1.9 (3) 0.0 ± 0.0 (3)e 50 49.0 ± 8.1 (3)e 27.0 ± 14.9 (3)d 20 81.8 ± 5.0 (3) 58.5 ± 1.9 (3)e (–)-isosamidin (12) 100 38.0 ± 17.1 (3)c 5.4 ± 2.8 (3)e 87.8 ± 1.1 (3) 50.3 ± 15.9 (3)c 50 45.8 ± 19.3 (3) 30.7 ± 15.1 (3)d 20 69.3 ± 6.6 (3) 68.9 ± 9.6 (3) 10 76.9 ± 5.4 (3) 77.4 ± 5.5 (3) 5 79.9 ± 4.3 (3) 83.4 ± 3.4 (3) (+)-peuformosin (13) 100 56.8 ± 7.7 (3)c 14.0 ± 9.7 (3)e 82.1 ± 2.4 (3)c 32.9 ± 13.3 (3)c (+)-cis-3’-acetoxy-4’-(2-methylbutyroy-loxy)-3’,4’-dihydroseselin (15) 100 53.4 ± 10.5 (3) d 0.0 ± 0.0 (3)e 74.4 ± 2.8 (3)e 0.0 ± 0.0 (3)e 50 76.1 ± 2.0 (3)e 45.4 ± 9.3 (3)e 22.7 ± 9.3 (3)e 20 83.4 ± 1.1 (3)e 70.4 ± 4.9 (3)d (–)-cis-3’-hydroxy-4’-(2-methylbutyry-loxy)-3’,4’-dihydroseselin (18) 100 73.9 ± 7.0 (3) 66.4 ± 3.9 (3) e 81.0 ± 3.7 (3) 19.5 ± 9.0 (3)e 50 66.1 ± 4.9 (3)e p-hydroxyphenethyl ferulate (22) 100 0.0 ± 0.0 (3)e 0.0 ± 0.0 (3)e 43.8 ± 8.7 (3)e 0.0 ± 0.0 (3)e 50 0.0 ± 0.0 (3)e 0.0 ± 0.0 (3)e 80.6 ± 6.7 (3) 9.5 ± 7.7 (3)e 20 0.0 ± 0.0 (3)e 0.0 ± 0.0 (3)e 74.1 ± 5.1 (3)c 10 0.0 ± 0.0 (3)e 0.0 ± 0.0 (3)e 5 3.1 ± 2.5 (3)e 25.1 ± 8.9 (3)e 2 34.5 ± 17.4 (3)c 69.5 ± 8.7 (3)c 1 74.7 ± 3.1 (3)d 85.8 ± 2.8 (3) (+)-lomatin (25) 100 81.0 ± 1.4 (3) 84.7 ± 0.5 (3)e 81.4 ± 7.6 (3) 73.6 ± 5.7 (3)c (+)-3’-hydroxymarmesin (29) 100 83.6 ± 3.1 (3) 90.8 ± 1.6 (3) 83.4 ± 3.0 (3) 83.3 ± 2.6 (3)c (+)-rutaretin (30) 100 64.4 ± 7.5 (3)d 66.6 ± 3.0 (3)e 73.0 ± 8.3 (3) 86.0 ± 1.5 (3) aspirinb 100 0.0 ± 0.0 (3)e 81.3 ± 0.5 (3) 50 11.7 ± 10.1 (4)e 20 84.3 ± 0.6 (4)e
a Platelets were preincubated with each compound or DMSO (0.5 %, control) at 37°C for 3 min, then the inducer arachidonic acid (AA),
collagen, thrombin or PAF was added. Values are presented as mean ± s.e.m. (n).
bPositive control.
Vasorelaxant active constituents from the roots of
Angelica furcijuga Kitagawa: structure of hyuganins
A, B, C, and D. Chem. Pharm. Bull. 48: 1429-1435. 11. Jong, T. T., Hwang, H. C., Jean M. Y., Wu, T. S. and
Teng, C. M. 1992. An antiplatelet aggregation principle and X-ray structural analysis of cis-khellactone diester from Peucedanum japonicum. J. Nat. Prod. 55: 1396-1401.
12. Fujioka, T., Furumi, K., Fujii, H., Okabe, H., Mihashi, K., Nakano, Y., Matsunaga, H., Katano, M. and Mori, M. 1999. Antiproliferative constituents from Umbel-liferae plants. V. A new furanocoumarin and falcarin-diol furanocoumarin ethers from the roots of Angelica
japonica. Chem. Pharm. Bull. 47: 96-100.
13. Kong, L. Y., Li, Y., Min, Z. D., Li, X. and Zhu, T. R. 1996. Coumarins from Peucedanum praeruptorum. Phytochemistry 41: 1423-1426.
14. Lee, C. K., Lee, P. H. and Kuo, Y. H. 2001. The chemical constituents from the aril of Cassia fistula L. J. Chin. Chem. Soc. 48: 1053-1058.
15. Darwish, F. M. M. and Reinecke, M. G. 2003. Ecdys-teroids and other constituents from Sida spinosa L. Phytochemistry 62: 1179-1184.
16. Lemmich, J. and Shabana, M. 1984. Coumarin sulphates of Seseli libanotis. Phytochemitry 23: 863-865.
17. Panichayupakaranant, P., Noguchi, H., De-Eknamkul, W. and Sankawa, U. 1995. Naphthoquinones and coumarins from Impatiens balsamina root cultures. Phytochemistry 40: 1141-1144.
18. Haraldssin, G. G., Gudmundsson, B. O. and Almarsson, O. 1995. The synthesis of homogeneous triglycerides of eicosapentaenoic acid and docosahexaenoic acid by lipase. Teterhedron 51: 941-952.
19. Lemmich, J., Havelund, S. and Thastrup, O. 1983. Dihydrofurocoumarin glucosides from Angelica
arch-angelica and Angelica silvestris. Phytochemistry 22:
553-555.
20. Reisch, J. and Voerste, A. A. W. 1994. Natural product chemistry. Part 181. Investigations on the synthesis of dihydropyrano- and dihydrofurano-coumarins by application of catalytic enantioselective cis-dihydroxyl-ation. J. Chem. Soc. Perkin Trans. 1: 3251-3256.
21. Ricardo, T. M., Raul, C. G., Norma, F. S. S. and Pedro, J. N. 1998. Isolation, total synthesis, and relative stereo-chemistry of a dihydrofurocoumarin from Dorstenia
contrajerva. J. Nat. Prod. 61: 1216-1220.
22. Lu, M., Nicoletti, M., Battinelli, L. and Mazzanti, G. 2001. Isolation of praeruptorins A and B from
Peuce-danum praeruptorum and their general
pharmacologi-cal evaluation in comparison with extracts of the drug. Farmaco 56: 417-420. (C. A. 136: 79275).
23. Ye, J., Zhang, H. and Yuan, C. 1982. Isolation and identification of coumarin praeruptorin E from the root of the Chinese drug Peucedanum praeruptorum Dunn. (Umbelliferae). Yaoxue Xuebao 17: 431-434.
24. Takata, M., Okuyama, T. and Shibata, S. 1988. Studies
on coumarins of a Chinese drug “Qian-Hu” VIII. Structures of new coumarin glycoside of Bai-Hua Qian-Hu. Planta Med. 54: 323-327.
25. Okuyama, T., Takata, M. and Shibata, S. 1990. Struc-tures of angular pyranocoumarins of Bai-Hua Qian-Hu, the root of Peucedanum praeruptorum. Planta Med. 56: 307-311.
26. Kong, L. Y., Pei, Y. H., Li, X., Wang, S. X., Hou, B. L. and Zhu, T. R. 1993. The isolation and identification of qianhucoumarin B and C from Peucedanum
praerup-torum. Yaoxue Xuebao 28: 772-776.
27. Kong, L., Pei, Y., Li, X. and Zhu, T. 1993. New compounds from Peucedanum praeruptorum. Chin. Chem. Lett. 4: 37-38 (C. A. 121: 31073).
28. Chang, H. and Li, X. 1999. Coumarins from
Peuce-danum praeruptorum Dunn. Shenyang Yaoke Daxue
Xuebao 16: 103-106.
29. Kong, L., Li, Y. and Masatake, N. 2003. A new pyrano-coumarin from Peucedanum praeruptorum. Hetero-cycles 60: 1915-1919.
30. Ghen, Z. X., Hung, B. S., She, Q. L. and Zeng, G. F. 1979. Study on the chemical constituents of the Chinese medicinal plant, Peucedanum praeruptorum Dunn. Structures of four new coumarins. Yao Hsueh Hsueh Pao 14: 486-496.
31. Okuyama, T. and Shibata, S. 1981. Studies on coumarins of a Chinese drug “Qian-Hu”. Planta Med. 42: 89-96.
32. Okuyama, T., Takata, M. and Shibata, S. 1989. Studies on coumarins of a Chinese drug “Qian-Hu” IX. Struc-tures of linear furano- and simple-coumarin glycosides of Bai-Hua Qian-Hu. Planta Med. 55: 64-67.
33. Kong, L. Y., Pei, Y. H., Li, X., Yu, R. M., Min, Z. D. and Zhu, T. R. 1994. Isolation and structure elucidation of baihuaqainhuoside and Pd-C-I from Peucedanum
praeruptorum. Yaoxue Xuebao 29: 276-280.
34. Kong, L, Pei, Y., Li, X. and Zhu, T. 1993. Chemical constituents of the root of whiteflower hogfennel
(Peu-cedanum praeruptorum). Zhongcaoyao 24: 401-404.
35. Kong, L., Li, Y. and Min, Z. 1996. Study on analysis of Pd-Ia in Peucedanum praeruptorum and Pd-C-I in
Peu-cedanum decursivum by RP-HPLC. Zhogguo Yaoke
Daxue Xuebao 27: 215-218. (C. A. 125: 323017).
36. Arima, J. 1929. The constitution of nodakenin, a new glucoside from Peucedanum decursivum Maxim. I. Bull. Chem. Soc. Japan 4: 113-119.
37. Kong, L. and Yao, N. H. 2000. Coumarin-glycoside and ferulate from Peucedanum decursivum. Chin. Chem. Lett. 11: 315-318.
38. Sakakibara, I., Okuyama, T. and Shibata, S. 1982. Studies on coumarins of a Chinese drug “Qian-Hu”. III. Coumarins from “Zi-Hua Qian-Hu”. Planta Med. 44: 199-103.
39. Yao, N. and Kong, L. 2001. Chemical constituents of
Peucedanum decursivum. Yaoxue Xuebao 36: 351-355.
40. Yao, N., Kong, L. and Ling, Y. 1999. New coumarins from Peucedanum decursivum. Chin. Chem. Lett. 10:
477-480.
41. Kong, L., Yao, N. H. and Masatake, N. 2000. Two new xanthyletin-type coumarins from Peucedanum
decursi-vum. Heterocycles 53: 2019-2025.
42. Asahara, T., Sakakibara, I., Okuyama, T. and Shibata, S. 1984. Studies on coumarins of a Chinese drug “Qian-Hu” V. Coumarin-glycosides from “Zi-Hua Qian-Hu”. Planta Med. 50: 488-492.
43. Sakakibara, I., Okuyama, T. and Shibata, S. 1984. Studies on coumarins of a Chinese drug “Qian-Hu”, IV. Coumarins from “Zi-Hua Qian-Hu” (supplement). Planta Med. 50: 117-120.
44. Jung, N. I., Yook, C. S. and Lee, H. K. 1994. Coumarins from the roots of Angelica
decursiva-albiflora. Saengyak Hakhoechi 25: 311-318. (C. A. 122:
101662).
45. Sano, K., Yosioka, I. and Kitagawa, I. 1973. Stereo-structures of decursin, decursidin, and a new coumarin isolated from Angelica decursiva. Chem. Pharm. Bull. 21: 2095-2097.
46. Avramenko, L. G., Nikonov, G. K. and Pimenov, M. G. 1970. Andelin, a new dihydropyranocoumarin from
Angelica decursiva roots. Khimiya Prirodnykh
Soed-inenii 6: 190-194. (C. A. 73: 87814).
47. Hata, K. and Sano, K. 1969. Coumarins from the root of Angelica decursiva. I. Structure of decursin and decursidin. Yakugaku Zasshi 89: 549-557. (C. A. 71: 38832).
48. Yen, K. Y., Lai, H. M. and Chen, W. S. 1966. Chemical constituents of umbelliferous plants of Taiwan. VIII. Coumarins in the roots of Peucedanum japonicum. Taiwan Yaoxue Zazhi 18: 75-77.
49. Yamada, Y., Hsu, C. S., Iguchi, K., Suzuki, M., Hsu, H. Y. and Chen, Y. P. 1974. Two new khellactone esters from Peucedanum japonicum. Tetrahedron Lett. 29: 2513-2516.
50. Shigematsu, N., Kouno, I. and Kawano, N. 1982. On the isolation of (+)-samidin from the roots of
Peuceda-num japonicum Thunb. Yakugaku Zasshi 102: 392-394.
51. Cisowski, W. 1983. Flavonoid compounds of the herb
Peucedanum japonicum Thunb. Pol. J. Chem. 57:
1283-1286. (C. A. 102: 218357).
52. Duh, C. Y., Wang, S. K. and Wu, Y. C. 1991. Cytotoxic pyranocoumarins from the aerial parts of Peucedanum
japonicum. Phytochemistry 30: 2812-2814.
53. Duh, C. Y., Wang, S. K. and Wu, Y. C. 1992. Cytotoxic pyranocoumarins from roots of Peucedanum
japonicum. Phytochemistry 31: 1829-1830.
54. Ikeshiro, Y., Mase, I. and Tomita, Y. 1992. Dihydropy-ranocoumarins from roots of Peucedanum japonicum. Phytochemistry 31: 4303-4306.
55. Ikeshiro, Y., Mase, I. and Tomita, Y. 1993. Dihydropy-ranocoumarins from Peucedanum japonicum. Phyto-chemistry 33: 1543-1545.
56. Ikeshiro, Y., Mase, I. and Tomita, Y. 1994. Coumarin glycosides from Peucedanum japonicum. Phytochemis-try 35: 1339-1341.
57. Lee, G., Park, H. G., Choi, M. L., Kim, Y. H., Park, Y. B., Song, K. S., Cheong, C. and Bae, Y. S. 2000. Falcarindiol, a polyacetylenic compound isolated from
Peucedanum japonicum, inhibits mammalian DNA
topoisomerase I. J. Microbiol. Biotechn. 10: 394-398. 58. Hisamoto, M., Kikuzaki, H., Ohigashi, H. and
Nakatani, N. 2003. Antioxidant compounds from the leaves of Peucedanum japonicum Thunb. J. Agric. Food Chem. 51: 5255-5261.
59. Hisamoto, M., Kikuzaki, H. and Nakatani, N. 2004. Constituents of the leaves of Peucedanum japonicum Thunb. and their biological activity. J. Agric. Food Chem. 52: 445-450.
60. Lee, S. O., Choi, S. Z., Lee, J. H., Chung, S. H., Park, S. H., Kang, H. C., Yang, E. Y., Cho, H. J. and Lee, K. R. 2004. Antidiabetic coumarin and cyclitol compounds from Peucedanum japonicum. Arch. Pharm. Res. 27: 1207-1210.
61. Teng, C. M., Kuo, S. C., Ko, F. N., Lee, J. C., Lee, L. G., Chen, S. C. and Huang, T. F. 1989. Antiplatelet actions of panaxynol and ginsenosides isolated from ginseng. Biochim. Biophys. Acta 990: 315-320.
62. Yoko, A., Toshio, K., Naoki, T. and Seisho, T. 1995. The antagonistic effects of khellactones on platelet-activating factor, histamine, and leukotriene D4. Chem. Pharm. Bull. 43: 859-867.