行政院國家科學委員會專題研究計畫成果報告
氧化還原蛋白 CXXC 主題結構中的第一個 X 如何調控氧化還原性
質?
計畫編號:NSC 89-2311-B-009-008
執行期限: 90 年 8 月 1 日至 91 年 7 月 31 日
主持人:林苕吟 國立交通大學生科所
一、中文摘要 屬於 Thioredoxin 家族的氧化還原酵素 之活性區含一帶雙胱氨酸(bis(cysteinyl))的 一致性(consensus)序列 Cys-X-X-Cys。這些 結構相似之蛋白質的氧化還原電位卻有極 度差別。為了解氧化還原蛋白主題結構 CXXC 中之第一個 X 的重要性,我們將 E. coli thioredoxin 活性區 Gly 突變為 Lys。令 我們驚訝的是此一突變並不影響到酵素的 氧化還原電位。在氧化還原性質方面,其 硫醇的有效濃度(Ceff)與野生種皆約為 91 M,突變株與野生株間的直接平衡常數比 (K12)為 1.1。然而在 E. coli thioredoxin reductase 所催化的氧化還原系統中,此突 變株 thioredoxin 比野生蛋白的 kcat/Km下降 2.8 倍。其主因在於 Km的上升。在對 insulin 的還原反應上,突變株野生株降低約兩 倍。突變株對噬菌體 T3/7 的平盤效率 (E.O.P.)於 37 oC 降低了五倍,而於 42 oC 下降了 106倍,顯示噬菌體基因 V 蛋白和 thioredoxin 間的交互作用受到突變的阻 撓。圓形(CD)光譜及螢光研究結果顯示活 性區 GlyLys 的改變並不造成 thioredoxin 結構的變化。因此第一個 X 加上正電荷並 不改變蛋白質的氧化還原電位或結構,其 主要的效應在於影響了 thioredoxin 與其他 蛋白質間的交互作用。 關鍵詞:Thioredoxin、突變、Thioredoxin reductase、氧化還原 Abstract Oxidoreductases of thioredoxin superfamily possess the C-X-X-C motif. The redox potentials vary over a wide range for these structurally similar proteins. This has been attributed to the variation of the dipeptide sequence X-X. In this paper, we substitute Lys for Gly at the first X position of the primodial protein of this family, E. coli thioredoxin, to investigate how a positive charge would affect the redox potential. The substitution does not affect the redox potential of the protein; Ceff is 91 M for the mutant protein, which does not change from that of the wild-type protein. Equilibrium constant obtained from pair-wise reaction between the mutant and the wild-typeproteins shows a value of 1.1, indicating that the replacement does not significantly affect the thiol-disulfide redox equilibrium. However, the catalytic efficiency of thioredoxin reductase on G33K mutant decreases approximately 2.8 times compared to the wild-type protein. The mutation mainly affects Km with little effects on kcat. The mutant protein catalyzes disulfide reduction of insulin in the presence of NADPH and thioredoxin reductase with
approximately two-fold lower activity than the wild-type. The mutant gene was also tested whether it supports the growth of bacteriophage T3/7. Efficiency of plating of phage T3/7 on the mutant strain decreases 5 times at 37 oC, and reduced 106 orders at 42 o
C relative to the wild-type strain, suggesting interaction between phage gene V protein and thioredoxin is hindered. The global structure of the mutant protein does not show significant change as studied by CD and fluorescence spectra. Therefore, the positive charge at the first X does not affect the overall structure or redox potential of thioredoxin, but interferes with its interaction with other proteins.
Keywords: Thioredoxin, Mutation,
Thioredoxin reductase, Oxidoreduction
二、緣由與目的
Thioredoxin contains two redox-active half-cystines in its active site. The function of the protein includes reduction of
methionine sulfoxide and sulfate (1), donation of hydrogen to ribonucleotide reductase (2), regulation of photosynthetic enzymes such as spinach chloroplast fructose bisphosphatase and NADP-dependent malate dehydrogenase (3, 4). Thioredoxin is reduced by NADPH in a reaction catalyzed by thioredoxin reductase as follows (5), Trx-S2 + NADPH + H+ Trx-(SH)2 + NADP+ (1) Where Trx-S2 and Trx-(SH)2 refer to the oxidized and reduced thioredoxin, respectively.
The active site region is highly conserved with an amino acid sequence of Cys-Gly-Pro-Cys from bacteria to human,. The dipeptide sequence encompassed by two half-cystines is thought to be important in modulating the redox properties of the protein. Other thiol-disulfide
oxidoreductase of thioredoxin enzyme family also possesses Cys-X-X-Cys motif (6). The redox potentials of these enzymes vary in a large range. Our previous studies (7) however showed that thioredoxin with an Asp at the first X does not show profound effects on the redox potential of the protein. The replacement nevertheless reduces
catalytic efficiency of thioredoxin reductase by approximately 10 folds. Therefore, amino acid at position 33 (follows E. coli sequence) of thioredoxin appears to be important for the reactions catalyzed by thioredoxin reductase. In this report, we generate a Gly to Lys substitution at the first position to investigate the significance of the first X on the redox potential as well as other reactions involving this protein.
三、結果
Site Directed Mutagenesis for G33V Thioredoxin and Purification of Protein
Sequential PCR method was used to obtain G33K mutation. A plasmid, pET/trx, carries the wild-type thioredoxin gene was used as template. In the first PCR reaction for G33K mutation, the 5‘ primer was 5‘-TGGTGCAAGCCGTGCAAAATGATC-3’ (Underline is the position of nucleotide substitution.), and the 3’ primer was
5‘-TGATGGTGCATAAGGCCTGAACCA GATCAG-3’, which contains a StuI site. The nucleotide fragment obtained and the T7 primer
5’-TAATACGACTCACTATAGGG-3’ were used as 3‘ and 5’ primers for the second PCR reaction. The product of the second
reaction was ligated to pGEM-T Easy vector. After transforming E. coli DH5, white colonies were selected from X-gal and ampicillin plates. The size of the plasmid was checked, and the mutation the gene was confirmed by DNA sequencing. The plasmid was scissored with XbaI and EcoRI, and the mutant gene was ligated between the same sites of pET to yield pET/G33K. The mutant protein was purified by DEAE and G50 chromatography. Electrophoresis of the protein on SDS-polyacrylamide gel showed a single band.
Measurements of Ceff
Ceff of the wild-type and the G33K mutant protein were measured using
glutathione as the reference. The measured Ceff of wild-type protein was 9 M for G33K mutant protein (Table 1), which is the same as the wild-type protein.
Direct Equilibrium Ratio
Direct redox equilibrium ratio (K12) between G33K thioredoxin and the wild-type thioredoxin was measured. A value of 1.1 was obtained (Table 1). This demonstrates that G33K mutation does not affect the redox equilibrium ratio. The redox potential is not changed by the positive charge at the first X position.
Oxidoreduction Catalyzed by E. coli Thioredoxin Reductase
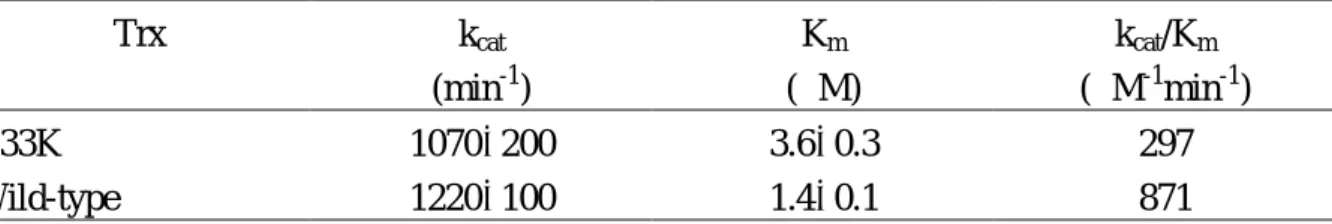
E. coli thioredoxin reductase catalyzes the electron transfer between NADPH and thioredoxin. Kinetic parameters of this reaction when using wild-type and G33K mutant proteins as electron acceptors were studied. G33K substitution does not significantly affect kcat, whereas it increases Km by 2.5 folds. Therefore, the catalytic efficiency decreases approximately 2.8 folds compared to the wild-type protein (Table 2).
Reduction of Insulin Disulfide
Reduction of insulin disulfide by thioredoxin was carried out in the presence of NADPH and thioredoxin reductase. The initial rate of reduction and the amount of insulin disulfide reduced in 30 minutes were measured. Table 3 shows that the initial rate (vi) of insulin reduction by the mutant was slower than the wild-type. Furthermore, the total amount of disulfide reduced by the wild-type protein within 30 minutes was about twice that of that by the mutant protein.
Efficiency of plating by T3/7 Phage Growth of the T3/7 phage on the wild-type and the G33K mutant strains were compared. The results (Table 4)
demonstrate that E.O.P. of T3/7 on the mutant strain decreases 5 times at 37 oC. E.O.P. of the mutant reduced by 106 orders at 42 oC relative to the wild-type strain. It suggests that the interaction between phage gene V protein and thioredoxin is hampered by the mutation.
Structural Studies
The structures of the oxidized and the reduced proteins of both the wild-type and the mutant proteins were studied by CD and fluorescence spectroscopies. The results indicate that the mutation does not alter the global structures of the protein in either the reduced or the oxidized state.
四、討論
Thioredoxin is an electron transport protein that takes part in many cellular oxidation-reduction reactions. The C-X-X-C motif in its active site is essential in carrying out the redox functions. The central residues in the motif are considered to be important in modulating the redox
potential. In this study, we performed a GlyLys substitution at the first X position of E. coli thioredoxin. Ceff measurements demonstrate that the mutant protein has the same value as the wild-type protein. The redox equilibrium between the G33K mutant and the wild-type protein shows a value of 1.1, in consistent with the Ceff determination. It again shows that G33K dose not
significantly affects the redox potential of the protein. A charge group near the active site could change the pKa of cysteines, and affects the redox potential of the protein. Our results demonstrate that the positive charge of lysine does not interfere with the
thiol-disulfide equiibrium, suggesting that the side chain of lysine is positioned away from the side of the thiol groups. This point is corroborated by the results of the interaction between the mutant protein and a variety of proteins as discussed below.
The catalytic efficiency of thioredoxin reductase catalyzed reduction of thioredoxin by NADPH is decreased for the G33K mutant. The substitution also alters the efficiency of the protein as an electron donor for insulin disulfides. Such decrease in the activity of thioredoxin cannot be attributed to a significant change of its redox potential as a result of the substitution in the active site. G33K mutant thioredoxin is not an efficient substrate for E. coli thioredoxin reductase compared to the wild-type protein. Its kcat
value is 1070 min-1, which is close to the measured wild-type value of 1220 min-1. The Km of the wild-type thioredoxin is 1.4
M. However, the Km of the G33K mutant
protein increases to 3.6 M. The reduced efficiency of thioredoxin reductase in
catalyzing reduction of the mutant
thioredoxin arises mainly from increase of Km value without altering kcat, suggesting the
side chain of lysine points to the interaction surface between thioredoxin reductase and thioredoxin.
In our previous report, the substitution of Asp for Gly at 33 position of thioredoxin
decreases the catalytic efficiency by approximately 9 folds, which can be
attributed to an increase of Km.
Comparison of the catalytic efficiency of thioredoxin reductase on G33K and G33D mutant thioredoxin demonstrates that a negative charge at 33 position is ~3 times more unfavorable than a positive charge at the same position. Computer modeling
according to the X-ray structure of
thioredoin-thioredoxin reductase complex shows that the environment around Asp33 is more acidic with Thr 44 side chain OH and K39 main chain carbonyl group at a distance of 3.95-4.27 Å to the side chain of Asp33. When lysine is positioned at 33, the amino side chain is at a distance of 4.08 Å to carbonyl group of Thr44, and 4.42 Å and 4.08 Å to carbonyl and amino groups of
Lys39, respectively. The acidic
surroundings of the Asp33 could account for the relatively low catalytic efficiency of thioredoxin reductase compared to Lys33.
An arginine has been inserted in the active site of thioredoxin to generate a sequence of -Cys-Gly-Arg-Pro-Cys-. This insertion supresses the ability of thioredoxin to serve as a substrate of thioredoxin reductase. The catalytic efficiency is reduced to 5 M-1 min-1, which is 137 times lower than that of the wild-type protein. The severity of this mutant relative to the G33K suggests that besides the effects of the
positive charged side chain, a large proportion of its decrease in kcat/Km coming from the expansion of the disulfide ring size. The severity of this mutant relative to the G33K suggests that expansion of the disulfide ring size could cause drastic reduction in the catalytic efficiency of the thioredoxin reductase. The redox potential has not been measured in this case. The redox potential and the structure of the disulfide are likely to be altered in this mutant protein, both of which can contribute to the loss of catalytic ability.
Assay of the reduction of insulin by thioredoxin in the presence of NADPH and large amount of thioredoxin reductase was carried out at 25 oC. The initial rate and the total amount of insulin disulfide reduced were decreased for the G33K mutant protein compared to the wild-type protein. As the redox potential of the mutant protein does not change significantly, the reduction is attributed to the inefficient interaction of the thioredoxin with the insulin disulfide when Lys is substituted for Gly.
Substitution of Lys for Gly at 33 also reduces the plating efficiency of T3/7, suggesting that the efficiency of DNA polymerase is decreases. This effect is
irrelevant to the redox potential of
thioredoxin as has been shown that the enzyme activity does not evoke the redox
crystallography of the bacteriophage T7 phage DNA replication complex shows that the active site of thioredoxin participates in the interaction with the protein encoded by gene 5 of the phage. Modeling on the basis of this structure suggests that the amino side group of Lys33 could make contacts with the main chain amino and carbonyl groups of Phe 274. However, it also clashes with side chains of Phe274 and Tyr 286. This accounts for the observed reduction in E.O.P. value of T3/7.
CD and fluorescence spectra indicate that the structure of either oxidized or reduced thioredoxin is not altered by the G33K substitution. The tryptophan residues (residue 28 and 31) are in close proximity to the active site. No significant change of the fluorescence spectra between the wild-type and the G33K mutant illustrates the conservation of the tertiary structure in the active site region. The relatively mild effects of G33K on the thioredoxin reductase catalyzed reaction also supports that the replacement does not alter the global structure of the protein. Therefore, the replacement of Gly33 with Lys primarily affects the kinetics of the cellular reactions that thioredoxin participates in, but not the redox potential and structure of the protein.
The Cys-X-X-Cys motif in the active site of thioredoxin has been found in other thiol/disulfide oxidoreductases. For
instance, DsbA from the periplasm of E. coli posseses a catalytic disulfide of sequence Cys-Pro-His-Cys. A recent paper by Huber-Wunderlich and Glockshuber (6) showed that when the dipeptide sequence PH in the wild-type DsbA was changed to those of other oxidoreductases, the redox potentials of the variants decreased and shifted
according to those of the natural enzymes. We have shown that substitution of Asp for Gly33 has quite mild effects on the redox potential. In the present study, the Gly33 to Lys replacement also shows little alteration in the redox potential. Therefore,
introducing a negative or positive charge at the first X of the dipeptide does not cause large variation of the redox potential. The main effects of these charged groups are on the interaction of thioredoxin with the other proteins, and not on the redox potential.
五、參考文獻
1. Black, S., Harte, E. M., Hudson, B., and Wartofsky, L. (1960) J. Biol. Chem. 235, 2910-2916.
2. Laurent, T. C., Moore, E. C., and Reichard, P. (1964) J. Biol. Chem. 239, 3436-3444.
3. Clancey, C. I., and Gilbert, H. F. (1987) J. Biol. Chem. 262, 13545-13549. 4. Scheibe, R., Fickenscher, K., and Ashton, A. R. (1986) Biochim. Biophys. Acta 870, 191-197.
5. Williams, C. H., Jr. (1976) Enzymes 13, 89-173.
6. Huber-Wunderlich, M., and Glockshuber, R. (1998) Fold Des. 3, 161-171.
7. Lin, T.-Y. (1999) Biochemistry 38, 15508-15513.
Table 1. Redox Properties of G33K and Wild-type Thioredoxin Trx Ceff (M) Direct equilibrium ratio between G33V and wild-type (K12) ΔEo’ (mV) G33K 91 1.10.1 -1.2 Wild-type 91
Table 2. Kinetic Parameters of the Thioredoxin Reductase Reaction with Thioredoxin at pH 7, 25 oC Trx kcat (min-1) Km (M) kcat/Km (M-1 min-1) G33K 1070200 3.60.3 297 Wild-type 1220100 1.40.1 871
Table 3. Reduction of insulin by thioredoxin Vi(M/min) Disulfide
reduced in 30 min (M)
Wild-type 8 12817
G33K 4 7010
Table 4. Growth of T3/7 phage on the wild-type and the mutant E. Coli
E.O.P.
37 oC 42 oC
Wild-type 1 1