行政院國家科學委員會補助專題研究計畫成果報告
※※※※※※※※※※※※※※※※※※※※※※※※※※
※
心臟血管疾病之蛋白質基因組研究實驗室:
※
※
擴張性心肌病變之基礎研究
※
※
Pr oteomics Labor ator y for Car diovascular Diseases :
※
※ The Studies of Dialated Car diomyopathy via Pr oteomics Appr oach ※
※※※※※※※※※※※※※※※※※※※※※※※※※
計畫類別:個別型計畫
計畫編號:NSC 89-2314-B-002-285-
執行期間:89 年 8 月 1 日至 90 年 7 月 31 日
計畫主持人: 許榮彬
共同主持人: 朱樹勳
執行單位: 台大醫學院附設醫院外科部
中
華
民
國
90 年
10 月
31 日
行政院國家科學委員會專題研究計畫成果報告
計畫編號:NSC 89-2314-B002-285 心臟血管疾病之蛋白質基因組研究實驗室:擴張性心肌病變之基礎研究 執行期限:89 年 8 月 1 日至 90 年 7 月 31 日 主持人:許榮彬 單位:台大醫院心臟外科 協同主持人: 朱樹勳 單位 : 台大醫院外科 壹、中文摘要 我們嘗試用蛋白基因體分析的方式,來研究複雜的心臟血管疾病的病因。我們以 擴張性心肌病變(dilated car diomyopathy; DCM) 作研究目標,以鑑別出與 DCM 相關的蛋 白質與蛋白質修飾基。我們使用差別性萃取法(Differ ential extr action)純化心肌纖維蛋白 後,使用二維膠質電泳(Two-dimensional gel electr ophor esis)分離這些蛋白質,再用質譜儀 作心肌纖維蛋白質的鑑定與修飾基的研究。舉 mlc-2 蛋白為例,我們發現此心肌蛋白之 Ser -14 有磷酸化發生;而另一心肌蛋白 ATP synthase subunit,則無修飾基產生。我 們將這些資料加以整理,以比較組織間修飾基種類差異,並且找出DCM 病人所特有之蛋白質修飾基。
關鍵詞:擴張性心肌病變、蛋白質基因組分析、質譜儀、二維膠質電泳
ABSTRACT
We employed proteomics methods to analyze myocardial proteins derived from DCM myocardium, particularly myofibril proteins. Dilated cardiomyopathy (DCM) is a severe heart disease leading to heart insufficiency and many patients require heart transplantation to correct the disease. We successfully isolated myofibril proteins using differential extraction method and then resolved them on a 2D gel electrophoresis system. The identities of proteins are verified using liquid chromatography-tandem mass spectrometry and specific modifications on these proteins can also be identified. Using myosin regulatory light chain 2 (mlc-2) as an example, we demonstrated that how modification is mapped using selected ion tracing approach. We also employed the similar approach to study proteins involved in energy metabolism. As an example, the ATP synthase subunit was identified on the 2D gel and analyzed for its post-translational modifications. Surprisingly, no modification was found beside the methionine oxidation. We have been investigating the post-translational
modification of other cardiac proteins. These data will be complied to identify myocardium-specific modifications as well as to find markers for DCM hearts.
Key word: dilated cardiomyopathy, proteomics, tandem mass spectrometry, two-dimensional gel electrophoresis
貳、源由與目的
Cardiovascular diseases, like dilated cardiomyopathy (DCM), are usually very complex and the elucidation of pathogenic mechanisms is very difficult. Dilated cardiomyopathy (DCM) is a severe heart disease leading to heart insufficiency and most heart transplantations are indicated by DCM. We proposed to employ proteomics methods to analyze cytoskeletal proteins derived from DCM myocardium, particularly the myofibril proteins. We aimed to identify the proteins that are differentially expressed in and to identify the protein
modifications that are increased or decreased in DCM myocardium.
As the major acting macromolecules in cells, the proteome will be eventually altered by almost all, if not all, of the pathological processes. Elucidation of these alterations will greatly enhance our ability to understand how cellular functions are impaired and how these impairments can be fixed to restore cellular function. The study of the proteomic change can also help us to recognize what process causes the cellular changes and how a pathologic process is evolved.
The major advantage of proteomics over genomics is in the area of post-translational modifications. It is now clear that the activity of a protein often depends on its modification state. While the expression of a gene may be the same at different situations, if its
modification status is different, the activity of a protein can be thus tuned on or off. Therefore, the study of protein modifications through a proteomics approach is a crucial and integral part to progress our understanding of living organisms.
參、結果與討論
Myofibril proteins are the major components of myocardial cytoskeleton. In order to explore their change in dilated cardiomyopathy, we employed a proteomics approach to monitor their expression as well as post-translational modifications.
First, we isolated the myofibril proteins by first extracting the non-myofibril proteins using high salt solutions. The insoluble portion is then solubilized and homogenized in the lysis buffer containing 0.1% of sodium dodecyl sulfate (SDS). Myofibril proteins in this
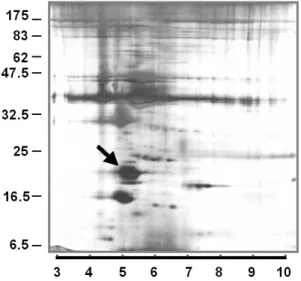
Figur e 1. Two-dimensional electr ophor esis of myofibr il pr oteins. Two hundred fifty micrograms of
purified myofibril proteins were loaded to IPG IEF 3-10 gel, and were resolved overnight for 19,000 V· hr. The gel was placed at the tope of a 11% polyacrylamide gel, which was run at 150 V until the dye front reached the bottom of the gel. The gel was developed using silver stain method. The arrow indicates the position of regulatory myosin light chain 2.
fraction were extracted using a methanol-chloroform method such that the detergents and salts were mostly removed. These myofibril proteins were then analyzed using two-dimensional electrophoresis, consisting of isoelectric focusing and SDS polyacrylamide gel electrophoresis (SDS-PAGE).
Figure 1 shows a typical pattern of myofibril proteins in a 2D gel electrophoresis system. The identities of these proteins were verified using liquid chromatography-tandem mass
spectrometry. The myofibril protein pattern was compared between donor and recipient hearts, but showing no distinct difference in terms of protein species and their relative abundance. In order to access whether the post-translational modifications were altered in DCM hearts, we employed a newly developed method to gauge the modification states.
It has been reported that phosphorylation was incorporated at Ser-14 of cardiac myosin regulatory light chain 2 (mlc-2), therefore we tested our method on this particular protein. On the 2D gel, this protein has an isoelectric point of ~5.2 and an apparent molecular weight of 20 k. These are comparable to the corresponding parameters deduced from its amino acid
sequence, which are 4.92 for the isoelectric point and 18,640 for the molecular mass. The gel spots containing the mlc-2 polypeptides were excised and subjected to in-gel trypsin digestion. The digestion product was then analyzed via liquid chromatography-tandem mass
spectrometry.
Figure 2 shows the identification of peptides derived from mlc-2 proteins. More than 90% of the amino acid sequence can be recovered, showing the good throughput of our method. Mostly importantly, all the phosphorylatable amino acids can be verified. Selected ion tracing method was used to identify potential phosphopeptides, which revealed only a phosphorylated peptide. Tandem mass spectrometry was then used to map the
phosphorylation site.
Figur e 2. The amino acid sequence of myosin r egulator y light chain 2. The recovered peptide
sequences were underlined with horizontal bars. The position of Ser-14 was highlighted. The coverage of the entire polypeptide is about 94% (155 out of 165 amino acids).
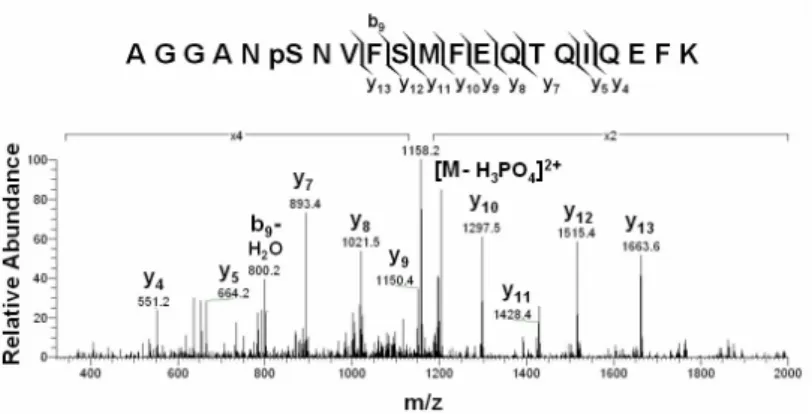
Figur e 3. Tandem mass spectr um of Ser-14 phosphopeptide. The Ser-14 phosphopeptide was
subjected to collision-induced dissociation experiment and the resulted fragment ions were resolved by a LCQ ion trap mass spectrometer.
In the tandem mass spectrum of this phosphopeptide, a predominant fragment ion of m/z
1158.2 was seen, which represented the parent ion with a loss of a charge-less phosphoric acid molecule. This signature feature indicates that this peptide is indeed a phosphopeptide. As y4
to y13 ions have no mass shift in the spectrum, this excludes the possibility that the phosphate
group is on either Ser-18 or Thr-23. Since Ser-14 is the only phosphorylatable residues
besides these two residues, this result strongly indicates that Ser-14 is the phosphorylation site. We also studied the modifications on proteins involved in energy metabolism. We first studied ATP synthase subunit, a key enzyme for ATP generation. As Fig. 4 indicated, about 67% of amino acid residues can be recovered using our method. All of these peptides were subjected to SIT analysis. However, no modification could be verified except methionine oxidation.
肆、計畫成果自評
In this report, we showed that myofibril proteins could be isolated and resolved using a 2D gel system. Liquid chromatography-tandem mass spectrometry is an appropriate method to identify the proteins in the gel. If we are interested in the modification state of a particular DCM-associated protein, it can be determined using this newly developed LC/MS method. Currently, we have no definite conclusion on what modification is related to DCM. This will await the collect samples from more patients.
Nevertheless, we will use this approach to do more detailed analysis on modification states of cardiac myofibrils as well as other important myocardial proteins at different disease conditions. Most of cardiovascular diseases are very complex and this might be one of the better strategies to solve this kind of biomedical problems. However, we also understand that it is more suitable for experiments using a larger sample number. Currently, several
cardiovascular diseases are being evaluated. We are also considering the animal models to do more precise experiments.
Figur e 4. Protein coverage of ATP synthase β subunit. The letters in dar k indicate the residues discovered by LC-MS method. The bar below indicated the regions that these residues reside.