NSC 88-2314-B-002-331
以心臟超音波組織特性預測急性心肌梗塞後冠狀動脈阻塞或再灌流
之研究
The Study of Ultrasonic Tissue Characterization for Evaluating Coronary
Stenosis or Reperfusion after Acute Myocardial Infarction.
個人基本資料: 姓名 高憲立 KAO HSIEN-LI 聯絡地址 台北市中山南路7號 台大醫院內科 聯絡電話 (公)(02)23970800-5874 傳真號碼 (02)23959933 E-MAIL cwu@ha.mc.ntu.edu.tw 服務機關 國立台灣大學醫學院內科
中文摘要: 關鍵詞:超音波組織特性分析、催迫式心臟超音波、心肌梗塞、冠狀動脈心臟病、再灌流治療。 評估急性心肌梗塞病患之預後時,多條血管之冠狀動脈病變是一重要因素。直至目前,冠 狀動脈攝影仍被視為標準檢查方式。然而,許多非侵襲性檢查,包括運動心電圖、鉈 201 心肌 灌流掃瞄及催迫式心臟超音波已逐步發展並應用於多血管冠狀動脈心臟病之預測。但以實用觀 點而言,仍有許多臨床上的限制。首先,對於心肌梗塞病患,這些催迫式檢查在急性期仍有相 當程度的危險性。其次,對於仍需留觀於加護病房之患者,運動心電圖及鉈 201 心肌灌流掃瞄 無法適用。第三,催迫式心臟超音波檢查常於某血管灌流範圍之心肌收縮異常時即停止下一階 段之檢查,因此,往往只能偵測到具有最嚴重缺氧程度之單一冠狀動脈,無法同時對三條冠狀 動脈進行評估。超音波組織特性分析乃利用心肌組織對於超音波音束之散射作用,將所有逆散 射之訊號予以總和,視為反射之能量顯示。於正常心肌組織,此反射能量之大小會隨心臟收縮 與舒張,而有週期性之變化,於收縮時達到最小,而於舒張時達到最大。但於心肌缺氧或梗塞 時。此週期變化之大小會減少,谷底值出現之時期亦會延後。然而,若即時予以再灌流治療, 此變化將比對心肌收縮程度之影響更早恢復。 本計畫乃利用超音波組織特性分析(UTC)及催迫性超音波心圖檢查(DSE),預測急性心肌梗 塞(AMI)病患是否併有多條血管之冠狀動脈病變,並且針對再灌流治療於梗塞血管之成效予以 評估。共有三十位病人於 AMI 後之 8.3±3 天內接受 UTC 及 DSE 檢查。在 UTC 檢查中, phase-weighted variation 可 分別出 一塊心 肌組織 是否還 存有冠 狀動脈 灌 流 不 足或已 結 疤 (p<0.001) 。在存活而不具缺氧狀況的心肌組織其數值則與正常心肌組織類似(p=0.453)。利用 5.8 作分界,用 UTC 之 phase-weighted variation 對存活但仍殘存心肌缺氧組織之判定,其診斷 性之靈敏度、特異性及精確度分別是 75%,100%及 90.2%。而當此心肌接受再灌流治癒後其 phase-weighted variation 數值極小,且與冠狀動脈血流是否暢通無關。
因此,吾人發現 UTC 對偵測不具狹窄冠狀動脈的梗塞心肌非常有效,它可用以篩檢急性心 肌梗塞後患者是否須接受導管或冠狀動脈穿通術之依據。
Abstr act:
Background The identification of viable myocardium and residual ischemia in
patients with acute myocardial infarction (AMI) has important prognostic implications. The ultrasonic tissue characterization (UTC) with integrated backscatter has been shown to be a sensitive marker of myocardial ischemia and infarction. After reperfusion of occluded coronary artery, the cardiac cyclic-dependent variation of integrated backscatter restores its amplitude modulation before the recovery of myocardial contractility. It implies that the promising method can be used to identify stunning myocardium.
Methods and Results UTC and dobutamine-atropine stress echocardiography (DSE)
were performed 8.3±3 days after AMI in 30 patients. The DSE was considered as a reference to identify viable myocardium and residual ischemia. After coronary intervention, both modalities were performed to evaluate the influences of residual stenosis. The parameter obtained from UTC, phase-weighted variation, could differentiate the myocardium with residual coronary stenosis or nonviable from the viable myocardium without residual coronary stenosis (p<0.001). The latter has the similar value to that of normal control group (p=0.453). Using the cutoff value of 5.8, the sensitivity, specificity, and accuracy for detecting viable myocardium without residual infarct-related artery stenosis were 75%, 100%, and 90.2%, respectively. The phase-weighted variation of viable infarction zone restored after the coronary stenosis was relieved. Contrarily, the nonviable myocardium had small phase-weighted variation irrelevant to the patency of infarct-related artery.
Conclusions The UTC was a very useful method to identify the viable myocardium
of which infarct-related artery was free from residual stenosis. It can be used as a predischarge screening tool for risk stratification and is helpful in constructing the intervention strategy.
Key Words • myocardial infarction • echocardiography • ischemia • stunning,
Background
In patients with acute myocardial infarction (AMI), early thrombolytic therapy can salvage a significant amount of myocardium as well as improve left ventricular function and prognosis.1-5 However, residual stenosis of the infarct-related artery is common after AMI 6-8 that may further jeopardize viable myocardium in the area at risk. 9-15 The elective revascularization of stenotic coronary arteries with viable myocardium after AMI improved the ventricular function. 15,16 Risk stratification for patients with AMI was based not only on left ventricular function but also on residual ischemia. Dobutamine-atropine stress echocardiography (DSE) can predict the recovery of ventricular function by low-dose testing 13,17-19 as well as detecting the residual coronary artery stenosis during high-dose infusion. 14,20 It may provide an objective evidence in making strategy for further intervention. 20-22 Ultrasonic tissue characterization (UTC) with integrated backscatter has been shown to be valuable in detecting myocardial infarction and ischemia. 23-25 In patients with AMI, the restoration of cyclic variation of integrated backscatter develops before the recovery of wall motion abnormalities and indicates the potentially salutary effects of coronary artery revascularization. 26
The aims of the present study were to (1) document the reservation of cardiac cyclic-dependent variation of integrated backscatter in the infarct zone that is viable without residual ischemia, (2) show the restoration of cardiac cyclic-dependent variation of integrated backscatter in viable myocardium after relieving residual coronary artery stenosis, (3) verify the accuracy of UTC in identifying the viable myocardium that is free from residual ischemia.
Methods
Patient Selection
Thirty consecutive patients fulfilling the following criteria were enrolled in the study: (1) first episode of AMI diagnosed on the basis of ischemic chest pain lasting more than 30 minutes, characteristic electrocardiographic changes and an increase in serum creatine phosphokinase (CK) and CK-MB myocardial enzymes of at least twice above the normal value; (2) a satisfactory precordial echocardiographic window; (3) no contraindication to dobutamine testing, such as unstable angina, major arrhythmia, or severe hypertension. No patients had any of the other criteria of exclusion: atrial fibrillation, multiple ventricular premature contractions, and cardiomyopathy. Fourteen patients received recombinant tissue-type plasminogen activator as the reperfusion therapy. Primary percutaneous transluminal coronary angioplasty was
performed in the other 5 patients, including one patient with primary stenting. When the UTC and DSE were performed, no patient was given any inotropic agent or experienced postinfarction angina. At the discretion of the attending physician, -blockers were administered.
Backscatter Data Acquisition and Analysis
The backscatter data was collected by 2 observers within 48 hours before the coronary angiography. UTC examinations were performed with a modified, commercially available, real-time, 2-dimensional imaging system (Acoustic Densitometry in Integrated Backscatter mode, SONOS 2500, Hewlett Packard, Andover, Massachusetts) capable of providing images in which gray levels were displayed proportional to the integrated backscatter amplitude obtained. A 64-element ultrasound transducer operating at a center frequency of 2.5 MHz was used in this study. The imaging frame rate was automatically set to 30 Hz and the dynamic range of the integrated backscatter signal was approximately 44 decibels (dB). In spite of the substantial anisotropy in the parasternal short-axis view, which can be compensated for with lateral gain compensation,26 we used this view at the papillary muscle level to obtain the two-dimensional image of integrated backscatter for evaluating all the three vessel territories simultaneously. The transmit power, time gain compensation27 and lateral gain compensation26 were adjusted to optimize image presentation and remained constant throughout the study for individual patient. Sixty frames were displayed and captured in digital format for each examination period. The integrated backscatter was quantified by placing a region of interest in the myocardium on the frozen image. The region of interest was configured with a different shape, size and orientation to fit within the boundaries of the myocardial wall at end-diastole. For all patients, the 21 × 21 pixel region of interest was used. The location of the region of interest was adjusted frame by frame to keep it well within the subendocardial myocardium 28,29 and to avoid specular endocardial and epicardial echoes throughout the cardiac cycle. For each patient, three regions of interest were chosen in the same parasternal short axis image. One was at the mid-anteroseptal area to represent the myocardium perfused by left anterior descending coronary artery; another was at the junction of mid-posterior with mid-lateral area to stand for the myocardium perfused by left circumflex artery; and the other was at the mid-inferior area for the myocardium perfused by right coronary artery. The serial changes in the magnitude of integrated backscatter (in dBs) within the region of interest were then obtained from each frame and displayed as a curve of integrated backscatter amplitude versus time with electrocardiographic R wave as a reference. The images and data sets were stored in the optic disc for off-line analysis. For most patients, the curve included more than 2 consecutive beats. The amplitude of the integrated backscatter was obtained by
averaging the values corresponding to the same time point in each cardiac cycle. Finally, the integrated backscatter power curve for a full cardiac cycle was plotted as amplitude versus time of 33-millisecond interval. The presence of interpatient acoustic variability (such as miscellaneous attenuation across the chest wall on transthoracic approach) and different gain setting to optimize the image made the standardization of integrated backscatter power difficult. By a dog study, however, Natio et al. 30 disclosed that there was no significant difference in the amplitude of cyclic variation of integrated backscatter between the open chest and closed chest setting. We wrote a program with the MATLAB (the Math Works, Inc.) and the first harmonic Fourier fitting of the power curve was obtained promptly.23,31
Two basic measurements were derived from the first harmonic Fourier fitting curve: the amplitude and phase. The amplitude and phase are separate, independent measures of the integrated backscatter power curve that relate to different aspects of myocardial dynamics.25 The results of UTC can be represented as a vector quantity, with a magnitude (amplitude of cyclic variation) and an orientation (phase).32 The procedure for phase weighting constitutes a vectorial approach to the determination of changes in dynamic backscatter variables. We designated the variation, by doubling the amplitude, to represent the difference between maximal and minimal values of integrated backscatter. By using the electrocardiographic R wave as the trigger reference, the phase-weight variation was derived from the variation and phase.23,32 It can be considered as an index of contractile dysfunction and appears to reflect accurately the severity of ischemic injury.32 The following criteria were used to decide the phase weighting factor:25
Factor= -1 if (0° ó phaseó 45°)
-cos[2(phase-45°)] if (45° ó phaseó 135°)
1 if (135° ó phaseó 225°)
cos[2(phase-45°)] if (225° ó phaseó 315°)
-1 if (315° ó phaseó 360°)
The criteria used in weighting variations were such that the variation with a phase value of approximately 180° (135° to 225°) was normal and had a weight factor of 1. On the contrary, variations with a phase value progressively smaller than 135° or > 225° were reduced by a factor gradually toward -1. The phase-weighted variation represent the alteration in variation and phase simultaneously and was considered as the parameter of UTC in our study. It took about half an hour to acquire the UTC, analyze the backscatter data and arrive at the final parameter for each study.
Dobutamine str ess echocar diogr aphy
Just after performing UTC, DSE was performed by another 2 observers with the same equipment and an image storage system (P90, Tomtec). Continuous 12-lead
electrocardiographic recording was carried out throughout the test and blood pressure was measured every 3 minutes. The dobutamine was administered intravenously by an infusion pump at the initial dosage of 5, 10, and 20 g/Kg/min for 3 minutes each as
low-dose testing.33 The dosage was then increased by 10g/Kg/min every 3 minutes up to a maximal dose of 40g/Kg/min. If there was no significant side effect and
the heart rate response was inadequate, 0.5 mg atropine was given twice with 1-minute interval34,35 to achieve peak heart rates of > 120 bpm.36 The test was prematurely terminated if severe angina, > 2 mm ST-segment depression or elevation, marked wall motion deterioration, major ventricular arrhythmia, systemic hypertension (blood pressure > 220/120 mmHg), systolic hypotension (defined as a drop in systolic hypotension > 20 mmHg compared to the previous step), or obvious adverse effects appeared.
Four standard views of the left ventricle (parasternal long- and short-axis, apical 4-and 2-chamber views) were recorded at baseline 4-and during dobutamine infusion. Wall motion was graded as: 1 = normal, 2 = hypokinetic, 3 = akinetic, and 4 = dyskinetic.18,36 The coronary lesions were predicted by using conventionally defined wall segments37 and a schema correlating the segmental wall motion abnormalities with the coronary artery distribution.36,38 The echocardiographic site of AMI was recognized by correlating the wall motion abnormalities with the evolutionary electrocardiographic changes. A patient was considered to have significantly viable myocardial segments within the infarction zone when an improvement in contractility ò 1 grade developed in at least 2 contiguous segments or ò 2 grades in 1 segment. DSE was considered positive for myocardial ischemia in the infarct zone when a deterioration of its thickening and motion at higher doses occurred after improvement at low doses was noted (biphasic response). A new wall motion abnormality in a basally normal segment or deterioration of a hypokinetic segment belonging to the infarct-related artery territories according to the scheme of coronary perfusion was also considered indicative of ischemia.14,20,39 When there was no improvement in myocardial thickening or motion during low-dose dobutamine infusion, the akinesic or dyskinetic segments were considered to be nonviable.40 Changes from dyskinesis to akinesis at low dose were considered to be unchanged.40 Akinesia deteriorating directly to dyskinesia was not considered indicative of myocardial ischemia.14,20,40,41 Agreement among the 2 observers was achieved in 96% (29 of 30) of the studies. Any disagreement among the 2 observers was resolved through discussion.
Coronar y angiogr aphy and angioplasty
After obtaining the consent forms from the patients, coronary angiography was performed with routine technique at 8.3 ñ 3 days after the onset of AMI. A computer-aided quantitative angiographic analysis system (DCI-S Automated Coronary
Analysis System, Philips Medical Systems, Eindhoven, The Netherlands) was used. After identifying the infarct-related artery, the patency was assessed by 2 observers having no knowledge of the result of UTC and DSE. A ò 50% diameter stenosis 42 was considered significant and angioplasty was performed. The success of angioplasty was defined to reduce more than 50% of the diameter stenosis.
Follow-up Ultr asonic Tissue Char acter ization and Dobutamine Stress Echocar diogr aphy
After successful angioplasty, the follow-up UTC and DSE were performed within 24 hours.
Data Reproducibility.
For each study, the UTC was performed twice to evaluate the intraobserver variability. To evaluate the interobserver variability, the another observer performed the integrated backscatters data acquisition and analysis independently.43 The mean absolute variabilities for variation were 0.6ñ0.5 dB (intraobserver) and 1.0 ñ 0.7 dB (interoberver). For phase, these were 5ñ7° (intraobserver) and 7ñ9° (interoberver). Performing correlation analysis of these repeated measurements by linear regression method, the coefficients of correlation for variation were 0.96 (intraobserver) and 0.90 (interoberver). The standard errors of estimate were 0.8 dB (intraobserver) and 1.1 dB (interoberver). As to phase, the coefficients of correlation were 0.90 (intraobserver) and 0.81 (interoberver). The standard errors of estimate were 8° (intraobserver) and 10 ° (interoberver).
Statistical analysis.
All results were given as mean ñ standard deviation. Continuous data were compared using one-way ANOVA test. Duncan‘s multiple range test was used to find those groups with statistically significant difference. The unpaired t test was used to compare continuous data between 2 different groups. Two-way ANOVA test was used to evaluate the changes of phase-weighted variation after successful angioplasty in different patient groups. McNemar’s test was applied to compare the concordance between DSE and UTC. A p value <0.01 (two tailed) was considered statistically significant. The specificity, sensitivity and accuracy for phase-weighted variation in identifying myocardium that was viable without residual ischemia were plotted to determine the cutoff value. Furthermore, the receiver operating characteristics curve analysis was also applied.44
Results
Patients Data
There were 27 men and 3 women enrolled in this study (mean age, 55ñ 12 years). Thrombolytic therapy was used in 14 patients and 7 patients received primary angioplasty. The reperfusion therapy was given within 4.8ñ2.6 hours after the onset of AMI.
Coronar y Angiogr aphy and Angioplasty
Coronary angiography was performed at 8.3ñ3 days after AMI. The infarct-related artery was the left anterior descending coronary in 16 patients, the left circumflex in 1, and the right coronary in 13. The residual stenosis of the infarct-related artery was 70±35%. Significant residual stenosis (ò 50% luminal diameter stenosis) was found in 73% of the patients (22 of 30), including 4 occluded arteries. Successful angioplasty was done in 15 patients. However, 4 patients refused the follow-up DSE after successful angioplasty. Two patients received coronary artery bypass graft surgery for multivessel disease and complete revascularization.
Dobutamine-Atropine infusion
For the 30 cases of DSE done before intervention, the peak dobutamine dose was 37ñ 6 g/Kg/min. Atropine (0.5 to 1.0 mg) was used in 16 patients. The peak heart rate and systolic blood pressure were 121ñ17 bpm and 146ñ22 mmHg, respectively. End points were heart rate of ò 120 bpm in 15 patients, maximal dose in 2, ST depression ò 2 mm in 4, angina in 5, vomiting in 1, multiple inducible wall motion deterioration in 1, non-sustained ventricular tachycardia in 1, and hypotension in 1. There were 11 patients receiving DSE after successful angioplasty. All were completed at maximal dose and atropine was used in 5 patients. The peak heart rate and systolic blood pressure were 136ñ14 bpm and 146ñ16 mmHg, respectively. No adverse effects were noted.
Dobutamine-Atropine Str ess Echocar diogr aphy
Analyzing the wall motion before coronary angiography, the infraction zone showed biphasic in 15 patients and worsened from basally normal or hypokinetic segments in 4. These were defined as viable myocardium with residual ischemia. Five patients have persistently improved wall motion in the infarction zone and were considered to have viable myocardium without residual ischemia. The infarction zone wall motion showed no improvement in 6 patients. That myocardium was considered to be nonviable. According to the inducible ischemic response, the sensitivity, specificity and accuracy for dobutamine-atropine stress echocardiography in detecting residual stenosis of infarct-related artery were 73%(16/22), 63%(5/8) and 70%(21/30),
respectively. Because the nonviable myocardium showed unchanged or worsened from akinesia to dyskinesia in wall motions during dobutamine infusion, even after successful angioplasty, the ability of DSE in detecting residual stenosis in nonviable myocardium was limited.20 After excluding the nonviable myocardium, the sensitivity, specificity and accuracy had been 94%(16/17), 57%(4/7) and 83%(20/24), respectively. After successful coronary angioplasty, 9 patients whose infarction zone were viable received follow-up DSE. There were no changes in baseline wall motion. The previous inducible ischemia become absence in 6.
Ultr asonic Tissue Char acter ization
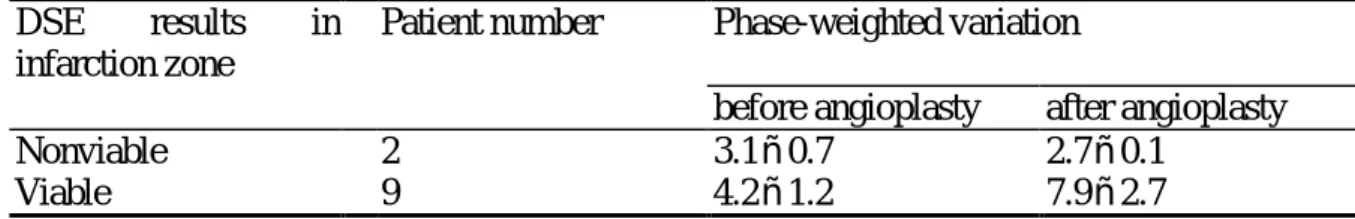
For evaluating the predictability of residual infarct-related artery stenosis on viable myocardium, we classified the results of UTC into 5 groups (Table 1). Group I was those vessel territories supplied by patent coronary arteries and without inducible ischemia in DSE. It was considered as control group. Group IIA was the infarction zone without dobutamine responsive contractile improvement and with residual infarct-related artery stenosis. If there was no residual coronary stenosis of the nonviable myocardium, including the 2 patients after successful angioplasty, the results were designated as group IIB. According to the presence of residual infarct-related artery stenosis of viable infarction zone, the results of UTC were classified into Group IIIA and IIIB, respectively. After relieving the residual coronary stenosis of viable infarction zone by angioplasty, the 9 follow-up studies were also classified into group IIIB. There was significant difference of phase-weighted variation among the 5 groups (p<0.001, by one-way ANOVA). The difference existed between group I, IIIB and IIA, IIB, IIIA. It meant that there was no difference of phase-weighted variation between control group and the infarction zone that was viable and without residual coronary stenosis (p=0.453). Contrarily, the phase-weighted variation of nonviable infarction zone, irreverent to the residual infarct-related artery stenosis, was fewer than that of control group (for group IIA and IIB, p<0.001 and p=0.008 versus control group, respectively). The phase-weighted variation of the viable infarction zone with residual coronary stenosis was also small (p<0.001 versus group I and IIIB). To evaluate the influence of residual infarct-related artery stenosis on UTC for infarction zone, two-way ANOVA was performed to compared the change of phase-weighted variation (Table 2, 9 patients with dobutamine responsive contractile reserve on infarction zone and 2 patients with nonviable myocardium). The statistic difference (P=0.0014) was attributed to the restoration of phase-weighted variation of viable infarction zone after relieving residual infarct-related artery stenosis (Fig 4 and 5). To identity the cut-off value of phase-weighted variation in predicting the residual coronary stenosis of viable infarction zone, the curves of sensitivity, specificity and accuracy were plotted. The receiver operating characteristic curve was also drawn.
Using the optimal cut-off value of 5.8, the sensitivity, specificity and accuracy for detecting viable infarction zone without residual infarct-related artery stenosis were 75%(12/16), 100%(25/25), and 90.2%(37/41), respectively. The concordance with DSE was good (McNemar‘s test, χ2
= 0, after Yate’s correction, P=NS). The area under the receiver operating characteristic curve was 0.84.
Discussion
There were three major findings in this study. First, although the wall motion was dyssynergic, the viable myocardium without residual ischemia had similar result of UTC to the normal control group. Secondly, in viable myocardium, the residual coronary stenosis would hamper the restoration of magnitude in backscatter power curve after AMI. After relieving the residual coronary stenosis, however, the parameter obtained from UTC, phase-weighted variation, would recover before the regional wall motion improved. Finally, the nonviable myocardium had small phase-weighted variation irrelevant to the patency of infarct-related artery. According to these findings, we could differentiate the myocardium with residual coronary stenosis or nonviable from the viable myocardium without residual coronary stenosis.
Ultr asonic Tissue Char acter ization of Infarction Zone Myocar dium
Previous studies28,45 have shown a cardiac cycle-dependent variation of integrated backscatter from the myocardium with the highest values recorded at end diastole and the lowest at end systole. According to a physiologically based model for the behavior of ultrasonic backscatter proposed by Wickline et al. 45 the time-varying change in backscattered energy during the cardiac cycle results from a changing acoustic impedance that is caused by variations in tissue elastic modules during sarcomere shorting and is influenced by ischemia. Recently, a “geometric” model has been proposed by O‘Brien et al. that is based primarily on changes in the density of scatterers with contraction.46
The occurrence of myocardial ischemia or infarction in animals or humans causes a reduction in the cyclic variation and an increase in the phase value obtained by first harmonic Fourier fitting.23-25,26,32,47,48 The reperfusion after temporary coronary clamping can restore the amplitude of cyclic variation and the phase delay to some extent. 23,26,32,47 However, after prolonged coronary occlusion (>5 hours), the phase delay did not return to normal baseline after reperfusion and was considered useful in distinguishing between ischemic and infarcted myocardium.23 Because these restorations of cyclic variation and phase develop before the recovery of regional wall motion abnormalities, the ultrasonic tissue characterization has been assumed to be a useful tool in detecting the stunned myocardium.26,49,50,51Recently, O‘Brien et al.52
have verified the acoustic propagation properties capable of identifying stunned and infarcted myocardium.
Milunski and coworkers26 have performed UTC in patients for detecting AMI and reperfusion. Within the first 24 hours after AMI, the magnitude of cyclic variation of integrated backscatter in infarct regions was lower than that in normal regions. The follow-up study showed that the magnitude increased over time in patients with documented vessel patency, although no recovery of wall thickening was noted. Patients with occluded infarct-related arteries exhibited no significant recovery of cyclic variation. However, no viability assessment or stress test for ischemia was done. In our study, Those myocardium that was viable without significantly residual coronary stenosis (group IIIB) had similar phase-weighted variation to that of control group (group I). Because these viable segments were dyssynergic but free from residual ischemia, it was conceivable that myocardial stunning was responsible for the dysfunction. It was compatible with the findings from animal studies of which the stunning effect was induced by temporary coronary occlusion.32,48,49 We found that there were two factors limiting the restoration of phase-weight variation: the residual coronary stenosis (Group IIA and IIIA)26 and nonviability (Group IIA and IIB). The latter has been mentioned in animal studies after prolonged coronary occlusion.23,32In our study, almost all the significant residual coronary stenosis of viable myocardium showed ischemic response to dobutamine infusion. It has been reported that the dobutamine stress inducible wall motion deterioration was related to impaired coronary flow reserve. 53 In this study, without the use of dobutamine stress, the diminished functional flow reserve also impaired the recovery of cyclic variation of integrated backscatter in 9 patients with residual stenosis between 50 to 90%. In which range, the coronary lesions were considered not severe enough to decrease resting coronary flow. Because we performed the UTC in the acute stage of AMI, the long-term evolutional changes of backscatter power curve for viable myocardium could not be well evaluated. The finding needs further investigation.
Restor ation of Car diac Cycle-dependent Var iation of Integr ated Backscatter After Relieving Residual Coronar y Stenosis of Viable Myocar dium
Although the terms “hibernation”54 and “stunning”50,51 represent uniquely different pathophysiological processes with distinct definitions, in clinical circumstances the boundaries between stunning and hibernation are often indistinct. Both process may occur in the same patient and even coexist in the same myocardial region.55 The residual ischemia will impair the recovery of dysfunction.14 It implicates that the viable myocardium will go into hibernation status and the successful revascularization is required for the recovery of regional function.13,15,56 Our study showed that the residual ischemia also hampered the restoration of cyclic backscatter variation of
viable myocardium (Group IIIA vs IIIB, p<0.001) and the successful revascularization could restore the phase-weighted variation. The restoration developed before the recovery of regional wall motion abnormalities.47-49 The improvement in function of these segments with low-dose dobutamine indicated their likelihood for eventual recovery,17-19 especially after successful revascularization and no ischemia induced by follow-up DSE.13-15 On the contrary, the intervention made no difference on phase-weighted variation of those nonviable myocardium(Table 2). It may be due to the large extent of ischemic injury23,32and implicate the irreversible wall motion abnormalities.39
Risk Str atification After Acute Myocar dial Infarction
Among the multiple factors that govern the risk after an AMI, 3 are recognized as principle: left ventricular function (the single most important variable), residual myocardial ischemia, and cardiac arrhythmia. The first 2 have been the most important in influencing management after AMI because of the available therapeutic modalities.57 DSE can identify the viable myocardium in infarction zone and predict the recovery of ventricular function by low-dose testing.13,17-19 If a segment has an inotropic reserve after dobutamine infusion, it is likely to recover and the left ventricular function will improve. The association of viability within the infarction zone with reduction in risk for cardiac events has been verified by DSE.21 The presence of residual ischemia can further jeopardize viable myocardium in infarction zone, which may act as an unstable substrate for further events11,12 or show impaired recovery of regional function unless be successfully revascularized.13-15 Carlos and coworkers21 provided evidence that supported the use of the noninvasive strategy with DSE for risk stratification of patients surviving an uncomplicated AMI regardless of whether thrombolytic therapy was used. Furthermore, the findings of DSE were better at risk-stratifying patients than was angiographic demonstration of multivessel disease. The widespread application of routine angiography and revascularization dose not improve survival or rates of reinfarction in patients with AMI.8,58,59 The use of coronary angiography and revascularization should be reserved for patients with angina and/or heart failure symptoms, with demonstrable ischemia by noninvasive stress testing, and patients with depressed left ventricular function in whom multivessel disease is suspected.21,22,60,61
In our study, the accuracy of UTC for detecting viable myocardium without residual coronary stenosis was satisfactory (90.2%) and the concordance with DSE was also good. Although the maximal dobutamine stress test is verified safe during the first week after AMI,20,21 Quinones suggested to wait until the seventh day before submitting a patient to a full dobutamine or exercise protocol. This means that some patients undergo testing as outpatients in today‘s climate of managed care.22 In
comparison with DSE, no stress method is needed for UTC. It can be performed in more acute stage after AMI.23,32,47-49 It means that we can apply the UTC on the patient with AMI after reperfusion therapy. If the phase-weighted variation recovers, the salutary effect of reperfusion therapy has been achieved. The myocardium within the infarction zone can be considered as viable and without residual ischemia. The patient may be classified into low-risk group and will have more preserved ventricular function. Intervention strategy should be held unless other indications are present. If the phase-weighted variation is still low, the infarction zone may become nonviable or the significantly residual infarct-related arterial stenosis may exist. Further evaluation and intervention should be considered.
Study Limitations This study has several limitations. Firstly, the myocardial viability
was assessed by DSE only. Although it has been assumed to be a valid method, long-term follow-up study is mandatory to verify our observation. Secondly, unlike DSE, the UTC could not afford a comprehensive view of the whole ventricle because of anisotrophy and difficult delineation of the endocardium in the apical segments.24 Only the midventricular myocardium was evaluated. In our study, there was no patient in whom the infarct area was too small to be delineated in this image. Besides, the wall motion response to dobutamine infusion at midventricular segments on which UTC was performed was all compatible with the result of DSE in evaluating the whole left ventricle. Indeed, the most significant wall motion changes developed at midventricle during DSE. However, the difference of myocardial viability between apical and mid- ventricle might influence our interpretation. Thirdly, the magnitude of ultrasonic energy backscattered from myocardial tissue, as well as the attenuation suffered by the ultrasonic waves, depends on the angle of insonification with respect to the predominant orientation of the myofibers at selected intramural levels. Although the property of ultrasonic anisotropy can be compensated by clinically applicable techniques, such as lateral gain compensation and time gain compensation,26,27 a large-scale study is necessary to disclose the ultrasonic angle dependency of the measurement obtained in the three different areas of the short axis image. Finally, the magnitude of cardiac cycle-dependent variation of integrated backscatter is reduced in the patients with pressure-overload ventricular hypertrophy62 and diabetes mellitus. 63 The magnitude is also affected by aging. 64 These factors are common in patients with AMI. Our study was too small in scale to reveal the possible effects of these factors. The further investigation is warranted.
Conclusion and Clinical Implication
infarct-related artery was free from residual stenosis. It is safe because no additional stress modalities are needed. It can be used as a predischarge screening tool for risk stratification and is helpful in constructing the intervention strategy. The UTC may also be applied to evaluate the beneficial effects of reperfusion if we perform the test promptly after therapy. Could diminished phase-weighted variation predict the restenosis of infarct-related artery of viable myocardium ? Could we select the patients for whom reperfusion therapy is really necessary by ruling out the development of spontaneous recanalization of infarct-related arteries after AMI? Further studies are mandatory.
References
1. Simoons ML, Serruys PW, van den Brand M, Bär F, van der Zwaan C, Res J, Verheugt FWA, Krauss XH, Remme WJ, Vermeer F, Lubsen J. Improved survival after early thrombolysis in acute myocardial infarction: a randomized trial by the Interuniversity Cardiology Institute in the Netherlands. Lancet 1985;ii:578-582. 2. Gruppo Italiano per lo Studio della Streptokinasi nell‘infarto Miocardico (GISSI).
Long-term effects of intravenous thrombolytic treatment in acute myocardial infarction: final report of the GISSI. Lancet 1987;i:871-874.
3. Lendenink T, Simoons ML, Van Es GA, Van de Werf F, Verstraete M, Arnold AER, for the European Cooperative Study Group. Benefit of thrombolytic therapy is sustained throughout five years and is related to TIMI perfusion grade 3 but not grade 2 flow at discharge. Circulation 1995;92:1110-1116.
4. Serruys PW, Simoons ML, Suryapranata H, Vermeer F, Wijns W, van den Brand M, Bär F, van der Zwaan C, Krauss XH, Remme WJ, Res J, Verheugt FWA, von Domburg R, Lubsen J, Hugenholtz PG. Preservation of global and regional left ventricular function after early thrombolysis in acute myocardial infarction. J Am Coll Cardiol 1986;7:729-742.
5. Yoshida K, Gould KL. Quantitative relation of myocardial infarct size and myocardial viability by positron emission tomography to left ventricular ejection fraction and 3-year mortality with and without revascularization. J Am Coll Cardiol 1993;22:984-997.
6. DeWood MA, Spores J, Notske R, Mouser LT, Burroughs R, Golden MS, Lang HT. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med 1980;303:897-902.
7. Verani MS, Roberts R. Preservation of cardiac function by coronary thrombolysis during acute myocardial infarction: fact or myth? J Am Coll Cardiol 1987;10:470-476.
8. The TIMI study group. Comparison of invasive and conservative strategies after treatment with intravenous tissue plasminogen activator in acute myocardial infarction- results of the thrombolysis in myocardial infarction (TIMI) phase II trial. N Engl J Med 1989;320:618-627.
9. Eitzman D, Al-Aouar Z, Kanter HL, vom Dahl J, Kirsh M, Deeb GM, Schwaiger M. Clinical outcome of patients with advanced coronary artery disease after viability studies with positron emission tomography. J Am Coll Cardiol 1992;20:559-565.
10. Gibson RS, Beller GA, Gheorghiade M, Nygaard TW, Watson DD, Huey BL, Sayre SL, Kaiser DL. The prevalence and clinical significance of residual myocardial ischemia 2 weeks after uncomplicated non-Q wave infarction: a prospective natural history study. Circulation 1986;73:1186-1198.
11. Lee KS, Marwick TH, Cook SA, Go RT, Fix JS, James KB, Sapp SK, MacIntyre WJ, Thomas JD. Prognosis of patients with left ventricular dysfunction, with and without viable myocardium after myocardial infarction- relative efficacy of medical therapy and revascularization. Circulation 1994;90:2687-2694.
12. Brown KA, Weiss RM, Clements JP, Wackers FJTh. Usefullness of residual ischemic myocardium within prior infarct zone for identifying patients at high risk late after acute myocardial infarction. Am J Cardiol 1987;60:15-19.
13. Barilla F, Gheorghiade M, Alam M, Khaja F, Goldstein S. Low-dose dobutamine in patients with acute myocardial infarction identifies viable but not contractile myocardium and predicts the magnitude of improvement in wall motion abnormalities in response to coronary revascularization. Am Heart J 1991:122:1522-1531.
14. Previtali M, Poli A, Lanzarini L, Fetiveau R, Mussini A, Ferrario M. Dobutamine stress echocardiography for assessment of myocardial viability and ischemia in acute myocardial infarction treated with thrombolysis. Am J Cardiol 1993;72:124G-130G.
15. Miketiæ S, Carlsson J, Tebbe U. Improvement of global and regional left ventricular function by percutaneous transluminal coronary angioplasty after myocardial infarction. J Am Coll Cardiol 1995;25:843-847.
16. Garot J, Scherrer-Crosbie M, Monin JL, DuPouy P, Bourachot ML, Teiger E, Rosso J, Castaigne A, Gueret P, Dubois-Randé JL. Effect of delayed percutaneous transluminal coronary angioplasty of occluded coronary arteries after acute myocardial infarction. Am J Cardiol 1996;77:915-21.
17. Salustri A, Elhendy A, Garyfallydis P, Ciavatti M, Cornel JH, Cate FJ, Boersma E, Gemelli A, Roelandt JRTC, Fioretti PM. Prediction of improvement of ventricular function after first acute myocardial infarction using low-dose
dobutamine stress echocardiography. Am J Cardiol 1994;74:853-856.
18. Smart SC, Sawada S, Ryan T, Segar D, Atherton L, Berkovitz K, Bourdillon PDV, Feigenbaum H. Low-dose dobutamine echocardiography detects reversible dysfunction after thrombolytic therapy of acute myocardial infarction. Circulation 1993;88:405-415.
19. Piérard LA, De Landsheere CM, Berthe C, Rigo P, Kulbertus HE. Indentification of viable myocardium by echocardiography during dobutamine infusion in patients with myocardial infarction after thrombolytic therapy: comparison with positron emission tomography. J Am Coll Cardiol 1990;15:1021-1031.
20. Smart SC, Knickelbine T, Stoiber TR, Carlos ME, Wynsen JC, Sagar KB. Safety and accuracy of dobutamine-atropine stress echocardiography for the detection of residual stenosis of the infarct-related artery and multivessel disease during the first week after acute myocardial infarction. Circulation 1997;95:1394-1401. 21. Carlos ME, Smart SC, Wynsen JC, Sagar KB. Dobutamine stress
echocardiography for risk stratification after myocardial infarction. Circulation 1997;95:1402-1410.
22. Quinones MA. Risk stratification after myocardial infarction. Circulation 1997;95:1352-1354.
23. Fitzgerald PJ, McDaniel MD, Rolett EL, Strohbehn JW, James DH. Two-dimensional ultrasonic tissue characterization: backscatter power, endocardial wall motion, and their phase relationship for normal, ischemic, and infarcted myocardium. Circulation 1987;76:850-859.
24. Saeian K, Rhyne TL, Sagar KB. Ultrasonic tissue characterization for diagnosis of acute myocardial infarction in the coronary care unit. Am J Cardiol 1994; 74:1211-1215.
25. Vitale DF, Bonow RO, Gerundo G, Pelaggi N, Lauria G, Leosco D, Coltorti F, Bordini C, Rengo C, Rengo F. Alterations in ultrasonic backscatter during exercise-induced myocardial ischemia in Humans. Circulation 1995;92:1452-1457.
26. Recchia D, Miller JG, Wickline SA. Quantification of ultrasonic anisotropy in normal myocardium with lateral gain compensation of two-dimensional integrated backscatter images. Ultrasound Med Biol 1993;19:497-505.
27. Milunski MR, Mohr GA, Perez JE, Vered Z, Wear KA, Gessler CJ, Sobel BE, Miller JG, Wickline SA. Ultrasonic tissue characterization with integrated backscatter. Acute myocardial ischemia, reperfusion, and stunned myocardium in patients. Circulation 1989;80:491-503.
28. Wickline SA, Thomas III LJ, Miller JG, Sobel BE, Perez JE. The dependence of myocardial ultrasonic integrated backscatter on contractile performance.
Circulation 1985;72:183-192.
29. Sagar KB, Rhyne TL, Warltier DC, Pelc L. Wann LS. Intramyocardial variability in integrated backscatter: effect of coronary occlusion and reperfusion. Circulation 1987;75:436-442.
30. Naito J, Masuyama T, Mano T, Yamamoto K, Doi Y, Kondo H, Hori M, Shiba A, Murakami K, Shimura T, Kamada T. Validation of transthoracic myocardial ultrasonic tissue characterization: comparison of transthoracic and open-chest measurements of integrated backscatter. Ultrasound Med Biol 1995;21:33-40. 31. Mottley JG, Glueck RM, Perez JE, Sobel BE, Miller JG. Regional differences in
the cyclic variation of myocardial backscatter that parallel regional differences in contractile performance. J Acoust Soc Am 1984;76:1617-1623.
32. Wickline SA, Thomas III LJ, Miller JG, Sobel BE, Perez JE. Sensitive detection of the effects of reperfusion on myocardium by ultrasonic tissue characterization with integrated backscatter. Circulation 1986;74:389-400.
33. Afridi I, Kleiman NS, Raizner AE, Zoghbi WA. Dobutamine echocardiography in myocardial hinernation: optimal dose and accuracy in predicting recovery of ventricular function after coronary angioplasty. Circulation 1995;91:663-670. 34. Fioretti PM, Poldermans D, Salustri A, Forster T, Bellotti P, Boersma E, McNeill
AJ, El-Said ESM, Roelandt JRTC. Atropine increases the accuracy of dobutamine stress echocardiography in patients taking beta-blockers. European Heart J 1994;15:355-360.
35. Ling LH, Pellikka PA, Mahoney DW, OH JK, McCully RB, Roger VL, Seward JB. Atropine augmentation in dobutamine stress echocardiography: role and incremental value in a clinical practice setting. J Am Coll Cardiol 1996;28:551-557.
36. Sawada SG, Segar DS, Ryan T, Brown SE, Dohan AM, Williams R, Fineberg NS, Armstrong WF, Feigenbaum H. Echocardiographic detection of coronary artery disease during dobutamine infusion. Circulation 1991;83:1605-1614.
37. Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgessel H, Reichek N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. Recommendation for the quantification of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr 1989;5:358-367.
38. Segar DS, Brown SE, Sawada SG, Ryan T, Feigenbaum H. Dobutamine stress echocardiography: correlation with coronary lesion severity as determined by quantitative angiography. J Am Coll Cardiol 1992;19:1197-1202.
39. Wu CC, Ho YL, Kao SL, Chen WJ, Lee CM, Chen MF, Liau CS, Lee YT. Dobutamine stress echocardiography for detecting coronary artery disease. Cardiology 1996;87:244-249.
40. Watada H, Ito H, OH H, Masuyama T, Aburaya M, Hori M, Iwakura M, HigashinoY, Fujii K, Minamino T. Dobutamine stress echocardiography predicts reversible dysfunction and quantitates the extent of irreversibly damaged myocardium after reperfusion of anterior myocardial infarction. J Am Coll Cardiol 1994;24:624-630.
41. Arnese M, Fioretti PM, Cornel JH, Postma-Tjoa J, Reijs AEM, Roelandt JRTC. Akinesis becoming dyskinesis during high-dose dobutamine stress echocardiography: a marker of myocardial ischemia or a mechanical phenomenon? Am J Cardiol 1994;73:896-890.
42. Baptista J, Arnese M, Roelandt JRTC, Fioretti PM, Keane D, Escaned J, Roersma E, DiMario C, Serruys PW. Quantitative coronary angiography in the estimation of the functional significance of coronary stenosis: correlations with dobutamine-atropine stress test. J Am Coll Cardiol 1994;23:1434-1439.
43. Stuhlmuller JE, Skorton DJ, Burns TL, Melton HE, Vandenberg BF. Reproducibility of quantitative backscatter echocardiographic imaging in normal subjects. Am J Cardiol 1992;69:542-546.
44. Wanger RF, Wear KA, Perez JE, McGill JB, Schechtman KB, Miller JG. Quantitative assessment of myocardial ultrasound tissue characterization through receiver operating characteristic analysis of Bayesian Classifiers. J Am Coll Cardiol 1995;25:1706-1711.
45. Wickline SA, Thomas III LJ, Miller JG, Sobel BE, Perez JE. A relationship between ultrasonic integrated backscatter and myocardial contractile function. J Clin Invest 1985;76:2151-2160.
46. O‘Brien PD, O’Brien WD, Rhyne TL, Warltier DC, Sagar KB. Relation of ultrasonic backscatter and acoustic propagation properties to myofibrillar length and myocardial thickness. Circulation 1995;91:171-175.
47. Glueck RM, Mottley JG, Miller JG, Sobel BE, Perez JE. Effects of coronary artery occlusion and reperfusion on cardiac cycle-dependent variation of myocardial ultrasonic backscatter. Circ Res 1985;56:683-689.
48. Barzilai B, Vered Z, Mohr GA, Wear KA, Courtois M, Sobel BE, Miller JG, Perez JE. Myocardial ultrasonic backscatter for characterization of ischemia and reperfusion: relationship to wall motion. Ultrasound in Med & Biol 1990;16:391-398.
49. Milunski MR, Mohr GA, Wear KA, Sobel BE, Miller JG, Wickline SA. Early identification with ultrasonic integrated backscatter of viable but stunned myocardium in Dogs. J Am Coll Cardiol 1989;14:462-471.
50. Braunwald E, Kloner RA. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation 1982;66:1146-1149.
51. Bolli R. Myocardial ‘stunning’ in man. Circulation 1992;86:1671-1691.
52. O‘Brien WD, Sagar KB, Warltier DC, Rhyne TL. Acoustic propagation properties of normal, stunned, and infarcted myocardium: morphological and biochemical determinants. Circulation 1995;91:154-160.
53. Severi S, Underwood R, Mohiaddin RH, Boyd H, Paterni M, Camici PG. Dobutamine stress: effects on regional myocardial blood flow and wall motion. J Am Coll Cardiol 1995;26:1187-1195.
54. Braunwald E, Rutherford JD. Reversible ischemic left ventricular dysfunction: evidence for the “hibernating myocardium”. J Am Coll Cardiol 1986;8:1467-1470.
55. Bonow RO. Identification of viable myocardium. Circulation 1996;94:2674-2680.
56. Meluzin J, Cigarroa CG, Brickner E, Cerny J, Spinarova L, Frelich M, Stetka F, Groch L, Grayburn PA. Dobutamine echocardiography in predicting improvement in global left ventricular systolic function after coronary bypass or angioplasty in patients with healed myocardial infarction. Am J Cardiol 1995;76:877-880.
57. Deedwania PC, Amsterdam EA, Vagelos RH. Evidence-based, cost-effective risk stratification and management after myocardial infarction. Arch Intern Med 1997;157:273-280.
58. SWIFT (Should We Intervene Following Thrombolysis?) Trial Study Group. SWIFT trial of delayed elective intervention ν conservative treatment after thrombolysis with anistreplase in acute myocardial infarction. BMJ 1991;302:555-560.
59. Rouleau JL, Moyé LA, Pfeffer MA, O. Arnold JM, Bernstein V, Cuddy TF, Dagenais GR, Geltman EM, Goldman S, Gordon D, Hamm P, Klein M, Lamas GA, McCans J, McEwan P, Menapace FJ, Parker JO, Sestier F, Sussex B, Braunwald E. A comparison of management patterns after acute myocardial infarction in Canada and the United States. N Engl J Med 1993;328:779-784. 60. Ellis SG, Mooney MR, George BS, Ribeiro da Silva EE, Talley JD, Flanagan WH,
Topol EJ. Randomized trial of late elective angioplasty versus conservative management for patients with residual stenosis after thrombolytic treatment of myocardial infarction. Circulation 1992;86:1400-1406.
61. Pitt B. Evaluation of the postinfarct patient. Circulation 1995;91:1855-1860. 62. Masuyama T, St. Goar FG, Tye TL, Oppenheim G, Schnittger I, Popp RL.
Ultrasonic tissue characterization of human hypertrophied hearts in vivo with cardiac cycle-dependent variation in integrated backscatter. Circulation 1989;80:925-934.
63. Perez JE, McGill JB, Santiago JV, Schechtman KB, Waggoner AD, Miller JG, Sobel BE. Abnormal myocardial acoustic properties in diabetic patients and their correlation with the severity of disease. J Am Coll Cardiol 1992;19: 1154-62. 64. Masuyama T, Nellessen U, Schnittger I, Tye TL, Haskell WL, Popp RL.
Ultrasonic tissue characterization with a real time integrated backscatter system in normal and aging human hearts. J Am Coll Cardiol 1989;14:1704-8.
TABLE 1. Ultr asonic Tissue Char acter ization of Infarction Zone in Relation to the Myocar dial Viability and Residual Stenosis of the Infarct-related Ar ter y
Group Viable myocardium identified by DSE
Residual stenosis of infarct-related artery
(coronary lesion severity)
Phase-weighted variation I ( control) (n=43) 8.1ñ2.6 IIA (n=5) - + (98ñ4%) 3.6ñ1.4* IIB (n=3) - -(33ñ21%) 3.5ñ1.3† IIIA (n=17) + + (90ñ8%) 4.2ñ1.1* IIIB (n=16) + -(10ñ16%) 7.5ñ2.7‡
DSE: dobutamine-atropine stress echocardiography; n: number of coronary vessel territory; +: positive finding; -: negative finding; *: p < 0.001 compared to Group I; †: p < 0.05 compared to Group I; ‡: p < 0.001 compared to Group IIIA
TABLE 2. Change of Ultr asonic Tissue Char acter izaiton of Infarction Zone after Successful Coronar y Angioplasty
DSE results in infarction zone
Patient number Phase-weighted variation
before angioplasty after angioplasty
Nonviable 2 3.1ñ0.7 2.7ñ0.1
Viable 9 4.2ñ1.2 7.9ñ2.7