Volume 2012, Article ID 486568,12pages doi:10.1155/2012/486568
Research Article
The Potential Utility of Curcumin in the Treatment of
HER-2-Overexpressed Breast Cancer: An
In Vitro
and
In Vivo
Comparison Study with Herceptin
Hung-Wen Lai,
1, 2Su-Yu Chien,
3, 4, 5Shou-Jen Kuo,
1, 4, 5Ling-Ming Tseng,
6, 7Hui-Yi Lin,
8Chin-Wen Chi,
2and Dar-Ren Chen
1, 41Comprehensive Breast Cancer Center, Changhua Christian Hospital, Changhua 50006, Taiwan
2Department and Institute of Pharmacology, School of Medicine, National Yang-Ming University, Taipei 11221, Taiwan 3Department of Pharmacology, Changhua Christian Hospital, Changhua 50006, Taiwan
4School of Medicine, College of Health Care and Management, Chung Shan Medical University, Taichung 40201, Taiwan 5School of Nutrition, College of Health Care and Management, Chung Shan Medical University, Taichung 40201, Taiwan 6Division of General Surgery, Department of Surgery, Taipei Veterans General Hospital, Taipei 11217, Taiwan
7School of Medicine, National Yang-Ming University, Taipei 11221, Taiwan
8Department of Pharmacology, School of Pharmacology, China Medical University, Taichung 40402, Taiwan
Correspondence should be addressed to Dar-Ren Chen,darren chen@cch.org.tw
Received 11 November 2010; Revised 21 March 2011; Accepted 2 May 2011 Academic Editor: Jae Youl Cho
Copyright © 2012 Hung-Wen Lai et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. HER-2 is an important oncoprotein overexpressed in about 15–25% of breast cancers. We hypothesized that the ability of curcumin to downregulate HER-2 oncoprotein and inhibit the signal transduction pathway of PI3K/Akt, MAPK, and NF-κB activation may be important in the treatment of HER-2-overexpressed breast cancer. To examine the effect of curcumin on breast cancer cells, MCF-7, MDA-MB-231, MCF-10A, BT-474, and SK-BR-3-hr (a herceptin resistant strain from SK-BR-3) cells were used for in vitro analysis. The in vivo effect of curcumin on HER-2-overexpressed breast cancer was investigated with the HER-2-overexpressed BT-474 xenograft model. Cell growth, cell cycle change, the antimobility effect, signal transduction, and xenograft volume analysis between groups treated with herceptin and/or curcumin were tested. Curcumin decreased the cell growth of various breast cancer cell lines (MCF-7, MDA-MB-231, MCF-10A, BT-474, and SK-BR-3-hr). In Western blot analysis, the phosphorylation of Akt, MAPK, and expression of NF-κB were reduced in BT-474 cells, but not in SK-BR-3-hr cells, after treatment with herceptin. When treated with curcumin, the HER-2 oncoprotein, phosphorylation of Akt, MAPK and expression of NF-κB were decreased in both BT-474 and SK-BR-3-hr cells. In the BT-474 xenograft model, though not as much as herceptin, curcumin did effectively decrease the tumor size. The combination of curcumin with herceptin was not better than herceptin alone; however, the combination of taxol and curcumin had an antitumor effect comparable with taxol and herceptin. The results suggested that curcumin has potential as a treatment for HER-2-overexpressed breast cancer.
1. Introduction
Around 15–25% of breast cancers are noted to overexpress the human epithelial growth factor receptor 2 (HER-2) [1–
3], patients with HER-2 overexpression were associated with a poor prognosis, more disease relapse, and distant metas-tasis [3–6]. Herceptin (generic name: Trastuzumab), which effectively inhibits the HER-2-related PI3k/Akt and MAPK
pathways, is the first targeted therapeutic agent developed and approved for the treatment of patients with HER-2-overexpressed breast cancer [7–12]. Despite the success of herceptin, a significant proportion of HER-2-positive breast cancer patients responded poorly to the treatment [13,14]. In addition, some patients that initially responded to the therapy became resistant within one year [14, 15]. The refractory state of these HER-2-positive breast carcinomas
illustrates the need to examine the mechanisms underlying tumor resistance and the necessity to seek novel treatment strategies.
Curcumin (diferuloylmethane) is a yellow pigment de-rived from the rhizome of the plant Curcuma longa L. The powdered rhizome of this plant, called turmeric, is commonly used in the preparation of curries. Curcumin, as a polyphenol with a diarylheptanoid structure containing twoα, β-unsaturated ketones, is considered to be the major active constituent of turmeric [16, 17]. In addition to its wide range of pharmacological activities, the anticancer properties of curcumin have attracted great interest [18–20]. The ability of curcumin to downregulate EGFR [19,21,22] and HER-2 [23] oncoproteins and affect the PI3K/Akt [24] and MAPK [25] pathways, with which herceptin interfered, raised interest in the potential utility of curcumin in the treatment of HER-2-positive breast cancer.
Taxol (generic name: Paclitaxel) combined with her-ceptin is one of the current preferred regimens for the treatment of HER-2-overexpressed breast cancer [11, 26]. NF-κB is an important factor related to proliferation and antiapoptosis [27, 28], and the activation of NF-κB plays an important role in the chemoresistance of taxol [29,30]. Curcumin has the well-known ability to downregulate
NF-κB [31,32]. The combination of curcumin with taxol could suppress taxol-related NF-κB activation and enhance the antitumor effect of taxol [31, 33]. The evolving concept of combining a monoclonal antibody (herceptin or per-tuzumab) for the extracellular domain of HER-2 and small molecule tyrosine kinase inhibitors (TKI) for EGFR (gefi-tinib, erlotinib) [34–36] or EGFR/HER-2 (lapatinib) [37] has shown benefit in some preclinical studies and patient trials. The multifunctions of curcumin in downregulating EGFR and HER-2 oncoproteins, reducing the phosphorylation of Akt and MAPK and suppressing NF-κB activation, led to interest in using curcumin in the treatment of HER-2-overexpressed breast cancer, along with herceptin and/or taxol.
The aim of this preclinical study was to explore the potential application of curcumin in the treatment of HER-2-overexpressed breast cancer and examine the interaction of curcumin and herceptin, which has rarely been reported.
2. Materials and Methods
2.1. Cell Culture and Reagents. To examine the effect of
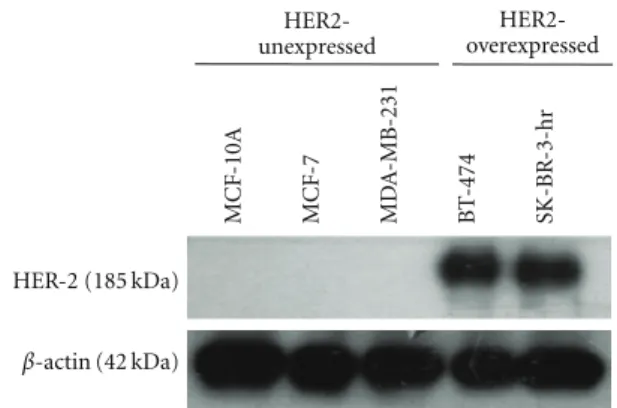
herceptin and curcumin on breast cancer cell lines with various estrogen receptors (ER) and HER-2 receptors, MCF-7 [ER(+), HER-2(−)], SK-BR-3-hr (a herceptin-resistant strain from SK-BR-3 breast cancer cells) [ER(−), HER-2(+)], BT-474 [ER(+), HER-HER-2(+)], MDA-MB-231 [ER(−), HER-2(−)], and normal breast epithelial cells, MCF-10A [ER(−), HER-2(−)], were chosen. The expression of HER-2 oncoprotein in MCF-7, MCF-10A, MDA-MB-HER-231, BT-474, and SK-BR-3-hr cells was verified by western blotting
(Figure 1).
The cell lines (MCF-7, BT-474, MDA-MB-231, and normal breast MCF-10A) used in this study were purchased
MCF -10A MCF -7 MD A -MB-231 B T -474 SK-BR -3-hr HER-2 (185 kDa) β-actin (42 kDa) HER2-unexpressed HER2-overexpressed
Figure 1: The expression of HER-2 oncoprotein in various human breast cancer cell lines. Cells were lysed and analyzed by western blot as described in methods. As revealed in figure, BT-474 and SKBr-3-hr were HER-2-overexpressed breast cancer cell lines. Whiles MCF-7, MCF-10A, and MDA-MB-231 cells were HER-2-unexpressed breast cancer cells.
from American Type Culture Collection (ATCC). SK-BR-3-hr, a herceptin-resistant strain, was a kind gift from Dr. L.-M. Tseng. The resistance of herceptin was induced by repeated culture of herceptin-treated SK-BR-3 cells, which were herceptin-sensitive and purchased from ATCC. The MCF-7 cell line was maintained in RPMI medium with 10% fetal bovine serum, 50 unit/mL penicillin, and 50 unit/mL streptomycin. The MDA-MB-231 cells were cultured in L15 medium containing 10% fetal bovine serum, 50 unit/mL penicillin, and 50 unit/mL streptomycin. BT-474 cells were maintained in DMEM/F12 medium with 10% fetal bovine serum, 50 unit/mL penicillin, and 50 unit/mL strep-tomycin. SK-BR-3-hr cells were maintained in DMEM/F12 medium with 10% fetal bovine serum, 50 unit/mL peni-cillin, and 50 unit/mL streptomycin. MCF-10A was main-tained in MEBM medium with 1% penicillin-streptomycin, 50μg/mL hydrocortisone, 1 μg/mL EGF, 500 μg/mL insulin, and 10μg/mL cholera toxin. Cells were maintained at 37◦C in a humidified atmosphere in the presence of 5% CO2.
2.2. Compounds. Curcumin (Sigma-Aldrich, Inc., St. Louis,
Mo, USA) was dissolved in DMSO at 50μg/mL as a stock solution. Herceptin was purchased from Roche and dissolved in PBS at 50μg/mL as stock. Taxol was dissolved in 49.7% ethanol at 6 mg/mL as stock.
2.3. Growth and Cell Proliferation Analysis. Cell proliferation
was measured using sulforhodamine B (SRB) colorimetric analysis, which is used for cell density determination, based on the measurement of cellular protein content [38]. The method described here has been optimized for the toxicity screening of compounds to adherent cells in a 96-well format. After an incubation period, cell monolayers were fixed with 10% (wt/vol) trichloroacetic acid and stained with SRB for 30 min after which the excess dye was removed by washing repeatedly with 1% (vol/vol) acetic acid. The protein-bound dye was dissolved in 10 mM Tris base solution
for OD determination at 510 nm using a microplate reader. The results were linear over a 20-fold range of cell numbers.
The trypan blue exclusion test was also used to confirm the cell proliferation result in herceptin- and curcumin-treated BT-474 and SK-BR-3-hr cells. Cells were stained with 4% trypan blue (Sigma), and viable cells counted with a hemocytometer under a light microscope.
2.4. Determination of Combinatorial Effects. The ability of
herceptin and curcumin to act in a synergistic, additive, or antagonistic matter with regard to growth inhibition was determined by a combination index (CI) as proposed by Chou and Talalay [39,40]. The Calcusyn software (Biosoft, Great Shelford, Cambridge, UK) was used to determine the CI for each concentration of drug mixture used. A CI value
<1 represented a case where synergism of herceptin and
curcumin was present. CI values of 1 and >1 represented additive and antagonistic effects, respectively.
2.5. Cell Cycle Analysis. Breast cancer cells were plated at a
density of 5×105/dish in 60×15-mm culture dishes and
allowed to adhere overnight. Cells were then incubated with media containing herceptin and/or curcumin at different concentrations as indicated. Following 72 hours (h) of incubation, the cells were washed with PBS, trypsinized, and collected by centrifugation at 1,500 rpm for 10 minutes. After centrifugation, the supernatant was removed, and the cell pellets were fixed with 75% alcohol at −20◦C. After 1 h of incubation, the cell pellets were collected by centrifugation at 1,500 rpm for 10 minutes. The pellets were incubated with propidium iodide (PI) solution (10μg/mL) for 30 minutes. The cell cycle phase was determined by Cytomics FC500 flow cytometry (Beckman Coulter).
2.6. Mobility Test. Cell migration is necessary in many
physiological processes, such as wound healing, and is a characteristic of cancer cell metastasis [41]. The inhibitory effect of herceptin and/or curcumin on different breast cancer cell lines was tested by wound healing assay. Upon inflicting a scratch wound, these cells were treated with various concentrations of curcumin and/or herceptin for 4 h and returned to standard media in an attempt to minimize any cytotoxic effects that could potentially confound our observations. Following 20 h of further incubation, the areas of the wounds were measured using Image J software
(http://rsb.info.nih.gov/ij/).
2.7. Western Blot Analysis. Breast cancer cells were allowed
to incubate with curcumin and/or herceptin at various concentrations and time points as indicated and harvested after treatment. Whole cell lysate was prepared by resus-pending the cells in RIPA buffer supplemented with protease inhibitors cocktail (PIERCE) and incubating the cells on ice for 30 minutes. Cell lysates were centrifuged at 13,000 x g for 10 minutes and the supernatant collected. The protein concentration was measured using the Bradford assay (Bio-Rad Laboratories, Hercules, Calif, USA).
An aliquot of protein lysate (10μg) from each sample was mixed with 2X Laemmli sample buffer (Bio-Rad, Hercules, Calif, USA), and the protein lysate was separated in 10% SDS-polyacrylamide gels for 1 h. After transferring the sample to a nitrocellulose membrane, the membrane was blocked with 5% milk in 1X TBST buffer (10 mM Tris, 150 mM NaCl, 0.5% Tween-20, pH 7.4) for 1 h at room temperature and immunoblotted using the following antibodies: HER-2, phosphorylated Akt (p-Akt), total Akt, phosphorylated MAPK (p-MAPK), total MAPK, and NF-κB (Cell Signaling Technology). The proteins were probed with HER-2 (Cell Signaling Technology), p-Akt, anti-total Akt, anti-p-ERK1/2, anti-ERK 1/2, anti-NF-κB, and anti-β-actin (Sigma) at 4◦C overnight, followed by incuba-tion with horseradish peroxidase-conjugated secondary anti-bodies (Sigma). Protein visualization was performed using the enhanced chemiluminescence kit (PIERCE) according to the manufacturer protocol. Equal loading of total protein was normalized with theβ-actin signal.
2.8. In Vivo Model: Xenograft of HER-2-Overexpressed Breast Cancer in Nude Mice. HER-2-overexpressed BT-474 cells
(1 ×107 cells per mice) were injected into 4- to
6-week-old female, athymic nude mice subcutaneously (s.c.) in the right flank region to form xenografts. Prior to tumor cell inoculation, all mice were primed with 17β-estradiol pellets introduced subcutaneously in a biodegradable carrier binder 7 days before inoculation of the tumor (1.7 mg of estradiol per pellet, Innovative Research of America, Inc.) to promote tumor growth. Then, 1 × 107 BT-474 cells, suspended in
(200μL) growth-factor-reduced matrigel (BD Bioscience, Bedford, MA), were injected s.c. into the right flank region. A period of 14 to 21 days elapsed to allow formation of tumor nodules. Tumor nodules were monitored twice weekly by a single observer using serial micrometer (mm) measurements, with tumor volume calculated as the product of length×width2/2. Six animals were randomly assigned
to each treatment group. Statistical tests were performed to assure uniformity in starting volumes between treatment and control groups at the beginning of each experiment.
Treatment groups included the control, herceptin alone, curcumin alone, and the combination of herceptin and curcumin. To evaluate the effect of curcumin and/or her-ceptin with chemotherapeutic agents, taxol alone, taxol and herceptin, taxol and curcumin, and taxol + herceptin + curcumin regimens were tested. Treatment with different protocols was initiated 21–28 days postxenograft inoculation status, at which time the xenograft volume was measured at around 50–100 mm3. Differences in xenograft volume on
the 28th posttreatment day between groups were analyzed by single-factor analysis of variance (ANOVA). The treatment protocol is summarized.
(a) Control group: sterile 0.1% DMSO intra-peritoneally (i.p.) injection once per week for 4 consecutive weeks. (b) Herceptin-only group: loading dose of 4 mg/kg her-ceptin in sterile PBS, administered by i.p. injection, then a dose of 2 mg/kg maintained at once per week, for 4 consecutive weeks.
(c) Curcumin only: curcumin dissolved in 0.1% DMSO injected i.p. at a dose of 45 mg/kg twice per week for 4 consecutive weeks.
(d) Combined curcumin and herceptin: curcumin dis-solved in 0.1% DMSO injected i.p. at a dose of 45 mg/kg twice per week, combined with a loading dose of 4 mg/kg herceptin in sterile PBS, admin-istered by i.p. injection, then a dose of herceptin 2 mg/kg maintained at once per week, for 4 consec-utive weeks.
(e) Taxol-alone group: taxol 10 mg/kg i.p. once per week for 4 consecutive weeks.
(f) Taxol + herceptin: taxol 10 mg/kg i.p. once per week, combined with a loading dose of 4 mg/kg herceptin in sterile PBS, administered by i.p. injection, then a dose of herceptin 2 mg/kg maintained at once per week, for 4 consecutive weeks.
(g) Taxol and curcumin: taxol 10 mg/kg i.p. once per week, curcumin dissolved in DMSO injected i.p. at a dose of 45 mg/kg twice per week for 4 consecutive weeks.
(h) Taxol, curcumin, and herceptin: taxol 10 mg/kg i.p. once per week, curcumin dissolved in 0.1% DMSO injected i.p. at a dose of 45 mg/kg twice per week, combined with a loading dose of 4 mg/kg herceptin in sterile PBS, administered by i.p. injection, then a dose of herceptin maintained at 2 mg/kg once per week, for 4 consecutive weeks.
After completing 4 weeks of treatment, the mice were sacrificed before recording body weight and tumor volume. The tumor was harvested and processed for various ana-lytical purposes. The animal use protocol were reviewed and approved by the Institutional Animal Care and Use Committee of Changhua Christian Hospital, Changhua, Taiwan.
2.9. Statistical Analysis. Analyses were performed using the
Statistical Analysis System (SAS 9.1). Data are presented as mean ± standard deviation, except where indicated. Comparisons between groups were analyzed using the chi-square test, Student’s two-tailed t-test, or one-way ANOVA with Bonferroni’s correction, as appropriate. A value ofP < 0.05 is considered statistically significant.
3. Results
3.1. Effects of Herceptin or Curcumin on Cell Proliferation.
To examine the biological effect of herceptin and curcumin, breast cancer cell lines were treated with different con-centrations of herceptin (0.1–10μg/mL) or curcumin (1– 25μg/mL) for 72 h. Cell proliferation change was assayed with an SRB assay. As shown in Figure 2(a), in HER-2-overexpressed BT-474 breast cancer cells, cell proliferation was inhibited by herceptin in a dose-dependent manner. Cell proliferation decreased to 60% after treatment with 1μg/mL of herceptin and reached a plateau when with
0 20 40 60 80 100 120 0 1 2 3 4 5 6 7 8 9 10 Ce ll gr o w th (%) Herceptin (μg/mL) (a) 0 20 40 60 80 100 120 Ce ll gr o w th (%) 0 5 10 15 20 25 Curcumin (μg/mL) MCF-7 MDA-MB-231 BT-474 SK-BR-3-hr MCF-10A (b)
Figure 2: The effect of herceptin and curcumin on growth of different breast cancer cell lines. Cells were incubated with different levels of herceptin (a) or curcumin (b) for 72 h, respectively, and cell growth was measured using SRB assay.
a concentration >1 μg/mL. In SK-BR-3-hr breast cancer cells, an HER-2-overexpressed and herceptin-resistant breast cancer cell line, cell growth was not inhibited by herceptin, even at a 10μg/mL high concentration. The growth of MCF-7 and MCF-10A, which were non-HER-2-overexpressed cells, was not affected by herceptin treatment. The growth of MDA-MB-231 cells decreased to 64% of the control after 10μg/mL of herceptin treatment (Figure 2(a)).
In examining the effect of curcumin treatment on these cell lines, we found that the cell proliferations of these five cell lines (MCF-7, BT-474, SK-BR-3-hr, MCF-10A, and MDA-MB-231) were all decreased after treatment with curcumin, with different sensitivities (Figure 2(b)). The SK-BR-3-hr, MCF-10A, and MDA-MB-231 cells were more sensitive to curcumin than BT-474 and MCF-7 cells. After treatment
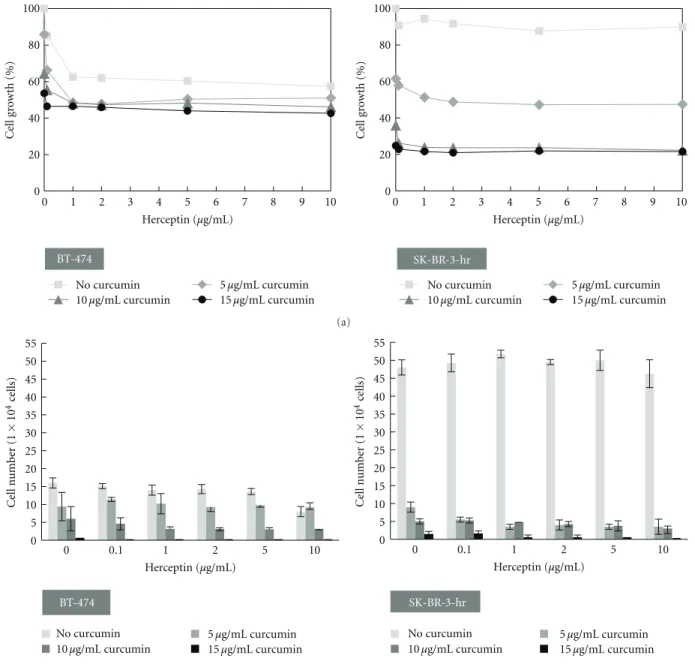
0 20 40 60 80 100 0 1 2 3 4 5 6 7 8 9 10 C el l gr o w th (%) Herceptin (μg/mL) BT-474 No curcumin 5μg/mL curcumin 15μg/mL curcumin 0 20 40 60 80 100 0 1 2 3 4 5 6 7 8 9 10 C el l gr o w th (%) Herceptin (μg/mL) 10μg/mL curcumin No curcumin 5μg/mL curcumin 15μg/mL curcumin 10μg/mL curcumin SK-BR-3-hr (a) BT-474 No curcumin 5μg/mL curcumin 10μg/mL curcumin 0 5 10 15 20 25 30 35 40 45 50 55 0 0.1 1 2 5 10 Ce ll num b er (1 × 10 4ce lls) Herceptin (μg/mL) SK-BR-3-hr 0 5 10 15 20 25 30 35 40 45 50 55 0 0.1 1 2 5 10 Ce ll num b er (1 × 10 4ce lls) Herceptin (μg/mL) 15μg/mL curcumin No curcumin 5μg/mL curcumin 10μg/mL curcumin 15μg/mL curcumin (b)
Figure 3: Cell growth of HER-2-overexpressed breast cancer cells with combined treatment of herceptin and curcumin. (a) The combined effects of herceptin and curcumin on the growth of BT-474 and SK-BR-3-hr cells. Cell growth was analyzed by SRB assay after drug treatment for 72 h. (b) The combinational effects of herceptin and curcumin on BT-474 and SK-BR-3-hr cells were analyzed by trypan blue exclusion assay after drug treatment for 72 h.
with 10μg/mL of curcumin, the cell proliferation of HER-2-overexpressed BT-474 cells and herceptin-resistant SK-BR-3-hr cells decreased to 65% and 20%, respectively.
3.2. Combination Treatment of Cells with Herceptin and Curcumin. Figure 3(a) shows the proliferation of different
cell lines after treatment with a combination of herceptin and curcumin. In the HER-2-overexpressed BT-474 cells, we found that the antiproliferative effect of herceptin was not affected by the addition of curcumin. Curcumin could further decrease the proliferation of BT-474 cells when combined with herceptin. No apparent synergistic effect was
presented when herceptin was combined with curcumin in the SK-BR-3-hr cells. The antiproliferative effect was mainly from curcumin, and the combination of herceptin with curcumin did not reveal a more antiproliferative effect than curcumin alone.
Trypan blue exclusion assay confirmed that both her-ceptin and curcumin inhibited the BT-474 cells dose-dependently. The combination of herceptin and curcumin further effectively decreased the cell viability of BT-474 cells (Figure 3(b)). The SK-BR-3-hr cells that we used here were resistant to herceptin but sensitive to curcumin. The combination of herceptin and curcumin effectively decreased
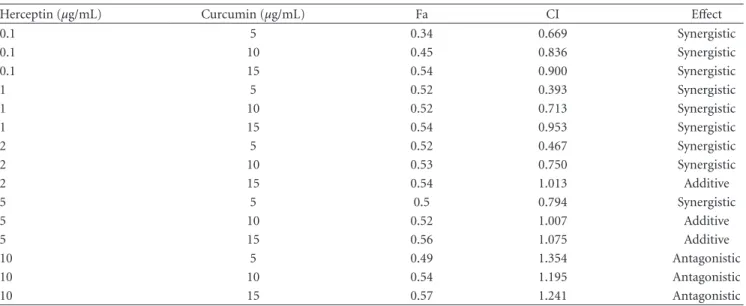
Table 1: The combination index of herceptin and curcumin treatment of the growth of BT-474 cells.
Herceptin (μg/mL) Curcumin (μg/mL) Fa CI Effect
0.1 5 0.34 0.669 Synergistic 0.1 10 0.45 0.836 Synergistic 0.1 15 0.54 0.900 Synergistic 1 5 0.52 0.393 Synergistic 1 10 0.52 0.713 Synergistic 1 15 0.54 0.953 Synergistic 2 5 0.52 0.467 Synergistic 2 10 0.53 0.750 Synergistic 2 15 0.54 1.013 Additive 5 5 0.5 0.794 Synergistic 5 10 0.52 1.007 Additive 5 15 0.56 1.075 Additive 10 5 0.49 1.354 Antagonistic 10 10 0.54 1.195 Antagonistic 10 15 0.57 1.241 Antagonistic
A value of CI<1 represents a case where synergism of herceptin and curcumin was present. CI values of 1 and >1 represent additive and antagonistic effects, respectively. Fa, fraction affected; CI, combination index.
the cell viability of SK-BR-3-hr cells. However, no apparent synergistic or antagonistic effect was present in SK-BR-3-hr cells when a combination of herceptin and curcumin was used.
The combination effect of herceptin and curcumin on the growth of BT-474 breast cancer cells was further analyzed with Calcusyn software for calculation of the CI. The combination of herceptin with curcumin exerted a biphasic interaction in BT-474 cells. The CI was less than 1 and showed a synergistic effect in the following conditions: a low dose of herceptin (0.1–1μg/mL) with curcumin (5– 15μg/mL), 2 μg/mL herceptin with curcumin (5–10 μg/mL), or 5μg/mL herceptin with curcumin 5 μg/mL. The CI was larger than 1 and showed an antagonistic effect when using a high dose of herceptin (>10 μg/mL) with curcumin (5– 15μg/mL). The CI and interaction between herceptin and curcumin in BT-474 cells are summarized inTable 1.
3.3. Effect of Herceptin and/or Curcumin on HER-2-Related Akt and MAPK Pathways in BT-474 and SK-BR-3-hr Cells.
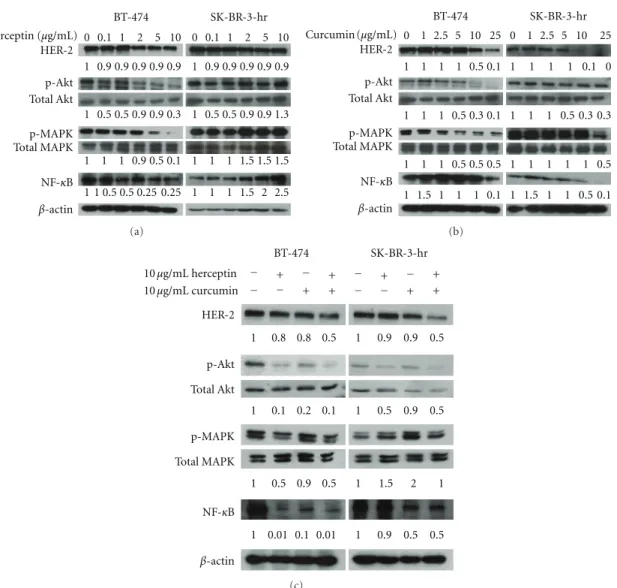
In the HER-2-overexpressed BT-474 and SK-BR-3-hr cells, phosphorylation of Akt and MAPK was observed (Figure 4). Herceptin dose-dependently inhibited the phosphorylation of Akt and MAPK in BT-474 breast cancer cells (left panel,Figure 4(a)). HER-2 oncoprotein was not depleted by herceptin treatment, even at high concentrations (10μg/mL). The addition of herceptin on SK-BR-3-hr cells did not decrease the expression of HER-2 oncoprotein nor decrease the phosphorylation of Akt and MAPK, even at a 10μg/mL concentration (right panel,Figure 4(a)). This was compati-ble with the fact that this SK-BR-3-hr cell line was a strain resistant to herceptin. When treated with curcumin, the phosphorylation of Akt and MAPK was decreased, combined
with the downregulation of HER-2 oncoprotein, in a dose-dependent manner in both the BT-474 and SK-BR-3-hr cells
(Figure 4(b)).
The level of NF-κB in BT-474 cells was decreased along with the decreased phosphorylation of Akt and MAPK after treatment with herceptin. The level of NF-κB was increased, even in high concentrations of herceptin-treated SK-BR-3-hr cells. When treated with curcumin, the level of NF-κB was decreased in a dose-dependent manner in both BT-474 and SK-BR-3-hr cells. The combination of herceptin and curcumin treatment resulted in a decreased level of HER-2 oncoprotein, p-Akt, p-MAPK, and NF-κB in both BT-474 and SK-BR-3-hr cells (Figure 4(c)).
3.4. Effect of Curcumin and/or Herceptin on BT-474 Breast Cancer Cell Cycle. To further characterize the effects of
curcumin and/or herceptin on breast cancer cell growth, analyses of cell cycle phase distribution were conducted. The cell cycle of BT-474 cells without drug treatment showed a G0/G1 phase of 74%, S phase of 19%, and G2/M phase of 7%. When treated with herceptin, the G0/G1 phase increased from 74% to 79%, the S phase decreased from 19% to 11%, and the G2/M phase increased from 7% to 10%. When BT-474 cells were treated with curcumin, the G0/G1 phase remained at 74%, the S phase decreased from 19% to 9%, and G2/M increased from 7% to 20%. The combination of 1μg/mL of herceptin with 10 μg/mL curcumin showed a decrease in the S phase (from 19% to 12%) without an apparent change in the G0/G1 (from 74% to 78%) and G2/M phases (7% to 10%). The results indicated that no significant change in cell cycle progression was observed in BT-474 cells after treatment with herceptin and/or curcumin.
In SK-BR-3-hr cells, the cell cycle was not changed after treatment with herceptin, which is compatible with
0 0.1 1 2 5 10 1 0.9 0.9 0.9 0.9 0.9 1 0.5 0.5 0.9 0.9 0.3 1 1 1 0.9 0.5 0.1 1 1 0.5 0.5 0.25 0.25 Herceptin (μg/mL) HER-2 p-Akt Total Akt p-MAPK Total MAPK NF-κB β-actin BT-474 SK-BR-3-hr 0 0.1 1 2 5 10 1 0.9 0.9 0.9 0.9 0.9 1 0.5 0.5 0.9 0.9 1.3 1 1 1 1.5 1.5 1.5 1 1 1 1.5 2 2.5 (a) 0 1 2.5 5 10 25 0 1 2.5 5 10 25 1 1 1 1 0.1 0 1 1 1 0.5 0.3 0.3 1 1 1 1 1 0.5 1 1.5 1 1 0.5 0.1 1 1 1 1 0.5 0.1 1 1 1 0.5 0.3 0.1 1 1 1 0.5 0.5 0.5 1 1.5 1 1 1 0.1 Curcumin (μg/mL) HER-2 p-Akt Total Akt p-MAPK Total MAPK NF-κB β-actin BT-474 SK-BR-3-hr (b) 10μg/mL herceptin 10μg/mL curcumin 1 0.8 0.8 0.5 1 0.1 0.2 0.1 1 0.5 0.9 0.5 1 0.01 0.1 0.01 1 0.9 0.9 0.5 1 0.5 0.9 0.5 1 1.5 2 1 1 0.9 0.5 0.5 − + − + − + − + − − + + − − + + HER-2 p-Akt Total Akt p-MAPK Total MAPK NF-κB β-actin BT-474 SK-BR-3-hr (c)
Figure 4: The level of HER-2, phosphorylated Akt, MAPK, and NF-κB after treatment with herceptin and/or curcumin in HER-2-overexpressed breast cancer cells. The dosage effects of herceptin (a), curcumin (b), and the combination of herceptin and curcumin (c) on the BT-474 and SK-BR-3-hr cells, respectively. The number at the bottom of each lane indicates the relative fold change of control.
the resistance of this cell line. When treated with curcumin for 48 h, the cell cycle of SK-BR-3-hr showed a decrease in the G1 phase (65% to 37%) and an increase in the S phase (28% to 37%) and G2/M phase (6% to 25%). Compared with the control, the combination of 1μg/mL herceptin and 10μg/mL curcumin showed a decrease in the G1 (65% to 53%), no apparent change in the S phase (28% to 27%), and an increase in the G2/M phase (6% to 19%).
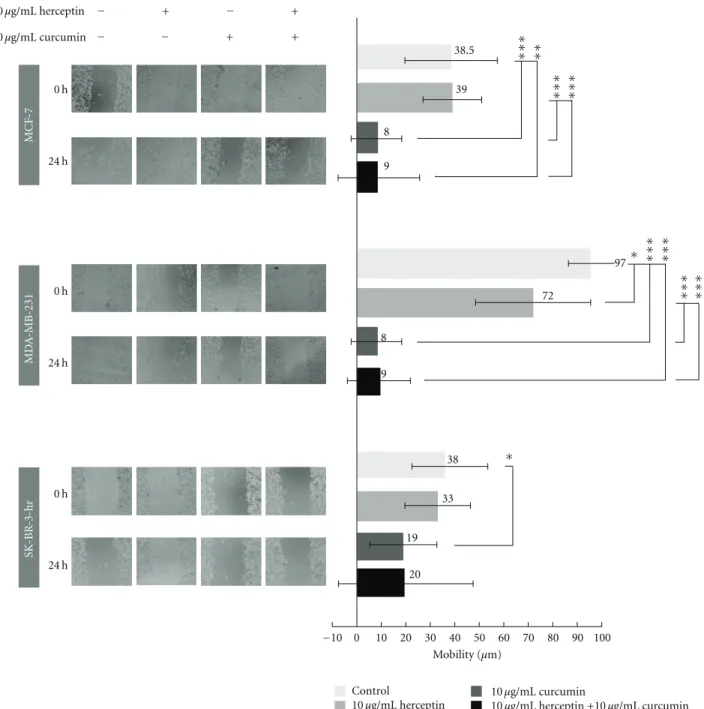
3.5. Effect of Curcumin and/or Herceptin on the Mobility of Different Breast Cancer Cells. The inhibitory effect of
her-ceptin and/or curcumin on different breast cancer cell lines was tested by wound-healing assay. Curcumin showed an apparent antimobility effect as illustrated in the MCF-7, MDA-MB-231, and SK-BR-3-hr cells (Figure 5). No effect
of herceptin and/or curcumin on the mobility of BT-474 cells was observed due to the lack of migration of these cells. In the SK-BR-3-hr cells, cell migration persisted despite
treatment with herceptin, which is consistent with herceptin resistance. When treated with curcumin, cell migration was greatly inhibited as compared with the control, revealing that curcumin had an apparent antimobility effect on SK-BR-3-hr cells. The combination of herceptin and curcumin did not have a better antimobility effect than curcumin alone. In the wound healing assay, curcumin showed an apparent antimobility effect in MCF-7, SK-BR-3-hr, and MBA-MB-231 breast cancer cells.
3.6. Curcumin and/or Herceptin Inhibited Tumor Growth in the Xenograft Animal Model. Compared with the
con-trol group, the mice treated with curcumin, herceptin, or combined herceptin and curcumin all had a smaller mean xenograft tumor volume after 4 weeks of treatment (control group 273.6±190.1 mm3, curcumin group 63.6±
25.7 mm3, herceptin group 36.3±7.8 mm3, and combined
0 h 24 h 0 h 24 h 0 h 24 h MCF -7 MD A -MB-231 SK-BR -3-hr −10 0 10 20 30 40 50 60 70 80 90 100 Mobility (μm) Control 10μg/mL herceptin 10μg/mL curcumin 10μg/mL herceptin +10 μg/mL curcumin ∗ ∗∗∗ ∗∗∗ ∗∗∗ ∗∗∗ ∗∗∗ ∗∗ ∗∗∗ ∗∗∗ ∗ 10μg/mL herceptin 10μg/mL curcumin 20 9 9 19 8 8 33 72 39 38 97 38.5 − + − + − − + +
Figure 5: Changes in mobility of MCF-7, MDA-MB-231, and SK-BR-3-hr cells after treatment with herceptin and/or curcumin. The antimobility effect of herceptin and/or curcumin on breast cancer cells were tested by a wound-healing assay. The anti-mobility effect was illustrated in the MCF-7, MDA-MB-231 and SK-BR-3-hr cells, respectively.∗P < 0.05;∗∗P < 0.01;∗∗∗P < 0.001.
(Figure 6(a)). The curcumin alone group had a larger mean
xenograft tumor size than the herceptin-alone group (63.6±
25.7 mm3versus 36.3±7.8 mm3,P=0.003). The combined
herceptin and curcumin group showed a smaller mean tu-mor volume than the curcumin-alone group (34.1 ±25.0 versus 63.6 ± 25.7 mm3, P = 0.079) or herceptin-alone
group (34.1 ± 25.0 versus 36.3 ± 7.8 mm3, P = 0.324),
although without statistical significance.
The effect of combining curcumin and/or herceptin with taxol was tested by comparing the effects of taxol, taxol + herceptin, taxol + curcumin, and taxol + herceptin
+ curcumin regimens in the BT-474 xenograft model. In another set of experiments, 24 mice inoculated with 1 ×
107BT-474 cells were randomized into 4 groups and treated
with taxol, taxol + herceptin, taxol + curcumin, or combined taxol + herceptin + curcumin. After 4 weeks of treatment, the mean xenograft tumor volumes were taxol 58.3±11.2 mm3,
taxol + herceptin 35.0 ±13.4 mm3, taxol + curcumin 44.5 ± 6.2 mm3, and combined taxol + herceptin + curcumin
group 31.3 ± 27.7 mm3. The antitumor effect of taxol +
herceptin was apparent, and combined taxol and curcumin had a comparable antitumor effect (44.5 ± 6.2 versus
0 50 100 150 200 250 300 0 1 2 3 4
Time after treatment (week)
T
umor
vol
ume
(mm)
Control Herceptin + curcumin
Herceptin Curcumin
(a)
0 1 2 3 4
Time after treatment (week) 0 20 40 60 80 100 T umor vol ume (mm)
Taxol Taxol + herceptin
Taxol + curcumin Taxol + herceptin + curcumin
(b) 0 3 6 9 12 15 18 21 24 W eig ht (g) 0 1 2 3 4
Time after treatment (week)
Control Herceptin + curcumin
Herceptin Curcumin
(c)
Figure 6: In vivo effects of curcumin on the herceptin and/or taxol-treated overexpressed breast cancer xenografts. The HER-2-overexpressed BT-474 cells were injected in 4–6-week-old, female, athymic, nude mice subcutaneously at 1×107cells/tumor in the right
flank region to form xenografts. Six mice per group were treated with different protocols with tumor volume monitored biweekly for consecutive 4 weeks. (a) The mean xenograft tumor volume change of control (0.1% DMSO), herceptin, curcumin, and combined curcumin and herceptin. (b) The mean xenograft tumor volume of taxol, taxol + herceptin, taxol + curcumin, and combined taxol + herceptin + curcumin. (c) The body weight change of these mice treated with herceptin and/or curcumin. DMSO, dimethyl sulfoxide.
35.0 ± 13.4 mm3,P = 0.884). The combination of taxol,
herceptin, and curcumin resulted in the smallest tumor volume, but this was not statistically different from that of the taxol and herceptin regimen (31.3 ± 27.7 versus 35.0
±13.4 mm3,P = 0.079) (Figure 6(b)). The body weight of
these mice treated with herceptin and/or curcumin was quite stable during the 4-week period (Figure 6(c)).
4. Discussion
This study was designed to test the efficacy of curcumin in HER-2-overexpressed breast cancer, with a direct com-parison with herceptin in the in vitro cell line and in vivo xenograft animal model. Our preclinical result revealed that curcumin reduced the cell viability of different breast cancer cell lines, including MCF-7 (ER-positive, HER-2-negative),
MDA-MB-231 (ER-negative, HER-2-negative), HER-2-over-expressed BT-474 (ER-positive, HER-2-positive), and her-ceptin-resistant SK-BR-3-hr (ER-negative, HER-2-positive) cells. The level of HER-2 oncoprotein, p-Akt, p-MAPK, and NF-κB were decreased in a dose- and time-dependant manner in BT-474 and SK-BR-3-hr cells when treated with curcumin. The cell cycle perturbation by curcumin was mainly found in the increase in the G2/M phase. The apparent antimobility effect of curcumin was also revealed in the MCF-7, MDA-MB-231, and SK-BR-3-hr cells. The combinational effect of herceptin with curcumin was a biphasic interaction on the growth of BT-474 cells. When a low dose of herceptin was used with curcumin, there was a synergistic effect, but an antagonistic effect was observed when a high dose of herceptin was used. In the BT-474 xenograft model, curcumin treatment effectively decreased
the tumor size, and the combination of taxol with curcumin had an antitumor effect comparable with that of taxol and herceptin treatment.
The action of herceptin in inhibiting ErbB2 (HER-2) signaling involves the reduced phosphorylation of Akt but not endocytic downregulation of ErbB2 [42]. HER-2 oncoprotein was not depleted by herceptin, even at a high concentration (Figure 4). The exact mechanism of herceptin resistance is not clear, and possible mechanisms include obstacles to herceptin-binding to HER-2, upregulation of HER-2 downstream signaling pathways, signaling through alternative pathways, or failure to trigger immune-mediated mechanisms to destroy tumor cells [14]. In the herceptin-resistant SK-BR-3-hr cells, the persistent activation of PI3K/Akt and MAPK pathways, despite treatment with
herceptin, may have been acquired through repeated cultures of herceptin-treated SK-BR-3 cells.
NF-κB activation played an important role in chemother-apy resistance [29, 30], and targeting NF-κB showed im-proved benefits in some preclinical HER-2-overexpressed cancer cells [28,43]. In HER-2-overexpressed breast cancer, ErbB2 activates NF-κB via signaling that includes PI3K, PDK1, Akt, protein kinase 2 (CK2), and CKBBP1 [44]. In herceptin-sensitive HER-2-overexpressed BT-474 cell lines, herceptin treatment effectively decreased the phosphoryla-tion of Akt, MAPK and the expression of NF-κB (Figure 4). Whether the resistance to herceptin was also related to
NF-κB activation is not clear. In the herceptin-resistant
SK-BR-3-hr cells, the expression level of NF-κB increased in parallel with the incremental dose of herceptin. When treated with curcumin, the expression of NF-κB was decreased, accom-panied with increased cell death. The ability of curcumin to inhibit herceptin-resistant SK-BR-3 cells may be related to the downregulation of HER-2 oncoprotein, and suppression of related Akt, MAPK, and NF-κB signaling pathways. The ability of curcumin to downregulate EGFR and HER-2 oncoproteins and inhibit the phosphorylation of Akt and MAPK and NF-κB activation suggests that curcumin has potential in the treatment of HER-2-overexpressed and/or herceptin-resistant breast cancer.
The interaction of curcumin and herceptin in HER-2-overexpressed cancer has rarely been reported. Whether the combination of herceptin with curcumin had any therapeu-tic advantage over herceptin alone is unknown. In the in
vitro BT-474 cell line study, the combination of herceptin and
curcumin showed an advantage over either treatments alone
(Figure 3). The combination of herceptin and curcumin
exerted a biphasic interaction on the growth of BT-474 cells. A synergistic effect was present when a low dose of herceptin (0.1–1μg/mL) was combined with curcumin, while a high dose (>10 μg/mL) of herceptin would exert an antagonistic effect when combined with curcumin (Table 1). In the SK-BR-3-hr cells, the combination of herceptin with curcumin exerted neither a synergistic nor antagonistic effect. This biphasic interaction observed in vitro warrants caution when herceptin is to be used with curcumin or other medications. In the xenograft animal study, curcumin treatment effec-tively reduced the tumor volume by 76.7%, compared with the control; however, it was not as effective as of herceptin,
which achieved an 86.7% tumor reduction (Figure 6(a)). The combination of herceptin and curcumin showed a greater antitumor effect than curcumin alone (87.5% versus 76.7% in tumor regression) but a similar effect to that of herceptin (87.5% versus 86.7%). The anticipated synergistic effect of combining herceptin with curcumin was not observed in our in vivo xenograft animal model. Although curcumin might not interfere with herceptin in the normal physiologic concentration, from our in vivo study, combined herceptin and curcumin was not better than herceptin alone. More solid evidence might be needed to support the rationale of combing herceptin with curcumin in the treatment of HER-2-overexpressed breast cancer.
It has been reported that curcumin could suppress taxol-induced NF-κB, and curcumin combined with taxol showed greater antitumor effects than taxol alone [31,33]. In our
in vivo xenograft study, the combination of curcumin and
taxol had therapeutic effects comparable with taxol and herceptin, one of the current preferred regimens for HER-2-overexpressed breast cancer (Figure 6(b)). The combination of taxol + herceptin + curcumin was associated with the smallest mean tumor volume although this was not statis-tically different from that of the taxol and herceptin regimen. Curcumin was rated safe and well-tolerated [45]; how-ever, the application of curcumin might be limited by its low bioavailability [16, 17, 45, 46]. To reduce the impact of the low bioavailability of oral intake, we used an intra-peritoneal injection to treat xenograft nude mice. In our study, the body weight of herceptin and curcumin-treated mice did not vary greatly during the entire treatment period (Figure 6(c)), demonstrating the relative safety and tolerability of curcumin as previously reported in animal and human studies [16, 47, 48]. The main limitation of our in vivo study was that we had only one fixed dose protocol for herceptin and/or curcumin treatment, and the serum concentration of the drugs was unknown. The dose of herceptin used here was in accordance with the current practice guideline [26], while the dose of curcumin was derived from a previous report [49]. The optimal dose of curcumin for HER-2-overexpressed breast cancer is unclear and needs to be determined for maximum therapeutic effect. In this study, we showed that curcumin could reduce the cell viability of both HER-2-overexpressed herceptin-sensitive BT-474 cells and herceptin-resistant SK-BR-3-hr breast cancer cells. In the BT-474 xenograft model, though not as much as herceptin, curcumin did effectively decrease the tumor size. The combination of curcumin with her-ceptin was not better than herher-ceptin alone; however, the combination of taxol and curcumin had an antitumor effect comparable with taxol and herceptin. The results, both in
vitro and in vivo, suggested that curcumin has the treatment
potential for HER-2-overexpressed breast cancer.
Conflict of Interests
Acknowledgment
C.-W. Chi and D.-R. Chen equally contributed to the paper.
References
[1] S. Y. Jung, W. Han, J. W. Lee et al., “Ki-67 expression gives additional prognostic information on St. Gallen 2007 and adjuvant! online risk categories in early breast cancer,” Annals
of Surgical Oncology, vol. 16, no. 5, pp. 1112–1121, 2009.
[2] V. Ludovini, S. Gori, M. Colozza et al., “Evaluation of serum HER2 extracellular domain in early breast cancer patients: correlation with clinicopathological parameters and survival,”
Annals of Oncology, vol. 19, no. 5, pp. 883–890, 2008.
[3] D. J. Slamon, G. M. Clark, S. G. Wong, W. J. Levin, A. Ullrich, and W. L. McGuire, “Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene,” Science, vol. 235, no. 4785, pp. 177–182, 1987. [4] R. M. Hudziak, J. Schlessinger, and A. Ullrich, “Increased
expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells,”
Proceedings of the National Academy of Sciences of the United States of America, vol. 84, no. 20, pp. 7159–7163, 1987.
[5] M. F. Press, M. C. Pike, V. R. Chazin et al., “Her-2/neu expression in node-negative breast cancer: Direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease,”
Cancer Research, vol. 53, no. 20, pp. 4960–4970, 1993.
[6] R. Seshadri, F. A. Firgaira, D. J. Horsfall, K. McCaul, V. Setlur, and P. Kitchen, “Clinical significance of HER-2/neu oncogene amplification in primary breast cancer,” Journal of Clinical
Oncology, vol. 11, no. 10, pp. 1936–1942, 1993.
[7] D. J. Slamon, B. Leyland-Jones, S. Shak et al., “Use of chemo-therapy plus a monoclonal antibody against her2 for metastat-ic breast cancer that overexpresses HER2,” The New England
Journal of Medicine, vol. 344, no. 11, pp. 783–792, 2001.
[8] M. D. Pegram, A. Lipton, D. F. Hayes et al., “Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185(HER2/neu) monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment,” Journal
of Clinical Oncology, vol. 16, no. 8, pp. 2659–2671, 1998.
[9] M. A. Cobleigh, C. L. Vogel, D. Tripathy et al., “Multina-tional study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have anti- HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease,” Journal of Clinical
Oncology, vol. 17, no. 9, pp. 2639–2648, 1999.
[10] M. J. Piccart-Gebhart, M. Procter, B. Leyland-Jones et al., “Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer,” The New England Journal of Medicine, vol. 353, no. 16, pp. 1659–1672, 2005.
[11] E. H. Romond, E. A. Perez, J. Bryant et al., “Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer,” The New England Journal of Medicine, vol. 353, no. 16, pp. 1673–1684, 2005.
[12] H. Joensuu, P. L. Kellokumpu-Lehtinen, P. Bono et al., “Adju-vant docetaxel or vinorelbine with or without trastuzumab for breast cancer,” The New England Journal of Medicine, vol. 354, no. 8, pp. 809–820, 2006.
[13] C. L. Vogel, M. A. Cobleigh, D. Tripathy et al., “Efficacy and safety of trastuzumab as a single agent in first-line treatment
of HER2-overexpressing metastatic breast cancer,” Journal of
Clinical Oncology, vol. 20, no. 3, pp. 719–726, 2002.
[14] P. R. Pohlmann, I. A. Mayer, and R. Mernaugh, “Resistance to trastuzumab in breast cancer,” Clinical Cancer Research, vol. 15, no. 24, pp. 7479–7491, 2009.
[15] F. J. Esteva, V. Valero, D. Booser et al., “Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer,” Journal of Clinical
Oncology, vol. 20, no. 7, pp. 1800–1808, 2002.
[16] B. B. Aggarwal, A. Kumar, and A. C. Bharti, “Anticancer poten-tial of curcumin: preclinical and clinical studies,” Anticancer
Research, vol. 23, no. 1 A, pp. 363–398, 2003.
[17] R. A. Sharma, A. J. Gescher, and W. P. Steward, “Curcumin: the story so far,” European Journal of Cancer, vol. 41, no. 13, pp. 1955–1968, 2005.
[18] D. P. Chauhan, “Chemotherapeutic potential of curcumin for colorectal cancer,” Current Pharmaceutical Design, vol. 8, no. 19, pp. 1695–1706, 2002.
[19] A. Chen, J. Xu, and A. C. Johnson, “Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1,” Oncogene, vol. 25, no. 2, pp. 278–287, 2006.
[20] Z. M. Shao, Z. Z. Shen, C. H. Liu et al., “Curcumin exerts multiple suppressive effects on human breast carcinoma cells,”
International Journal of Cancer, vol. 98, no. 2, pp. 234–240,
2002.
[21] L. Korutla, J. Y. Cheung, J. Mendelsohn, and R. Kumar, “Inhi-bition of ligand-induced activation of epidermal growth factor receptor tyrosine phosphorylation by curcumin,”
Carcinogen-esis, vol. 16, no. 8, pp. 1741–1745, 1995.
[22] T. Dorai, N. Gehani, and A. Katz, “Therapeutic potential of curcumin in human prostate cancer. II. Curcumin inhibits tyrosine kinase activity of epidermal growth factor receptor and depletes the protein,” Molecular Urology, vol. 4, no. 1, pp. 1–6, 2000.
[23] R. L. Hong, W. H. Spohn, and M. C. Hung, “Curcumin in-hibits tyrosine kinase activity of p185neu and also depletes p185neu1,” Clinical Cancer Research, vol. 5, no. 7, pp. 1884– 1891, 1999.
[24] A. R. Hussain, M. Al-Rasheed, P. S. Manogaran et al., “Cur-cumin induces apoptosis via inhibition of PI3’-kinase/AKT pathway in acute T cell leukemias,” Apoptosis, vol. 11, no. 2, pp. 245–254, 2006.
[25] L. Camacho-Barquero, I. Villegas, J. M. S´anchez-Calvo et al., “Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis,” International Immunopharmacology, vol. 7, no. 3, pp. 333–342, 2007.
[26] R. J. Winn and J. S. McClure, “NCCN Clinical Practice Guid-lines in Oncology: Breast Cancer,” 2010,http://www.nccn.org/ professionals/physician gls/PDF/breast.pdf.
[27] A. Lin and M. Karin, “NF-κB in cancer: a marked target,”
Seminars in Cancer Biology, vol. 13, no. 2, pp. 107–114, 2003.
[28] D. K. Biswas, S. C. Dai, A. Cruz, B. Weiser, E. Graner, and A. B. Pardee, “The nuclear factor kappa B (NF-κB): a potential therapeutic target for estrogen receptor negative breast can-cers,” Proceedings of the National Academy of Sciences of the
United States of America, vol. 98, no. 18, pp. 10386–10391,
2001.
[29] C. Nakanishi and M. Toi, “Nuclear factor-κB inhibitors as sensitizers to anticancer drugs,” Nature Reviews Cancer, vol. 5, no. 4, pp. 297–309, 2005.
[30] D. K. Biswas and J. D. Iglehart, “Linkage between EGFR family receptors and nuclear factor KappaB (NF-κB) signaling in breast cancer,” Journal of Cellular Physiology, vol. 209, no. 3, pp. 645–652, 2006.
[31] B. B. Aggarwal, S. Shishodia, Y. Takada et al., “Curcumin sup-presses the paclitaxel-induced nuclear factor-κB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice,” Clinical Cancer Research, vol. 11, no. 20, pp. 7490–7498, 2005.
[32] A. M. Kamat, G. Sethi, and B. B. Aggarwal, “Curcumin poten-tiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-κB and nuclear factor-κB-regulated gene products in IFN-α-sensitive and IFN-α-resistant human bladder cancer cells,” Molecular
Cancer Therapeutics, vol. 6, no. 3, pp. 1022–1030, 2007.
[33] H. J. Kang, S. H. Lee, J. E. Price, and L. S. Kim, “Curcumin suppresses the paclitaxel-induced nuclear factor-κB in breast cancer cells and potentiates the growth inhibitory effect of paclitaxel in a breast cancer nude mice model,” Breast Journal, vol. 15, no. 3, pp. 223–229, 2009.
[34] N. Normanno, M. Campiglio, F. Perrone, A. De Luca, and S. Menard, “Is the gefitinib plus trastuzumab combination feasi-ble in breast cancer patients?” Annals of Oncology, vol. 16, no. 10, p. 1709, 2005.
[35] S. L. Moulder and C. L. Arteaga, “A phase I/II trial of trastuzumab and gefitinib in patients with metastatic breast cancer that overexpresses HER2/neu (ErbB-2),” Clinical Breast
Cancer, vol. 4, no. 2, pp. 142–145, 2003.
[36] S. L. Moulder, F. M. Yakes, S. K. Muthuswamy, R. Bianco, J. F. Simpson, and C. L. Arteaga, “Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo,” Cancer Research, vol. 61, no. 24, pp. 8887– 8895, 2001.
[37] W. Xia, C. M. Gerard, L. Liu, N. M. Baudson, T. L. Ory, and N. L. Spector, “Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells,” Oncogene, vol. 24, no. 41, pp. 6213–6221, 2005.
[38] V. Vichai and K. Kirtikara, “Sulforhodamine B colorimetric assay for cytotoxicity screening,” Nature Protocols, vol. 1, no. 3, pp. 1112–1116, 2006.
[39] T. C. Chou and P. Talalay, “A simple generalized equation for the analysis of multiple inhibitions of Michaelis-Menten kinetic systems,” Journal of Biological Chemistry, vol. 252, no. 18, pp. 6438–6442, 1977.
[40] T. C. Chou and P. Talalay, “Applications of the median effect principle for the assessment of low dose risk of carcinogenesis and for the quantitation of synergism and antagonism of chemotherapeutic agents in new avenues in development cancer chemotherapy,” in Bristol-Myers Symposium, K. R. Harra and C. TA, Eds., pp. 37–64, Academic Press, New York, NY, USA, 1987.
[41] D. Hanahan and R. A. Weinberg, “The hallmarks of cancer,”
Cell, vol. 100, no. 1, pp. 57–70, 2000.
[42] K. E. Longva, N. M. Pedersen, C. Haslek˚as, E. Stang, and I. H. Madshus, “Herceptin-induced inhibition of ErbB2 signaling involves reduced phosphorylation of Akt but not endocytic down-regulation of ErbB2,” International Journal of Cancer, vol. 116, no. 3, pp. 359–367, 2005.
[43] S. Singh, Q. Shi, S. T. Bailey et al., “Nuclear
factor-κB activation: a molecular therapeutic target for estrogen
receptor-negative and epidermal growth factor receptor family receptor-positive human breast cancer,” Molecular Cancer
Therapeutics, vol. 6, no. 7, pp. 1973–1982, 2007.
[44] N. R. Monks, D. K. Biswas, and A. B. Pardee, “Blocking anti-apoptosis as a strategy for cancer chemotherapy: NF-κB as a target,” Journal of Cellular Biochemistry, vol. 92, no. 4, pp. 646– 650, 2004.
[45] A. L. Chen, C. H. Hsu, J. K. Lin et al., “Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions,” Anticancer Research, vol. 21, no. 4 B, pp. 2895–2900, 2001.
[46] M. L ´opez-L`azaro, “Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a can-cer chemopreventive and chemotherapeutic agent,” Molecular
Nutrition and Food Research, vol. 52, no. 1, pp. S103–S127,
2008.
[47] C. V. Rao, A. Rivenson, B. Simi, and B. S. Reddy, “Chemo-prevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound,” Cancer
Research, vol. 55, no. 2, pp. 259–266, 1995.
[48] K. B. Soni and R. Kuttan, “Effect of oral curcumin adminis-tration on serum peroxides and cholesterol levels in human volunteers,” Indian Journal of Physiology and Pharmacology, vol. 36, no. 4, pp. 273–275, 1992.
[49] C. C. Su, J. S. Yang, C. C. Lu et al., “Curcumin inhibits human lung large cell carcinoma cancer tumour growth in a murine xenograft model,” Phytotherapy Research, vol. 24, no. 2, pp. 189–192, 2010.