Ionic Conductivity of Plasticized Polymer Electrolytes
WHA-TZONG WHANG* and CHOU-LING LUInstitute of Materials Science and Engineering, National Chiao Tung University, Hsin Chu 300, Taiwan, Republic of China
SYNOPSIS
Two polar polymers with different dielectric constants, poly(viny1idene fluoride) (PVDF) and poly (ethylene oxide) (PEO), were each blended with a chlorine-terminated poly(ethy1ene ether) (PEC) and one of the two salts, LiBF4 and LiCF3C02, to form PEC- plasticized polymer electrolytes. The room-temperature ionic conductivity of the PEC- plasticized polymer electrolytes reached a value as high as S/cm. The room-temperature ionic conductivity of the PVDF-based polymer electrolytes displayed a stronger dependence on the PEC content than did the PEO-based polymer electrolytes. In PVDF/PEC/LiBF4 polymer electrolytes, the dynamic ionic conductivity was less dependent on temperature and more dependent on the PEC content than it was in PEO/PEC/LiBF4 polymer elec- trolytes. The highly plasticized PVDF-based polymer electrolyte film with a PEC content greater than CF4 (CF4 defined as the molar ratio of the repeat units of PEC to those of PVDF equal to 4) was self-supported and nonsticky, while the corresponding PEO-based polymer electrolyte film was sticky. In these highly plasticized PVDF-based polymer elec- trolytes, the curves of the room-temperature ionic conductivity vs. the salt concentration were convex because the number of carrier ions and the chain rigidity both increased with increase of the salt content. The maximum ionic conductivity at 30°C was independent of the PEC content, but it depended on the anion species of the lithium salts in these highly plasticized polymer electrolytes. 0 1995 John Wiley & Sons, Inc.
INTRODUCTION
Poly(ethy1ene oxide) (PEO) is the most favored sol- vating medium in studies of ionic conductivity in solid polymer electrolytes (SPE) because of its abil- ity to combine effectively with the salt cations to form a homogeneous solution.' However, there are several disadvantages in using PEO. The major dis- advantages are that PEO shows a high tendency to crystallize or to form crystalline complexes when long chains are used and that acceptable levels of ionic conductivity can only be obtained at temper- atures above the melting point.2 Ion transport in SPE can be aided by segmental motion of the poly- mer matrix, which, in turn, can be promoted by the preparation of new polymers and the addition of in-
* To whom correspondence should be addressed. Journal of Applied Polymer Science, Vol. 56, 1635-1643 (1995)
0 1995 John Wiley & Sons, Inc. ccc o o ~ i - a s s ~ / s ~ / i ~ i ~ ~ ~ - o s
organic additives and organic soluble
These methods may suppress the crystalline phase and the glass transition temperature (T,) and en- hance the amorphous phase and the dielectric constant7.* of the polymer electrolytes. Polymer electrolytes with a high dielectric constant can re- duce the energy required to dissociate the salt in the medium so that ionic conductivity can be promoted. A polymer with a high polarity usually has a high dielectric constant, c.
In addition to PEO, polar polymers, such as poly(ethy1ene s u c ~ i n a t e ) ~ , ' ~ ( c = 5.0-5.5), poly(p- propiolactone)," poly(ethy1ene adipate)12 ( c = 5.2), p~ly(N-propylaziridine),~~ poly(alky1ene ~ulfide),'~ and poly(viny1idene f l ~ o r i d e ) ' ~ , ' ~ ( c = 8-13) have been used as the matrices in SPEs. The conduc- tivities of these polymers, however, are poor. The dielectric constant of poly(viny1idene fluoride) (PVDF) is much higher than that of PEO ( c = 5), but the conductivity of PVDF/salt SPE is even lower
1636 WHANG AND LU
than that of the corresponding PEO/salt SPE. This poor ionic conductivity might result from the high
Tg
and the high degree of crystallinity of PVDF. The addition of polar soluble organic additives has been used to improve the conductivity of the SPEs. Low molecular weight polar additives, e.g., N,N-di- methylformamide (DMF), ethylene carbonate (EC), and other additives, have been successfully dissolved in polymer to alter the physical and electrical prop- erties of the r n a t r i ~ . ’ ~ - ’ ~ However, these kinds of ad- ditives are volatile at elevated temperatures. Liquid poly(ethy1ene glycol) (PEG), which is nonvolatile, has been added to the polymer electrolytes to pro- duce a more stable system. However, the end groups of the PEG affect the ionic conductivity. Endo-acet- ylated PEGIOO (molecular weight 400) has a lower viscosity than does PEGIm, but polymer electrolytes made with endo-acetylated PEGIOO have a lower conductivity than do those made with PEGIOO. This implies that conductivity is more sensitive to the polarity of the additives than to their viscosity. However, PEG with hydroxyl end groups is not suitable for battery application, since it reacts easily with lithium metal.In this study, nonreactive polar chlorine atoms were used as a substitute for the hydroxyl groups in PEG. The chlorine-terminated poly(ethy1ene ether) (PEC) was expected to promote chain segmental motion and provide extra ion-transport channels in the PEC-rich phase to improve the ionic conductiv- ity. Previous reports showed that unplasticized PVDF-based polymer electrolytes have a much lower conductivity than do the corresponding PEO-based polymer electrolytes, even though the dielectric constant of PVDF is much higher than that of PEO. We anticipated that the liquid PEC might have dif- ferent effects on the conductivity of solid polymer electrolytes containing polymer matrices with dif- ferent dielectric constants and different thermal transition temperatures
(T,
and T m ) . Therefore, in this study, PVDF and PEO were used as polymer matrices; the inorganic salt LiBF4 (LB) and the or- ganic salt LiCF3C02 (LA), as electrolytes; and PEC, as a plasticizer.EXPERIMENTAL
Preparation of Chlorine-terminated Poly(ethy1ene ether) (PEC)
Ten milliliters of SOCl2 was added to 35 mL of PEG4,,,,. After the mixture was refluxed for 4 days under dry nitrogen, it was placed in a vacuum oven
a t 100°C for 48 h in order to remove all the volatile components. The liquid residue was PEC, which was identified by using Fourier transform infrared (FTIR) spectroscopy and nuclear magnetic reso- nance (NMR) spectroscopy. Both techniques con- firmed that all the hydroxyl groups had been re- placed by chlorine atoms.16
Preparation of PVDF/PEC, PVDF/PEC/LiBF4, and PEO/PEO/LiBF4 Films
PVDF, PEC, LiBF4, and LiCF3C02 are all commer- cially available. The solutions of polymer electrolytes were obtained by dissolving different amounts of these components in anhydrous N, N-dimethylacet- amide (DMA) for the PVDF system and in aceto- nitrile for the PEO system. The weighing and mixing of the chemicals were performed in a dry box. The solutions were cast on surface-treated glass plates, and then the solvents were evaporated in a vacuum oven. The resulting polymer electrolyte films were stored in an electronic humidity-controlled desic- cator before characterization. The glass plates were pretreated with a solution containing 1% (CH3)3SiC1 in CHC1,. The salt concentrations in the polymer electrolytes were fixed a t 16/1 and 4/1 of the molar ratios of the total monomeric units of the polymer matrix to the formula units of the salt. The mole number of the total monomeric units was the sum of the mole number of the monomeric units of PVDF or PEO and the number of units of PEC.
Nomenclature FLBx and OLBx
FLBx and OLBx denote the polymer electrolytes containing the salt LiBF4 and one of the two poly- mers PVDF and PEO, respectively, without the plasticizer PEC. “F” stands for PVDF; “0,” for PEO; and LB, for LiBF,. “x” is the molar ratio of the repeat unit of the polymer matrix to the formula unit of the salt.
FCmLBn and OCmLBn
The symbols “F,” “0,” and “LB” have the same meanings as in the above section. In addition, “C” stands for PEC; “m,” for the molar ratio of the re- peat unit of PVDF or PEO to that of PEC; and “n,” for the molar ratio of the total polymeric repeat units (including PVDF, PEO, and PEC) to the formula unit of the salt.
Table I Thermal Transition Data of PVDF/PEC/LB (LB16) Polymer Electrolyte Films
Sample Composition Tg ("(2) T m ("C) AH,,, (mJ/mg)
FLB16 "F" only, F/LB = 16 130.0 169.0 FC4LB16 F/C = 4, (F
+
C)/LB = 16 123.6 165.5 FC2LB16 F/C = 2, (F+
C)/LB = 16 122.4 163.6 FClLB16 F/C = 1, (F+
C)/LB = 16 122.6 163.3 CF2LB16 C/F = 2, (C+
F)/LB = 16 123.1 161.4 CF4LB16 C/F = 4, (C+
F)/LB = 16 - 156.2 49.0 45.2 41.9 39.7 26.1 20.0CFmLAn, CFmLBn, and COmLBn
All these symbols have the same meaning as those defined in the above section, except that "m" stands for the molar ratio of the repeat unit of PEC to that of PVDF or PEO, and LA stands for LiCF3C02. In the two above sections, the first character stands for the component having the higher moles of repeat units in the polymer matrix.
LA and LB
LA represents the pure salt LiCF3C02, and LB, the pure salt LiBF,.
Characterization
Dynamic ionic conductivities and dielectric con- stants of the polymer electrolytes were obtained us- ing a DuPont 2970 dielectric analyzer a t 300 KHz. The measurement was scanned a t 3"C/min in a dry nitrogen flow of 500 cc/min. Thermal transitions of the polymer electrolytes were characterized using a Seiko differential scanning calorimeter a t 10"C/min in a nitrogen stream.
RESULTS AND DISCUSSION
Effects of Polymer Matrix
Thermal transition data for the PVDF/PEC/LB films are listed in Table I. The melting point (156-
170°C) of the PVDF crystalline phase decreased as the PEC content increased. When the PEC content in the polymer electrolyte PVDF/PEC/LB16 films increased beyond the critical value of CF2 (the molar ratio of the repeat unit of PEC to that of PVDF equal to 2), there was a lower temperature subpeak near -12°C in the DSC curve of CF4LB16. This was the melting peak of the plasticizer PEC. As summarized in Table I, the AH,,, of the PVDF melt-
ing decreased with increase in the PEC content. This indicates that PEC facilitated the formation of the PVDF amorphous phase while suppressing the crystalline phase in these SPEs. In the PEO-based polymer electrolyte system, increasing the PEC content showed the same effect as that in the PVDF system: It suppressed the crystalline phase and en- hanced the amorphous phase.
As shown in Table 11, the Tg's of the polymer electrolyte PEO/LB films without PEC were much lower than those of the corresponding PVDF/LB films. This means that the PEO polymer electrolytes were much more flexible than were the correspond- ing PVDF polymer electrolytes. The Tg's of the
PEO/LB films with or without PEC were lower than -50°C. The amorphous phases of the PEO/PEC/ LB16 and PEO/PEC/LB4 films in the temperature range 0-100°C of the dynamic ionic conductivity measurement were rubbery. The PEO-rich crystal- line phase in the PEO/PEC/LB16 films started melting at about 20°C and reached a peak in the range of 3O-5O0C, as shown in Table 11. The PEO-
Table I1 Thermal Transition Data of PEO/PEC/LB (LB16) Polymer Electrolyte Films
OLB16 "0" only, O/LB = 16 -51.3 52.5
OC4LB16 O/C = 4, (0
+
C)/LB = 16 -47.9 50.4 OClLB16 O/C = 1, (0+
C)/LB = 16 -55.5 40.4 C02LB16 C/O = 2, (C+
O)/LB = 16 -58.4 34.6 C04LB16 C/O = 4, (C+
O)/LB = 16 -61.1 31.1 OC2LB16 O/C = 2, (0+
C)/LB = 16 -53.7 43.9 73.4 64.2 49.7 40.1 7.5 3.21638 WHANG AND LU
0
LB16*
LR40 20 40 60 80 100
PEC content / mole % Figure 1
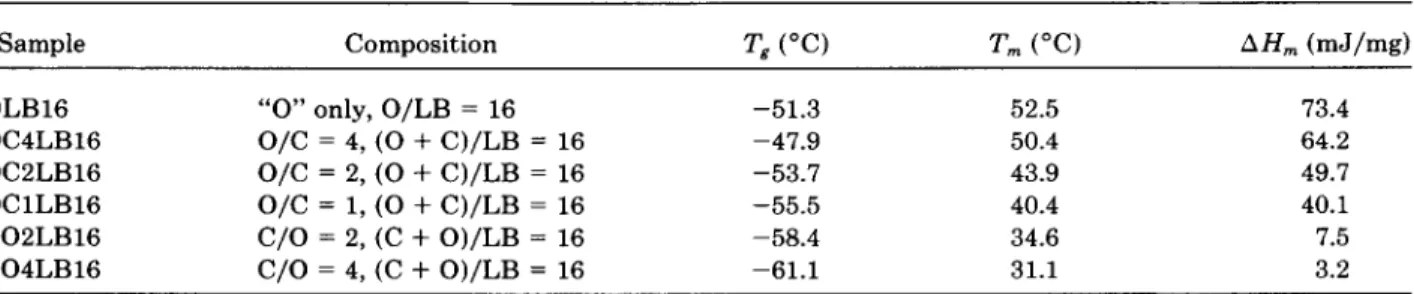
PEC content in PEO/PEC/LB polymer electrolytes. Dependence of ionic conductivity at 30°C on
salt complex crystalline phase of the PEO/PEC/LB4 films started melting from about 50"C, with a peak in the range of 100-115°C. The melting temperature of the PEO/PEC/LB films shifted to a lower tem- perature when more PEC was added to the polymer electrolytes. With a high PEC content in the poly- mer electrolytes, the PVDF-based film, e.g., CF4LB16, was self-supported and nonsticky, but the PEO-based film, e.g., C04LB16, was sticky because of the low
Tg
of the PEO system.Dependence of the ionic conductivity at 30°C on the PEC content in the polymer electrolyte PEO/ PEC/LB is illustrated in Figure 1. As the figure shows, the polymer electrolyte with a salt content of LB16 (lower concentration) had a higher ionic conductivity than that of the corresponding polymer electrolyte with a content of LB4. A higher concen-
tration of LiBF, in the polymer electrolytes resulted in the appearance of more complex phases. This, in turn, produced a more rigid hybrid film and reduced ion mobility in the polymer matrix; Therefore, poly- mer electrolytes with a higher salt content exhibited
a lower ionic conductivity. In the PEO/PEC/LB4
polymer electrolyte system, the addition of PEC ini- tially resulted in an increase in ionic conductivity, which then leveled off when the PEC content (the molar ratio of the monomeric units of PEC to the sum of the monomeric units of PEO and PEC) reached 33%. The same result occurred with PEO/ PEC/LB16, but the ionic conductivity leveled off at a higher PEC content of 50%.
Comparisons of the ionic conductivities of the hybrid films with the PEO matrix and the PVDF
-3 : -4
*
PEO/LM -8c
0 20 40 60 80 100PEC content / mole %
Figure 2 Dependence of ionic conductivity a t 30°C on PEC content in PVDF-based and PEO-based polymer electrolytes with constant LiBF, content (LB4).
matrix are shown in Figures 2 and 3. The dependence of ionic conductivity on PEC content in polymer electrolytes with the PVDF matrix was much stron- ger than it was in electrolytes with the PEO matrix. Figure 2 (with the fixed salt content LB4) shows that with a lower PEC content the ionic conductiv- ities of the PEO system were higher than those of the corresponding PVDF system, but with a PEC content of higher than 55 mol %, the ionic conduc-
--8
0 20 40 60 80 100
PEC content / mole %
Figure 3 Dependence of ionic conductivity a t 30°C on PEC content in PVDF-based and PEO-based polymer electrolytes with constant LiBFl content (LB16).
tivities of the PVDF system were higher than those of the corresponding PEO system. CF4LB4 (PEC content 80%) had an ionic conductivity of 4.4 X S/cm and was nonsticky and self-supporting. In comparison, C04LB4 had lower conductivity and was sticky. Figure 3 reveals that a t a lower PEC content the ionic conductivities of the PEO system were much higher than those of the corresponding PVDF system, but at 80 mol % of the PEC concen- tration, the polymer electrolyte of the PVDF system had the same ionic conductivity as that of the PEO system, i.e., near
lo-,
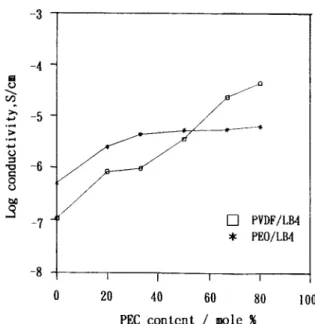
S/cm (8.2 X S/cm for CF4LB16 and 8.8 X S/cm for C04LB16). CF4LB16 film was self-supporting and nonsticky, whereas C04LB16 film was sticky. It might be useful for battery applications to blend CF4LB16 and C04LB16 in order to maintain good contact between the SPE and the electrode during charge-recharge cycles and to provide mechanical stability. Figure 3 also shows that the room-temperature ionic con- ductivity of the PVDF/PEC/LB16 film was much more dependent on PEC content than was the con- ductivity of the PEO/PEC/LB16 film.As shown in Figure 4, the dielectric constant (at 30°C and 300 kHz) of FLB16 (without PEC) was much lower than that of OLB16, although the di- electric constant of pristine PVDF is higher than that of pristine PEO. The
Tg
of the PVDF-based polymer electrolytes near 13OOC was much higher than that of the PEO-based polymer electrolytes near -50°C. The PEO polymer chain is more flexible700
0
PVDF/LBIG0 20 40 60 80 100
PEC content / mole X
Figure 4 Dependence of permittivity at 30°C on PEC content in PVDF-based and PEO-based polymer electro- lytes with constant LiBF4 content (LB16).
than is the PVDF chain. The polarization in a di- electric may be produced by the induction effect and the orientation effect. The total molar polarizability,
P,, is the combination of the induced polarizability
and the orientation polarizability. As shown in Fig- ure 4, the dielectric constant of OLB16 (without PEC) at about 135 was much higher than that of the pristine PEO at 5.
It was apparent that the salt contributes greatly to the polarization of the polymer electrolyte in an electric field. However, the effect of the salt on the polarization of the polymer electrolyte FLB16 was negligible, as the dielectric constant of the polymer electrolyte was close to, or even slightly lower than, that of the pristine PVDF. The main difference be- tween the PEO system and the PVDF system was that the PVDF system exhibited much higher Tg
and T , and a stronger coordination with the salt
LiBF,: The fluorine atoms in PVDF have greater electronegativity than that of the oxygen atoms in PEO. Therefore, the salt combined with PVDF more strongly than with PEO.
The combination of the chain flexibility of the polymer matrix and the interaction of the salt with the polymer matrix made the orientation of LiBF4 in the PVDF-based films much more difficult than it was in the PEO-based films in an electric field. At 30"C, the amorphous phase of FLB16 film was in the glassy state, so the polymer chain movement and the orientation of the salt were both limited. Due to coordination with the salt, the PVDF chain mobility in the polymer electrolyte FLB16 was more difficult than in the pristine polymer, so orientation of the polymer chains in FLB16 film in an electric field was more difficult than was orientation in the pristine PVDF film.
The low dielectric constant of FLB16 at 30°C was probably due to induced polarization. A t 30°C, the amorphous phase of the OLB16 polymer elec- trolyte film was in a rubbery state; the crystalline phase began to melt at 30"C, with a peak near 50°C. It is evident that the salt contributed greatly to the value of the dielectric constant, due to the orienta- tion polarization at 30°C in the OLB16 film. As more PEC plasticizer was added to the polymer electro- lyte, the crystalline phase was suppressed and the chain mobility was enhanced. Also, the orientation polarization of the polymer chains and the salts be- came easier, the value of the dielectric constant in- creased, and the room-temperature ionic conductiv- ity of the polymer electrolytes also increased. Ini- tially, the dielectric constant of the PVDF-based polymer electrolyte film rose slowly, but it increased rapidly when the PEC content was over 50 mol %.
1640 WHANG AND LU
However, the dielectric constant of the PEO/PEC/ LB16 polymer electrolyte increased rapidly with in- crease of the plasticizer content.
The conductivity of the PVDF/PEC/LB16 film was much more sensitive to the increase of the PEC content than was the PEO/PEC/LB16 film. It is clear that, in addition to the dielectric constant, there was another factor that affected the ionic con- ductivity of the polymer electrolytes. It was men- tioned above that the Tg and
T,,,
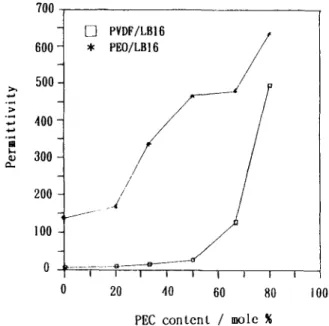
of the PVDF-based polymer electrolytes were high and the interaction between the fluorine atoms of PVDF and lithium ions was stronger than that between the oxygen at- oms of PEC and lithium ions, so that the weak-bond PEC-salt complex in the PVDF-based polymer electrolytes became a better conduction path. This explains why the ionic conductivity of the PVDF- based polymer electrolytes was much more sensitive to change in the PEC content than was the PEO- based polymer electrolytes.The dynamic ionic conductivities of the polymer electrolytes PVDF/PEC/LB and PEO/PEC/LB in the temperature range of 0-100°C are shown in Fig- ures 5-8. The conductivity of PVDF/PEC/LB poly- mer electrolyte films responded less sensitively to temperature but more sensitivity to PEC content than did the PEO/PEC/LB films. As mentioned be- fore, PVDF-based polymer electrolytes exhibited a high Tg of about 130"C, below which the polymer
electrolyte film was in a glassy state. On the other hand, in the PEO/PEC/LB films, the PEO-rich
crystalline phase and the PEO-salt complex crys- talline phase melted below 100°C. The melting of the crystalline phase resulted in a significant change in morphology. Therefore, the effect of temperature on the conductivity of PEO/PEC/LB films was more significant than it was on the conductivity of the corresponding PVDF systems. In PEO/PEC/LB16 films, there was a marked change in conductivity in the temperature range of 2O-4O0C, while in PEO/ PEC/LB4 films, the conductivity increased mildly in two ranges (from 20 to 40°C and from 75 to 100
"
C).Effects of Salt Concentration
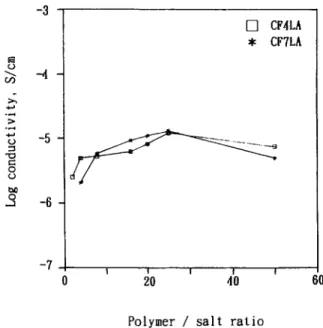
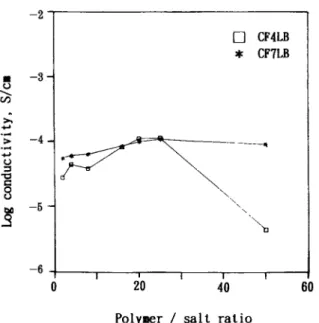
The curves of the room-temperature (30°C) ionic conductivity of highly plasticized polymer electro- lytes vs. the salt concentration are shown in Figure 9 for CF4LAn and CF7LAn and in Figure 10 for CF4LBn and CF7LBn. Both sets of curves are con- vex. As shown in Figure 9, at the same salt concen- tration of LA25, the CF4LAn and CF7LAn films showed the same value for the highest ionic con- ductivity, S/cm. In Figure 10, the dependence of the room-temperature ionic conductivity of CF7LBn films on the salt concentration was less than that of the ionic conductivity of CF4LBn films on the salt concentration of CF4LBn. At the salt concentration of LB25, both the CF4LBn and CF7LBn series exhibited the same maximum ionic conductivity of S/cm. Thus, the PEC concen- -3 1 -8
1
"0 20 40 60 80 1 Temperature(-C) 0 Figure 5PEC with constant LiBF4 content (LB16).
-4-
s
.z
3- \ . rA.-
4
-6t
-7-
-/-- ..’ /-’ , / / - / - .--- ,-- /-. /*- / - -,/---
,/-- / - . / * - //-- , . . ’ - / - - - + I ./. /,-- / - c_.
/ - - / ” ./- .---_----
/-.
/ - - / - - - -.-
/ / - - --
5-. -e-__----
><>.’
,-,.
<;
-
/’ /I- / - -.’
~ I .N-
/ - - / / ,@- , . ’ I’_---
I _ _ _ - * - 1.’ .G e- -/.
*/-. ,.0’.-
/ 5- /-*-I‘
_ * * * //-
’
- / - *_---
_ * - ----
tration had little effect on the maximum ionic con- ductivity of the highly plasticized polymer electro- lytes with the same salt. The maximum ionic con- ductivity was salt species-dependent. This result shows that, when the PEC concentration in PVDF- based polymer electrolytes is high enough, the effect of PEC content on ionic conductivity is no longer
important, except a t low salt concentration. The curves of the room-temperature ionic conductivities vs. the salt content showed a bell-shaped profile. This might be because of an increase in both the carrier number and the matrix rigidity with increas- ing salt concentration. The increase in the film rigidity might result from the formation of physical
FLW F C l W
- - _
FULM 1 x 2 ~ ~ 4 cF4LB4 - - - - _ _ __ _ _ _
FC2LB4 -a , 0 20 40 60 80 100 -3. I IFigure 7 Dynamic ionic conductivity of polymer electrolytes at various ratios of PEO/ PEC with constant LiBF, content (LB16).
1642 WHANG AND LU
Kl
Figure 8
PEC with constant LiBFl content (LB4).
Dynamic ionic conductivity of polymer electrolytes at various ratios of PEO/
crosslinking between fluorine atoms in PVDF or oxygen atoms in PEC and lithium ions. This rigid- ity hindered ion movement in the polymer electro- lytes and reduced the room-temperature ionic con- ductivity.
CONCLUSION
The conductivity of the unplasticized PVDF/LB polymer electrolytes is much lower than that of the unplasticized PEO/LB polymer electrolytes. The PEC plasticizer in the polymer electrolytes can change the morphology and the conductivity of the polymer electrolytes. The dependence of room-tem- perature ionic conductivities of the plasticized PVDF-based polymer electrolytes on the PEC con- tent was much more significant than that of the plasticized PEO-based polymer electrolytes on the PEC content. For PEC content in the plasticized polymer electrolytes higher than 55 mol %, the room-temperature conductivities of the plasticized PVDF/LB films are even higher than those of the corresponding PEO/LB films. At room temperature, the amorphous phase of PVDF-based polymer elec- trolytes was in the glassy state and that of PEO- based polymer electrolytes was in the rubbery state. In the plasticized PVDF-based polymer electrolytes, the PEC in the polymer electrolytes provided an ex- cellent ion conduction path through the amorphous
PEC domain since the PEC-salt complex bond is weak in the PVDF-based polymer electrolytes. The chemical structure similarity and the low Tg and low T , of the crystalline phase made the conductivity
of plasticized PEO-based polymer electrolytes less sensitive to the PEC content.
E 0 \ v) Y > Y V 3 -u I2 0 0 .- .A
3
-3 -4 -5 -60
CF4LA*
CF7LA1
-7 0 20 40Polymer / salt ratio
0
Figure 9 Dependence of ionic conductivity at 30°C on LiBF3C02 salt content at a constant ratio of PVDF/PEC -
h -+ .d .r( c1 V z
s
*
8
4
-6- -6 -2 [1
- \ \ ‘h-‘--
‘
I I 1 I II
*
CF7LB cF4LBI
Polymer / s a l t ratioFigure 10 Dependence of ionic conductivity at 30°C on LiBF, salt content a t a constant ratio of PVDF/PEC
- -
a
or $.The dynamic ionic conductivity of the plasticized PVDF-based polymer electrolyte was less temperature- dependent than that of the plasticized PEO-based polymer electrolytes. This was because the PEO-based films have low T,’s (below 100°C) and PVDF-based films have high T,’s, as high as 150°C. The maximum measurement temperature of the dynamic ionic con- ductivity was 100°C. Therefore, there was no signifi- cant phase transition in the plasticized PVDF-based polymer electrolytes. The highly PEC-plasticized PVDF-based polymer electrolytes showed a maximum room-temperature ionic conductivity as the salt in- creased. It was due to two different effects on the conductivity: an increased number of carrier ions and enhanced polymer chain rigidity with increased salt concentrations. The maximum ionic conductivity of the polymer electrolytes was independent of the PEC content, but was dependent on the anion species of the lithium salt.
The authors would like to express their appreciation to the National Science Council of the Republic of China for financial support on this study and its related patent ap- plication under Grant NSC 81-0405-E-009-07.
REFERENCES 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19.
M. B. Armand, Solid State Zonics, 9 / 1 0 , 745 (1983). M. Watanabe, M. Kanba, H. Matsuda, K. Tsunemi, K. Mizoguchi, E. Tsuchida, and I. Shinohara,
Macromol. Chem. Rapid Commun., 2, 741 (1981).
M. L. Kaplan, E. A. Reitman, and R. J. Cava, Polymer, 3 0 , 504 (1989).
J. Plocharski, J. Przyluski, W. Wiexzorek, and K. Such, Appl. Phys. A , 4 9 , 5 5 (1989).
M. Watanabe, M. Kaanba, K. Nagaoka, and I. Shi- nohara, J. Polym. Sci. Polym. Phys. Ed., 2 1 , 939
(1983).
B. Scrosati, Ed., Second International Symposium o n
Polymer Electrolytes, Elsevier, New York, 1990.
F. M. Gray, in Polymer Electrolyte Reviews I , J. R. MacCallum and C. A. Vincent, Eds., Elsevier, New York, 1987.
J. R. Owen, in Electrochemical Science and Technology
of Polymers, R. G. Linford, Ed., Elsevier, New York,
1989.
M. Watanabe, M. Rikukawa, K. Sanui, N. Ogata, H. Kato, T. Kobayashi, and Z. Ohtaki, Macromolecules, 17, 2902 (1984).
R. Dupon, B. L. Papke, M. A. Ratner, and D. F. Shriver, J . Electrochem. Soc., 1 3 1 , 586 (1984).
M. Watanabe, M. Togo, K. Sanui, N. Ogata, T. Ko- bayashi, and Z. Ohtaki, Macromolecules, 1 7 , 2908 (1984).
R. D. Armstrong and M. D. Clarke, Electrochim. Acta, 2 9 , 1443 (1984).
K. R. Baldwin, A. J. Golder, and J. Knight, RAE Technical Report 84036, 1984.
S. Clancy, D. F. Shriver, and L. A. Ochrymowycz,
Macromolecules, 1 9 , 6 0 6 (1986).
E. Tsuchida, H. Ohno, and K. Tsunemi, Electrochim.
Acta, 28(5), 591 (1983).
K. Tsunemi, H. Ohno, and E. Tsuchida, Electrochim.
Acta, 28(6), 833 (1983).
E. Tsuchida, H. Ohno, K. Tsunemi, and N. Kobayashi,
Solid State Zon., 1 1 , 227 (1983).
I. E. Kelly, J. R. Owen, and B. C. H. Steele, J . Power Sources, 1 4 , 13 (1985).
I. E. Kelly, J. R. Owen, and B. C. H. Steele, J. Elec- troanal. Chem., 1 6 8 , 4 6 7 (1984).
Received October 18, 1994