Effect of Poly (vinylidene fluoride) on the Ionic Conductivity
and Morphology
of
PEO-Salt Polymer Electrolytes
WHA-TZONG WHANG,* 11-HUY YANC, and YU-WEN FAN
Institute of Materials Science and Engineering, National Chiao Tung University, Hsin Chu 300, Taiwan, Republic of China
SYNOPSIS
The high dielectric constant polymer poly (vinylidene fluoride) (PVDF) was blended with PEO-salt polymer electrolytes to improve their ionic conductivity. PVDF did improve the room-temperature ionic conductivity of the polymer electrolytes PEO-LiCF3C02 and PEO- LiBFI, and the ionic conductivity at room temperature went up one order of magnitude with lO-'S/cm for LIB4M-PF4 being the most prominent. PVDF exhibited strong effects on the ionic conductivity and morphology of the polymer electrolytes, such as weakening the association of poly(ethy1ene oxide) ( P E O ) with the salt, hindering the formation of the PEO-salt complex, enlarging the crystalline PEO phase in LIAnM-PFm polymer elec- trolytes, and reducing the crystalline PEO phase in LIBnM-PFm polymer electrolytes. Both LIA4M-PF4 and LIB4M-PF4 polymer electrolytes formed a new PEO-PVDF-salt complex. The new complex in the former was crystalline, but that in the latter was amor- phous. 0 1994 John Wiley & Sons, Inc.
INTRODUCTION
Since the discovery of high ionic conductivity from blending PEO with potassium salts by Fenton et al.,' polymer electrolytes have attracted a lot of in- terest, especially in thin film batteries. Polymer electrolytes consist of polar polymers and ionizable salts. The Li' ion is the smallest metal ion, and lithium salts are among the most favored salts in polymer electrolytes. PEO is the most popular poly- mer used, due to its high solvating power with metal ions, good processability, and mechanical proper- Although the ionic conductivity of the PEO-salt polymer electrolytes at 100°C can be
up to
-
10-3S/cm, the conductivity a t roomtemperature is poor, usually hardly higher than 10-'S/cm. Therefore, much effort has been devoted to improving the ambient ionic conductivity of the polymer electrolytes by improving the cation's mi- gration in the amorphous domain or in the helical structure of the crystalline. Since ions primarily
* To whom correspondence should be addressed. Journal of Applied Polymer Science, Vol. 54, 923-933 (1994)
0 1994 John Wiley & Sons, Inc. CCC 0021-S995/94/070923-11
transport in the amorphous phase, many investi- gations aimed a t improving ionic conductivity have focused on increasing the amorphous phase of the material. The ambient ionic conductivity can also be improved by introducing flexible chains into the copolymer main chain7 or by adding ceramic powder.'
Munshi' and Rietman lo reported that the anions
of the salts strongly influenced the conductivity be- havior of the polymer electrolytes with the formation
of ion-pairs resulting in a decrease of conductivity
a t high salt concentration. Also the energy required to separate the ion-pair was inversely proportional to the dielectric constant of the medium, which can be as low as 5 in the PEO-salt polymer electrolyte system. When the salt concentration exceeded the threshold point, the ionic conductivity began to de- crease with salt concentration, due to the formation of ion-pairs. PVDF, a polymer with the high dielec-
tric constant E' = 8 - 13, has been used to prepare
polymer electrolytes."-'2 The ionic conductivity of PVDF-salt polymer electrolytes was somewhat lower than that of PEO-salt polymer electrolytes. We surmise that the stronger bonding of the metal ion to the fluorine atom than to the oxygen atom 923
924 WHANG, YANG, AND FAN
reduced the metal ion mobility in PVDF-salt poly- mer electrolyte, resulting in less conductivity than expected.
In this study, PVDF was blended as a minor com- ponent with PEO and salt to improve the ionic con- ductivity of the polymer electrolytes. T h e salts de- scribed in this paper include a n organic salt and a n inorganic salt. Both PVDF and PEO are polar poly- mers and have some compatibility with each other. We expected that the blending of PVDF with PEO might increase the amorphous phase and the di- electric constant of the medium, keeping the PEO contribution t o the ionic conductivity.
EXPERIMENTAL
T h e polymer electrolyte films containing lithium salts were prepared by casting the polymer electro- lyte solutions on surface-treated glass plates. The
lithium salts LiCF3C02 and LiBF4, acetonitrile, and
PEO ( molecular weight 200,000) were commercially
available from Aldrich. PVDF was obtained from Dynamit Nobel. Before preparing polymer electro- lytes, the lithium salts were dried in a vacuum oven
a t 90°C for 24 hours, and the polymers PEO and
PVDF were also vacuum-dried a t a temperature be- low the melting point of the polymers for 24 hours. Both the polymers and the salts were weighed in a
nitrogen dry box a t the desired molar ratios, denoted
as "n7" of monomeric units of both PEO and PVDF t o that of the salts. T h e "n" values were equal to 16 and 4 in this study. Acetonitrile was used a s a sol- vent. T h e polymer electrolyte solutions were stirred
for 48 hours to make them homogeneous. The molar
ratios of the monomeric units of PEO t o that of PVDF, denoted as "m,7' were 4,9, 15,24, and 49. In this paper the polymer electrolytes are denoted as follows:
Polymer Electrolyte Abbreviation
PEO-LiCF3C02 PEO/PVDF-LiCF,CO, LIAnM-PFm, PEO-LiBF4 LIBnM PEO/PVDF-LiBF4 LIBnM-PFm LIAnM, n = 16 or 4 m = 4, 9, 15, 24, 49
in which LIAnM and LIBnM represent the polymer electrolytes composed of polymer matrix PEO and
salt LiCF3C02
,
and of PEO and LiBF4, respectively;and PF represents the incorporation of the second polymer matrix PVDF.
After casting on the glass plates, the samples were vacuum-dried a t room temperature for 24 hours and
then a t 70°C for 30 hours. Dry air was used to release
the vacuum and the samples were immediately cov- ered and stored in a n electronic desiccator until tested. The sample preparation was handled with care in the dry box to exclude interference from wa- ter in air. The balance was also placed in the dry box for weighing. The dry box was first evacuated and then refilled with dry air, the operation was re- peated several times, and finally the box was further
dried with P205 powder.
The thermal transitions of the polymer electrolyte films were measured with a Seiko differential scan- ning calorimeter ( D S C ) a t 10"C/min. in a dry ni- trogen stream of 50 cc/min. The samples were pre- heated in the DSC cell a t 120°C for 5 minutes in the dry nitrogen stream t o evaporate any condensed water on site and then cooled to 0°C for DSC mea- surement. The dynamic ionic conductivity mea- surements of the solid polymer electrolytes were performed on a DuPont 2970 dielectric analyzer a t
300KHz. This test was scanned a t 3"C/min. under
a dry nitrogen flow of 500 cc/min. I t is generally
accepted that the high-frequency response of the polymer electrolytes in a n alternating electrical field reveals information on the electrical properties of the electrolytes themselves. The X-ray diffraction spectra were measured using a Siemens D5000 X- ray diffractometer. T h e X-ray sample chamber was
first dried with Pz05 powder overnight before a sam-
ple was inserted for measurement.
RESULTS A N D DISCUSSION
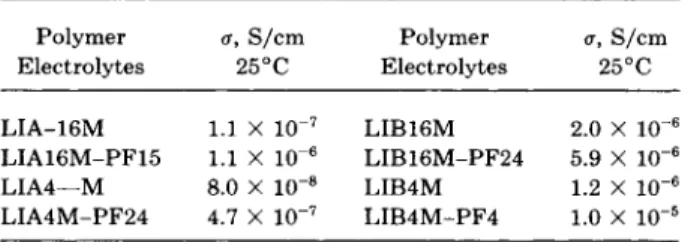
T h e ionic conductivities of PEO-salt polymer elec- trolytes with or without PVDF at room temperature are listed in Table I. T h e addition of PVDF to PEO- salt polymer electrolytes improved the room tem-
perature ionic conductivity by a factor of 2 to 9,
depending on the types and the concentration of salts. LIB4M-PF4 exhibited the highest conductiv-
ity of 10-5S/cm (at 25°C) among the tested samples.
Table I Electrolytes
The Ionic Conductivity of Polymer
Polymer a, S/cm P o 1 y m e r a, S/cm Electrolytes 25°C Electrolytes 25°C
2.0 x 10-6 LIA4-M 8.0 X lo-@ LIB4M 1.2 x 10-6 LIA4M-PF24 4.7 X LIB4M-PF4 1.0 x 10-5
LIA-16M 1.1 X LIB16M
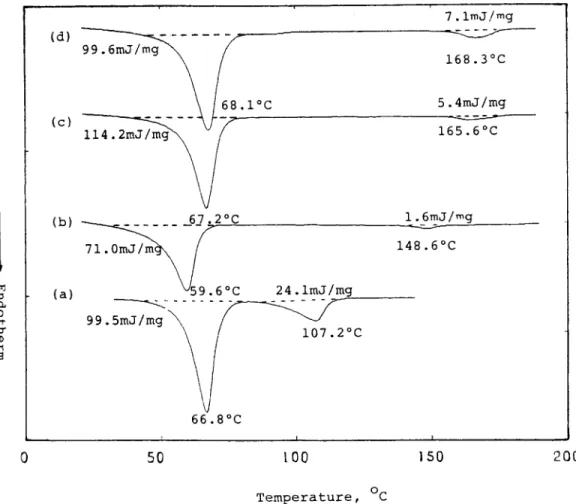
PEO-SALT POLYMER ELECTROLYTES 925 7.1mJ!mg 168.3"C 68.1OC 5.4mJ/mg 165.6OC 1.6mJ/mg ( b ) - - - 148.6OC ( a )
\I
66.8"C 0 50 100 1 5 0 2 0 0 Temperature, OCDSC thermograms of polymer electrolytes ( a ) LIAlGM, ( b ) LIA16M-PF24, Figure 1
( c ) LIA16M-PF15, and ( d ) LIA16M-PF9.
The cast sample of LIB4M is solid and non-sticky, but that of the LIB4M-PF4 films is soft and sticky even after drying in a vacuum oven for several days. No solvent loss was found on this sample by TGA. As PVDF is a semicrystalline polymer melting above 150"C, the softening of LIB4M-PF4 is not due to the inherent character of PVDF. It might be believed that PVDF exhibited strong interaction with PEO and LiBF4 and caused a strong effect on the mor- phology of the polymer electrolytes, which in turn affected the ionic conductivity.
The Thermal Transitions of PEO-salt Electrolytes
The DSC thermograms and data in Figures 1, 2, 4,
and 5 display three different thermal transitions in the polymer electrolytes. The lowest melting point (near 55-70°C) was from the crystalline PEO phase, the second melting point (near 105-130°C) was from the crystalline PEO-Li+ complex phase, and the
third melting point (near 150-170°C) was from the
crystalline PVDF phase. The addition of PVDF to
LIA16M polymer electrolyte resulted in the disap- pearance of crystalline PEO-LiCF3C02 complex (in
Fig. 1 ) , demonstrating that PVDF reduced the as-
sociation of PEO with the organic salt LiCF3C02 and disintegrated the crystalline phase in LIA16M. In these LIA16M-PFm polymer electrolytes the crystalline PVDF phase increases with the PVDF concentration, but the effect of PVDF on the crys- talline PEO phase is complicated and concentration- dependent. The PEO crystalline phase in LIA16M-
PF24 was less than that in LIA16M. More crystal-
line PEO phase was present in LIA16M-PF15 than in LIA16M-PF24 or even in LIA16M. The crystal- line PEO phase in LIA16M-PF9 was more than that in LIA16M-PF24 but less than that in LIA16M- PF15. We postulate that the blending of PVDF in
LIA16M-PFm leads to two competitive effects: ( 1 )
the disintegration of the original crystalline PEO phase by introducing PVDF into the polymer elec- trolyte, and ( 2 ) the formation of new PEO phase (both crystalline and amorphous) due to weakening the association of the PEO-salt complex (amor-
926 WHANG, YANG, A N D FAN
0 5 2 . 5 I05 1 5 7 . 5 2 1 0
0
Temperature, C
Figure 2
LIA4M-PF24, and ( d ) LIA4M-PF15, and ( e ) LIA4M-PF4.
DSC thermograms of polymer electrolytes ( a ) LIA4M, ( b ) LIA4M-PF49, ( c ) 7.1mJ/mg
- - -
* 4mJ/mg 4 9.
o-mJ_/_mq\&---
_ - -
-- (d)-6.9OC
\
7
128.7OC 162.8OCphous phase only; no crystalline phase was found in LIA16M-PFm) by increasing the dielectric constant of the polymer electrolytes with PVDF concentra- tion. This complication was also revealed in the se- ries of LIA4M-PF49, LIA4M-PF24, and LIA4M- PF15. According to Fedors13, one can estimate the solubility parameters of polymers by means of the group contributions to the cohesive energy and the molar volume: it is found that the solubility param- eters of PEO and PVDF are close to each other with 19.17 ( J / c m 3 ) 'I2 for PEO and 19.15 ( J / c m 3 ) ' I 2 for PVDF; and that the molar volumes of these two polymers are also close, with 36.0 cm3/mole for PEO and 36.9 cm3/mole for PVDF. This means that PEO and PVDF have excellent miscibility and good space fitting with each other. When PVDF was incorpo- rated into the PEO-salt polymer electrolytes, both the excellent polymeric miscibility and good space fitting of these two polymers easily caused the destruction of the PEO crystallinity and the en-
hancement of the dielectric constant to weaken the
association of PEO-salt complex and to raise the conductivity.
Thermal characteristics of LIA4M-PFm polymer
electrolytes are shown in Figure 2. The crystalline
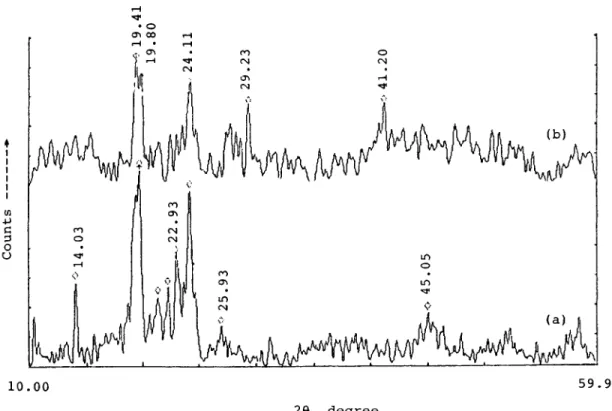
PVDF phase increased with the PVDF content but the crystalline PEO-LiCF,CO, complex phase first decreased, with PVDF content changing from PF49 to PF15 and then increasing a t PF4. However, the crystalline PEO phase of LIA4M-PFm went up to a maximum a t PF24 and then vanished a t PF4. T h e abnormal AHm of LIA4M-PF4 (melting peak at 128.7'C) might be due t o the melting of a new
crystalline PEO
/
PVDF/ LiCF3C02 complex, whichhas been confirmed by X-ray diffraction spectra in
Figure 3. The peaks a t 20 = 19.4' - 20.0" were the
characteristic peaks of the crystalline PVDF phase. T h e X-ray spectrum of LIA4M-PF4 showed two new peaks a t 29.23' and 41.20", which did not appear in the spectrum of LIA4M-PF49. The X-ray spec- trum of LIA4M-PF4 was quite different from that of LIA4M-PF49 except for the crystalline PVDF peaks. This might mean that there was a new crys- talline complex in LIA4M-PF4.
T h e DSC thermogram data on the thermal tran- sitions of LIB16M-PFm and LIB4M-PFm are
t
I I I I I (0 c, c 3 0 u In 0 In -f 10.00 5 9 . 9 5 28, degreeFigure 3 X-ray diffraction spectra of the polymer electrolytes ( a ) LIA4M-PF49 and ( b ) LIA4M-PF4.
0
1 5 6 . 6 " C 3.2mJ/mg 166 . l 0 C 5 0 1 0 0 150 Temperature, 0 c 2 0 0 Figure 4( c ) LIB16M-PF15, and ( d ) LIB16M-PF4.
928 WHANG, YANG, A N D FAN I I I I I I I -t I .f M 3 a 0 rt D- I D .
;I
1 6 5 . 2 " C 109.9"C 2 0 6 5 1 1 0 1 5 5 2 0 0 0 Temperature, c Figure 5( c ) LIB4M-PF15, and ( d ) LIB4M-PF4.
DSC thermograms of polymer electrolytes ( a ) LIB4M, ( b ) LIB4M-PF24,
shown in Figures 4 and 5, respectively. No PEO- LiBF, crystalline complex phase was found in LIBlGM, but a trace amount was found in LIB4M. A major peak for the crystalline PEO phase was
found in LIBlGM, but not in LIB4M. A higher
PVDF content in the LIB16M-PFm series led to a reduction in the crystalline PEO major peak and an increase in the crystalline PVDF minor peak, with a crystalline complex peak absent. In the LIB4M- PFm series, the crystalline PEO-LiBF4 complex phase of LIB4M-PF24 was much less than that of LIB4M; this phase was not found in LIB4M-PF15
or LIB4M-PF4 polymer electrolyte. No crystalline
PEO phase was found in the three LIB4M-PFm, either. The peak associated with the crystalline PVDF phase in LIB4M-PFm series increased with the PVDF content. The peak a t 151°C in LIB4M- PF4 was much larger. It revealed that the introduc-
tion of PVDF into LIB4M resulted in the disinte-
gration of the crystalline PEO-salt complex and fi-
nally led to the formation of a new PEO-PVDF-
salt complex in LIB4M-PF4, which is sticky. As we
have mentioned elsewhere, l4 the association of PEO
with LiBF4 was much weaker than with LiCF3C02 and the PEO-LiBF4 complex was much less than the PEO-LiCF3C02 complex in the polymer elec- trolytes. The formation of the new PEO phase, due to the dissociation of the PEO-salt complex of the electrolytes by introducing PVDF, became much less important in LIBnM-PFm than in LIAnM-PFm. Therefore, the introduction of PVDF in LIB16M and LIB4M led to a reduction in the crystalline PEO phase and the crystalline PEO-salt complex phase.
The sticky characteristic of LIB4M-PF4 is quite
peculiar. LIB4M is a solid polymer electrolyte. PEO
is highly crystalline with a melting point of 65°C
and a glass transition temperature of -60°C. PVDF is semicrystalline with a melting point higher than 150°C and a glass transition temperature of -40°C. Both polymers have high crystallizability. All the blends of PEO and PVDF ( a t various ratios) exhib-
ited two melting peaks: one corresponded to the
melting of the crystalline PEO phase and the other
PEO-SALT POLYMER ELECTROLYTES
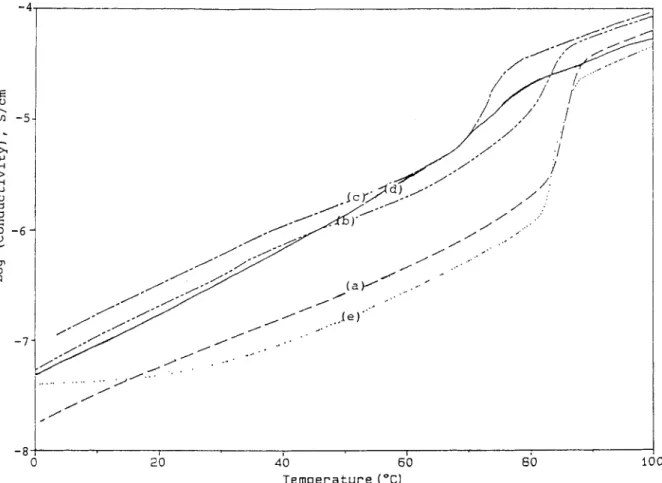
-
- 4 - 8 1 1 I 1 I 0 20 4 C 60 80 1 T e m p e r a t u r e ( “ C ) Figure 6PF24 (-) , ( c ) LIA16M-PF15 ( - - -) , and ( d ) LIA16M-PF9 ( - * -)
.
Ionic conductivities of polymer electrolytes ( a ) LIA16M ( -
-
-) , ( b ) LIA16M-929
I
I
I 10
phase. It is clear that the salt LiBF4 in the polymer electrolyte LIB4M-PF4 assisted the PVDF polymer in disintegrating the crystalline PEO phase and the crystalline PEO-salt complex into amorphous phases, and that sufficient PVDF could make the disintegration complete. In the LIA16M-PFm and LIA4M-PFm series, only LIA4M-PF4 formed a new complex phase. This showed that sufficient amounts of PVDF and salt were required to completely dis- integrate the crystalline PEO and complex in the polymer electrolytes to form a new complex. With the aid of the salt LiBF4 to hinder the crystallization of these two polymers in LIB4M-PF4, the amor- phous PEO phase which was miscible with the amorphous PVDF phase easily mixed to form a new complex phase with the salt. The complex phase was amorphous. The sticky characteristic of LIB4M-PF4 was due to the amorphous PEO and the amorphous PVDF being in the rubbery state a t room temperature. The endothermic peak in the DSC curve of LIB4M-PF4 was attributed to the thermal dissociation between the polymer matrix and the salt in the new complex.
Ionic Conductivity
The effect of PVDF on the ionic conductivity of LIAnM-PFm series is shown in Figures 6-9. At low temperatures, all the polymer electrolytes containing PVDF displayed a higher ionic conductivity than the corresponding polymer electrolytes without PVDF. The definition of “low temperature” de-
pended on the polymer electrolytes: 55°C for
LIA16M-PFm, 75°C for LIA4M-PFm, 35°C for
LIB16M-PFm, > 100°C for LIB4M-PFm. At tem-
peratores higher than 65°C for LIA16M-PFm and 85°C for LIA4M-PFm, the effect of PVDF on the ionic conductivity for these two types of polymer electrolytes became negligible. The low- temperature ionic conductivity of LIA16M-PFm polymer electrolytes followed this order: LIA16M-
PF15 >LIAlGM-PFS > LIA16M-PF24. In the
meantime, the fraction of the crystalline PEO phase in the electrolytes followed the same order. The room-temperature ionic conductivities of LIA4A- PFm followed the same order as the fraction of their
930 WHANG, YANG, A N D FAN
Figure 7 Ionic conductivities of polymer electrolytes ( a ) LIA4M ( - - -) , ( b ) LIA4M- PF49 ( - . .-), ( c ) LIA4M-PF24 (- - - ) , (d) LIA4M-PF15 (-),and ( e ) LIA4M-PF4 ( .
-
.).PF49 > LIA4M-PF15 > LIA4M-PF4. T h e high di-
electric constant PVDF can increase the dielectric constant of LIAnM-PFm polymer electrolytes and weaken the association of the PEO-LiCF3C02 com- plex. The reduction of the PEO-salt association in the polymer electrolytes may have caused the for- mation of some new PEO phase released from the PEO-LiCF3C02 complex and higher ionic conduc- tivity. The fraction of PEO phase might also be used
as a n indicator of the strength of PEO-salt associ-
ation in LIAnM-PFm. T h e greater the fraction of the PEO phase, the lower the strength of the PEO- salt association and the higher the ionic con- ductivity.
T h e effect of PVDF on the ionic conductivities of LIB16M-PFm polymer electrolytes was compli- cated. At low temperature the conductivities fol-
lowed this order: LIB16M-PF4
-
LIB16M-PF24> LIB16M; but at high temperature ( > 40°C) the
conductivities followed the same order as the
fraction of the PEO crystalline phase in the poly-
mer electrolytes: LIB16M-PF4 < LIB16M-PF24
< LIB16M.
T h e addition of PVDF t o LIB16M polymer elec- trolyte improved the low-temperature ionic conduc- tivity, possibly due to a n increase in the dielectric constant of the matrix and a reduction in the as- sociation of PEO with the salt. However, the ionic association in LIBnM polymer electrolytes was much weaker than that in the corresponding LIAnM polymer electrolytes, making them insensitive to the presence of more PVDF within the homologous se- ries. This explains why the low-temperature ionic conductivities of LIB16M-PF24 and LIB16M-PF4 are similar. At temperatures higher than 45°C the crystalline PEO phase melted and changed to amor- phous phase, but PVDF did not melt until above 150°C. Therefore the polymer electrolyte hav- ing the more amorphous PEO phase gave higher
ionic conductivity: LIB16M > LIB16M-PF24
In LIB4M-PFm homologues, all the polymer electrolytes containing PVDF exhibited higher ionic
conductivity than LIB4M (shown in Figure 9),
which did not contain PVDF, in the testing tem- perature range of 0°C to 100°C. It was found that > LIB16M-PF4.
PEO-SALT POLYMER ELECTROLYTES - 3 I
/
I /
- 7!
' I I I 1 0 20 40 6 0 8 0 1 T e m o e r a t u r e ("C) Figure 8 PF24 ( - ) , a n d ( c ) L I B l G M - P F 4 ( - - * * - - ) .Ionic conductivities of polymer electrolytes ( a ) LIB16M ( - - -) , ( b ) LIB16M-
93 1
0
the ionic conductivity of LIB4M-PFm polymer electrolytes increased with the PVDF concentration at temperatures from 0°C to 100°C except a t tem- peratures higher than 80"C, where the ionic con- ductivity of LIB4M-PF24 polymer electrolyte crossed a bit over that of the LIB4M-PF15 sample. As shown in Figure 5, the crystalline PEO-salt complex was found in LIB4M, but it disappeared in LIB4M-PF15 and LIB4M-PF4. PVDF promoted the dielectric constant of LIB4M-PFm polymer electrolytes and reduced the association of PEO- salt complex; therefore, the ionic conductivity of LIB4M-PFm increased with the PVDF concentra- tion. LIB4M-PF4 even became amorphous and sticky. Owing to its peculiar and favorable mor- phology, LIB4M-PF4 exhibited the best room tem- perature ionic conductivity of 10-5S/cm.
CONCLUSION
PEO and PVDF have excellent miscibility and good space fitting with each other. When PVDF was in-
corporated into the PEO-salt polymer electrolytes, both the excellent polymeric miscibility and good space fitting of these two polymers easily caused the destruction of the PEO crystallinity and the en- hancement of the dielectric constant to weaken the association of PEO-salt complex and to raise its conductivity. The addition of PVDF to PEO-salt polymer electrolytes significantly improved the room
temperature ionic conductivity by a factor of 2 to 9.
The highest conductivity was 10-5S/cm for LIB4M- PF4. The association of PEO with LiBF4 was much
weaker than with LiCF3C02, and PEO-LiBF4 com-
plex was much less than PEO-LiCF3C02 complex in the polymer electrolytes. The formation of the new PEO phase due to the dissociation of PEO-salt complex of the electrolytes by introducing PVDF became much less important in LIBnM-PFm than in LIAnM-PFm. Therefore, the introduction of PVDF in LIB16M and LIB4M led to a reduction in the crystalline PEO phase and the crystalline PEO- salt complex phase, but in the LIAnM-PFm series the effect of PVDF on the crystalline PEO phase was more complicated.
932 WHANG, YANG, AND FAN - 3
,
0 20 40 60 8 0 100 T e m p e r a t u r e ( " C ) Figure 9 PF24 ( - - - - ) , ( c ) LIB4M-PF15 ( - ) , a n d L I B 4 M - P F 4 ( - - - ) .Ionic conductivities of polymer electrolytes ( a ) LIB4M ( - * -) , ( b ) LIB4M-
Both the LIAnM-PFm and LIBnM-PFm series with a sufficient amount of PVDF ( P F 4 ) and salt ( 4 M ) formed a new complex. The new complex of LIA4M-PF4 was crystalline, but that of LIB4M- PF4 was amorphous and sticky. In addition to the effect of blending on reducing the crystallization of the polymer matrices, LiBF4 salt might also assist in hindering the crystallization of these two polymer matrices. At room temperature the amorphous PEO and PVDF are in the rubbery state. The amorphous PEO is miscible with the amorphous PVDF and the two easily mixed with each other to form one new complex with the salt. LiCF,CO, interacted with the
polymer matrices more strongly than LiBF4, so it
formed crystalline complexes with the polymer ma- trices more easily than LiBF4. The high room-tem- perature conductivity of LIB4M-PF4 was related to its amorphous and sticky morphology.
The fraction of PEO phase could be used as an
indicator of the strength of the PEO-salt association
in LIAnM-PFm. The lower the PEO-salt associa- tion, the higher the fraction of the PEO phase. The ionic conductivity of LIAnM-PFm increased with
the fraction of the PEO phase. LIBnM polymer
electrolytes had much weaker association of PEO- salt than LIAnM polymer electrolytes. The ionic conductivity of LIB16M-PFm was less sensitive to the presence of more PVDF within the homologous
series than that of LIAnM-PFm. A t room temper-
ature the ionic conductivity of LIB16M-PF24 was close to that of LIB16M-PF4. In the LIB4M-PFm series, the ionic conductivity increased with the PVDF content. The high room-temperature con- ductivity of LIB4M-PF4 is related to its peculiar morphology.
The authors would like to express their appreciation to the National Science Council of the Republic of China for financial support of this study under grant NSC 81-0405- E-009-07.
PEO-SALT POLYMER ELECTROLYTES 933
REFERENCES
1. B. E. Fenton, J. M. Parker, and P. V. Wright, Polym.
J ., 1 4 , 589 (1973).
2. M. L. Kaplan, I. E. Reitman, and R. J. Cava, Polym. J., 30, 504 (1989).
3. M. Gauthier, M. Armond, and D. Muller, “Aprotic Polymer Electrolytes and Their Applications,” in Electroresponsive Molecular and Polymeric Systems, Terje and A. Skotheim, Eds., Marcel Dekker, New York, 1988.
4. R. G. Linford, Electrochemical Science and Technology
of Polymers, Vol. I, Elsevier Applied Science, New York, 1987.
5. J. R. MacCallum and C. A. Vincent, Polymer Electro- lyte Reviews I, Elsevier Applied Science, New York,
1987.
6. J. R. MacCallum and C. A. Vincent, Polymer Electro- lyte Reviews II, Elsevier Applied Science, New York,
1989.
7. M. C. Wintergill, J. J. Fontanella, M. K. Smith, S. S.
Greenbaum, K. J. Adame, and C . C. Andeen, Polym.
J., 2 8 , 6 3 3 ( 1987).
8. J. Procharski, W. Wieczorek, J. Przyluski, and K. Such, Appl. Phys., A 4 9 , 5 5 (1989).
9. M. Z. A. Munshi and B. B. Owens, Polym. J., 2 0 , 5 7 7 (1988).
10. E. A. Rietman, M. L. Kaplan, and R. J. Cava, Solid State Ionics, 1 7 , 67 ( 1985).
11. K. Tsunemi, H. Ohno, and E. Tsuchida, Electrochim. Acta, 2 8 , 8 3 3 (1983).
12. E. Tsuchida, H. Ohno, and K. Tsunemi, Electrochim. Acta, 2 8 , 5 9 1 ( 1 9 8 3 ) .
13. D. W. Van Krevelen, “Cohesive Properties and Sol- ubility” in Properties of Polymers, Elsevier, New York, 14. L. H. Yang, Master’s Thesis, National Chiao Tung
1976, pp. 138-140. University, 1991.
Received December 16, 1992 Accepted March 25, 1994