Face selectivity in the reactions of 2,4-disubstituted adamantanes
and their modification by inclusion in b-cyclodextrin solutions
Jean-Ho Chu,
aWan-Sheung Li,
bIto Chao
b,* and Wen-Sheng Chung
a,*
a
Department of Applied Chemistry, National Chiao Tung University, Hsinchu 30050, Taiwan, ROC
b
Institute of Chemistry, Academia Sinica, Taipei 11529, Taiwan, ROC Received 27 April 2004; revised 9 July 2004; accepted 21 July 2004
Abstract—Sodium borohydride reduction reactions on 4-X-adamantan-2-ones (where XZethyleneketal 11, ethylenethioketal 12, and
methylene 15) were studied, which gave Z-alcohols 16 and 17 (from en-face attack) as the predominant products for ketones 11 and 12, but gave 1:1 mixture of Z- and E-18 alcohols for ketone 15. The en/zu face selectivity of 15 in sodium borohydride reduction was enhanced to 32/ 68 in b-CD solution. Both 1,3-dipolar addition and dichlorocarbene addition reactions on 4-ethyleneketal-2-methyleneadamantane 13 underwent again predominant en-face attack to give products in an E/Z ratio of O99:1 and 92:8, respectively. The exceptional high zu-face
selectivity on the dichlorocarbene addition reaction of 15 may be explained by a temporal complexation between the carbene and the C4-oxo
group. In the epoxidation reaction of 13 and 15 the zu-face attack products were favored despite their steric congestions suggesting that
hydrogen bonding interaction between the peroxide reagent and the C4-oxo or 4-ethyleneketal is involved.
q2004 Elsevier Ltd. All rights reserved.
1. Introduction
Many experimental probes have been devised to identify the various steric and electronic factors that influence p-facial selectivity in nucleophilic, electrophilic, and cycloaddition reactions, among them, sterically unbiased systems offer intrinsic advantage in isolating and evaluating electronic effects.1–4Relatively fewer studies have been reported on the reactions of 4-substituted adamantan-2-ones 1- and 2-X comparing to the very popular and more thoroughly studied 5-substituted adamantan-2-ones 3-X. One barrier for using 4-substituted-adamantan-2-ones 1- and 2–X is the multi-step syntheses involved in the preparation of these probes. Furthermore, an axial (but not equatorial) 4-substituent is expected to have a strong steric influence on the chemical reactivity of a nearby trigonal center; rendered it difficult in studying pure electronic effects. Despite the difficulties involved, there are some scattered reports5,6 on the face selectivity of 4-substituted adamantanes.
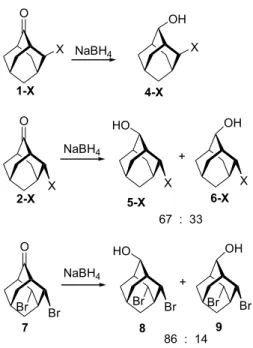
The steric effect of an axial substituent makes itself felt with even the smallest fluoro substituent: sodium borohydride attacks 1-F exclusively at the en face to give the pure diaxial alcohol 4-F. Similar data were found for the reduction of 1-Br.5,6 An equatorial fluoro substituent in 2-F, however, give the diequatorial alcohol 5-F and isomer 6-F in a ratio of 67:33 which resembles the face selectivity in 3-F.1aIn the sodium borohydride reduction of 7, adamantan-2-one with two equatorial b-bromosubstituents, a higher face selectivity was found (8:9Z86:14) and the results were reconciled with Cieplak’s model (seeChart 1).1a,5
Adamantane derivatives have received considerable atten-tion because of their diverse biological activity;7especially when substituted with spiro-cyclopropane or spiro-pyrroli-dine groups, they are known to have antiviral activity.7cWe report here facile syntheses of some 2,4-disubstituted adamantanes 11–15 and face selectivity studies on these probes that yielded various spiro acetals, cyclopropanes, oxiranes, and isoxazolines. Despite the difficulties in
0040–4020/$ - see front matter q 2004 Elsevier Ltd. All rights reserved. doi:10.1016/j.tet.2004.07.075
Keywords: Face selectivity; 2,4-Disubstituted adamantanes; b-Cyclodex-trin; Inclusion complex; Neighboring group participation.
dissecting electronic effects from these sterically biased probes, we found evidences to support ‘neighboring group participation’ in some of the reactions, furthermore, an unexpected syn-face enhancement in sodium borohydride reduction reactions of a 15$b-CD complex is observed.
2. Results and discussion
Adamantane-2,4-dione 10 was first synthesized by Wynberg8in 1968 and latter by McKervey9and Duddeck10 all through multiple-step syntheses. In 1985 Gilbert reported11a a direct oxidation of adamantan-2-one 3-H by CrO3 in acetic anhydride gave 10 in 20% yield. We
followed the procedures by Gilbert and obtained a good
yield (typical yields are in 37–50% range) of 10.11b Compounds 11 and 12 were prepared through the protection of carbonyl group by ethylene glycol and 1,2-ethanedithiol, respectively. Compounds 13 and 14 were prepared in high yields by the Wittig reaction of 11 and 12, respectively. The acid catalyzed deprotection of 13 gave 15 in 85% yield. The synthetic pathways are outlined inScheme 1.
Sodium borohydride (or LAH) reduction of 4-ketaladaman-tan-2-one 11 or 4-thioketal-adaman4-ketaladaman-tan-2-one 12, through en-face attack by hydride, gave Z-alcohols (Z-16 or Z-17) as the only products. On the other hand, the reduction of 4-methyleneadamantan-2-one 15 gave Z- and E-18 alcohols as a 1:1 mixture (Scheme 2andTable 1). The reduction of 11that led to Z-16 as the only product has been reported in literature.12a Similarly, the major reduction product of thioketal-12 is expected to be Z-17 due to severe steric hindrance caused by the 4-thioketal group. The configur-ation of the reduction products Z- and E-18 can be easily judged from their 1H NMR spectra where the 4-methyl-eneprotons show two doublets (AB pattern) in Z-18 but a singlet in E-18 due to the magnetic anisotropy effect exerted by the 2-hydroxy group. Furthermore, the structure of Z-18 can be independently synthesized from the acid-catalyzed deprotection of Z-16 to Z-19 followed by a Wittig reaction12bto give Z-18 exclusively (seeScheme 3). Thus, 4-methylene group seems to play no effect on the reduction
Chart 1. Scheme 2.
Table 1.Sodium borohydride and lithium aluminum hydride reduction reactions of 4-substituted-adamantan-2-ones 11, 12, and 15
Compound Reaction conditionsa
Z/E ratiosb Isolated yield,
% 11 NaBH4/MeOH 16(O99:1) 98 98
11 LiAlH4/THF 16(O99:1) 70 c
12 NaBH4/MeOH 17(O99:1) 98
12 LiAlH4/THF 17(O99:1) 71
15 NaBH4/MeOH 18(51:49) 98
15 LiAlH4/THF 18(49:51) 73 a
Reaction was carried out at 25 8C for 1 h.
b Note that en attack of hydride leads to Z-alcohol. Product ratios were
analyzed by GC and the error bars were estimated to be G2%.
c Data is consistent with that reported in Ref.12a.
of 4-methylene-adamantan-2-one 15. Similar results have been reported by Duddeck10c where tert-butyllithium addition of 15 gave a 1:1 mixture of E- and Z-alcohols. To our delight, the face selectivity on the reduction of 15 can be altered by inclusion of itself into b-CD cavity, but the results are opposite to our expectation based on previous model of 3-X in b-CD13a(vide infra).
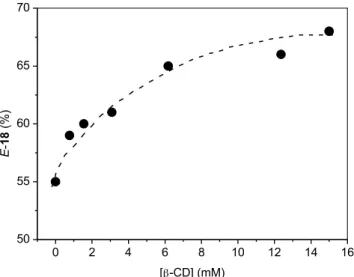
The 50/50 en/zu face selectivity of the sodium borohydride reduction of 15 in THF or methanol becomes 45/55 in water. The effect of b-CD complexation on the en/zu selectivity of 15in sodium borohydride reduction is shown inFigure 1, which reaches a maximum value of 32/68 at 15 mM of b-CD. The yields of Z- and E-18 alcohols from these reactions were in the range of 75–82% when b-CD was below 3 mM, but slightly decreased to 70–73% when b-CD concentration was above 6 mM. The product ratio varies with the concentration of b-CD in the way expected from the fact that saturation will be approached if the concen-tration of b-CD is made sufficiently high.13,14Based on the binding constants (260 MK1) of 15 with b-CD (vide infra) and assuming a 1:1 complex, one can calculate the percentage of compound 15 bound by b-CD to be 75% if the starting concentration of 15 is 5 mM and b-CD is 15 mM. Accordingly, after correcting for the unbound 15 the theoretical value of en/zu face selectivity in the reduction of 15$b-CD should be 22:78 instead of the observed 32:68. The enhanced zu-face attack in 15$b-CD complex is surprising because one would have expected the opposite had its conformation been similar to that of the reported 3-X$b-CD.13aIn order to gain some insights on the structures of 15$b-CD complexes, both1H NMR titration experiments and molecular dynamic calculations were carried out (see Supporting Information).
Evidences for complexation of 15 by b-CD were obtained from 1H NMR spectra, which show that H3
(DdZK0.055 ppm) and H5 (DdZK0.087 ppm) of b-CD
(which are oriented toward the interior of the CD cavity) are shifted upfield considerably in the presence of 15. By contrast, H1, H2, and H4, all located on the exterior wall of
CD, either have small downfield shifts or are unaffected (Fig. 2).15 On the other hand, the H10, H30, and H50 of
4-methyleneadamantan-2-one 15 are substantially down-field shifted in the presence of b-CD and their chemical shift difference Dd is: C0.18, C0.11, and C0.13 ppm, respect-ively (Fig. 3).15These observations are consistent with the notion that a complex is formed between b-CD and 15 and they most likely have 1:1 stoichiometric ratio, similar to those of adamantane derivatives found in several X-ray crystallography data.16The binding constant for complexes of 15 with b-CD was determined to be 260G20 MK1by Benesi–Hilderbrand plot (Figs. S-1 and S-2),14,17where the reciprocal chemical shift differences of guest 15 are plotted with the reciprocal concentration of b-CD.
Four of the most likely conformations of 15 in b-CD are shown inChart 2and they are complexes A–D. The results of sodium borohydride reduction reactions on the complex of 15 in b-CD is out of our expectation, because if complexes C and D are the major conformations (similar to those reported for 5-substituted-adamantan-2-ones 3-X$b-CD)13aone would expect that predominant Z-alcohol 18 be
Figure 1.The percentage E-18 product obtained in the sodium borohydride reduction reactions of 15 (5 mM) in aqueous solution as a function of added b-CD.
Figure 2.Effects of 15 on the1H NMR spectra of b-CD in D2O; where the
concentration of 15 was fixed at 5 mM but the concentration of b-CD decreased gradually from bottom (15 mM) to top (2 mM). Spectra were measured at 300 K.
Figure 3.Effects of b-CD on the1H NMR spectra of 15 (5 mM) in D 2O
solution; where the concentrations of b-CD increases gradually from bottom (0 mM) to top (15 mM). The signals of protons on the C4
-methylidene of 15 were buried in the huge water peak and were omitted. Spectra were measured at 300 K.
formed. On the contrary, E-alcohol 18 became the major product when 3 equiv. of b-CD vs. 15 was used. Alternatively, if complexes A and B are the major conformations of the 15$b-CD complexes, one may easily explain why more E-18 was formed at high [b-CD] because the torus of b-CD protects the en-face of 15 from hydride attacks. Theoretical calculations were thus carried out to gain more insight about the conformations of 15$b-CD complexes.
Snapshots from the MD simulations showed that preferred complexes are C and D, both with the hydrophobic methylidene groups pointing towards the CD cavity. The results are in accord with the previous proposed model 3-X$b-CD, where a dramatic reversal in face selectivity was achieved by partial blockage of the p-face zu to the bulky 5-substituent of a 3-X$b-CD complex by the CD host.13a Thus, the results from the simulations would predict the reduction reaction to yield the Z-18 alcohol as the dominant product by partial blockage of the zu-face of 15 from hydride attack. Yet, the predominant formation of the E-18 may indicate that b-CD has mediated the reaction through hydrogen bonding interaction of its hydroxyl groups with the metal hydride; it therefore favors a zu-face attack.17b The 1,3-dipolar cycloaddition reactions of the 4-substituted-2-methyleneadamantane 13–15 with benzonitrile oxide
were studied next (Scheme 4).18 Only E-isoxazolines 20 and 21 were formed in the reaction of 13 and 14, whereas, a 1:1 mixture of E- and Z-isoxazolines 22 were obtained in the reaction of 15 (Table 2). The E-adducts 20 and 21 were obtained from the expected attack of benzonitrile oxide on the less-hindered side, namely, the en-face that is opposite to the 4-X substituents. The face selectivity in the 1,3-dipolar reactions of 13 and 14 is similar to that of reduction in 11 and 12, but the reaction of 14 gave a very poor yield of product. Most of the starting material 14 could be recovered from the 1,3-dipolar reaction due to its poor reactivity.
The E and Z configuration of isoxazolines 20 and 21 are assigned by inspecting the splitting patterns of the methylene protons on C11, in which a larger chemical
shift difference DnAB is expected for the E-isomer than
for the Z-isomer due to their closer interaction with 4-ketal or 4-thioketal groups. For example, the DnAB of the
methylene protons on C11 of E-21 was found to be
0.62 ppm but was 0.26 ppm for the Z-21. The assignments of E- and Z-22 were further confirmed by an independent synthesis of E-22 through PTSA catalyzed conversion of E-20 to E-22. Lightner et al. reported12athat the magnetic anisotropy of the C4-oxo group can deshield the C11carbon
in a very similar structure and our observations are consistent with their statements; for example, the chemical shift of C11is 43.6 ppm as an axial substituent (E-22) but is
43.0 ppm as an equatorial one (Z-22). The 1:1 face selectivity of 15 by nitrile oxide is unexpected if one considers the C4-oxo to be an electron-withdrawing group,
where the Cieplak’s model19 would have predicted a favored Z-22 product (from zu-face attack). On the other hand, an electrostatic repulsion between the nitrile oxide and the C4-oxo group of 15 should disfavor a zu-face attack,
thus counter-balanced the face preference by hyperconju-gative effect. The photoreactions of 15 with acetone and benzophenone were reported by Mlinaric´–Majerski20a to give E-oxetanes (from en-face attack) as the major product (in 70:30 ratios); where, both steric effect and the electronic effect of C4-oxo group were used to rationalize the observed
products.
The electrophilic addition reactions of dichlorocarbene on 4-substituted-2-methylene-adamantanes 13–15 were carried out next (Scheme 5 and Table 2). The addition of
Table 2. Product ratios and yields in the 1,3-dipolar addition, carbene addition, and mCPBA epoxidation reactions of 4-substituted-2-methyle-neadamantanes 13–15
Substrate E/Z product ratioa(yield, %)
1,3-Dipolar addition Dichlorocarbene addition mCPBA epoxidation 13 E-20:Z-20 E-23:Z-23 E-26:Z-26
O99:1 (49%) 92:8 (98%) 49:51 (80%) 14 E-21:Z-21 E-24:Z-24 E-27:Z-27
O99:1 (trace) No reaction Complex mixture 15 E-22:Z-22 E-25:Z-25 E-28:Z-28
49:51 (51%) 1:O99b(48%) 33:67b(71%)
a
Product ratios determined by1H NMR spectroscopy with an estimated error of G5% unless otherwise specified. Note that in the three types of reactions, en attack of reagents leads to E-products.
b Ratios determined by GC with an estimated error of G2%.
Chart 2. Scheme 4.
dichlorocarbene with 13 gave E- and Z-spirocyclopropanes 23in 98% yield, in a ratio of 92:8 (determined by1H NMR analysis). However, no reaction was found when 14 replaced 13 in a similar reaction conditions for carbene additions. To our surprise, the addition of dichlorocarbene with 4-methylene-2-adamantanone 15 gave Z-spirocyclo-propanes 25 as the predominant product (based on GC analysis, E/Z-25Z1:O99) in 48% isolated yield. The configuration assignment of E- and Z-spirocyclopropanes 23–25 can again be judged from the splitting pattern of the methylene protons of C11 on the spirocyclopropanes, in
which a larger chemical shift difference DnABis expected
for the E-isomer than the Z-isomer due to their closer interaction with 4-ketal or 4-thioketal groups. Furthermore, the structure of E-25 can be independently synthesized from the acid catalyzed deprotection of E-23. The exclusive formation of Z-25 reminds us about the Simmons–Smith reaction of homoallylic 4-cyclohexenols21 which gave specifically syn cyclopropane product. The results imply that the C4-oxo group of 15 may have directed the
dichlorocarbene to the zu-face; therefore, leads to high yield of Z-25.
In all reactions carried out on 13, the en-face attack had almost always been the predominant one; we were therefore a bit surprised to find that the epoxidation of 13 by mCPBA gave E- and Z-oxiranes 26 as a 1:1 mixture (Scheme 6). Moreover, complex mixtures were obtained in the epoxida-tion of 14 presumably due to the attack of mCPBA on the sulfur atoms of sulfide, because the 2-methylene group was found to be intact by1H NMR analysis. For comparison, the epoxidation of 15 gave E- and Z-oxiranes 28 (33:67) in 71% yield (Table 2). The somewhat high zu-face reactivity on 13 and 15 despite their steric congestions, suggests that hydrogen-bonding interaction between the mCPBA and C4-oxo or ketal groups is quite likely. Remember that the
hydroxyl group of an allylic alcohol is well-known to direct mCPBA in a highly stereoselective syn epoxidation reaction,22here, the C4-oxo seems to play a similar role. It
is worth noting that the zu-face epoxidation is more favored
on 15 than on 13 and we believe that the results are consistent with the Cieplak’s model.19In other words, since C4-oxo is considered to be stronger electron-withdrawing
than the ethylene ketal group, therefore, the reaction on CZ C double bond of 15 (or 13) is expected to occur preferentially from a direction anti to the more electron-rich C–C bonds.
The configuration of E- and Z-26 was judged from the 1H NMR spectrum of the ketal group, where the splitting pattern of Z-26 is more complex than E-26 due to its close interaction with oxirane. On the other hand, the configur-ation assignments of E- and Z-28 can also be judged from the splitting pattern of the methylene protons (of C11) of the
oxirans, in which a larger chemical shift difference DnABis
expected for the E-isomer (0.11 ppm) than for the Z-isomer (0.08 ppm) due to their closer interaction with C4-oxo
group. Furthermore, the protons on C11are more downfield
for the E-28 than those for the Z-28 due to their interactions with C4-oxo. Independent syntheses of E- and Z-28 from
acid-catalyzed deprotection of 26 with PTSA were unsuccessful because the oxirane rings tend to be opened by the acid too.
3. Conclusion
The results studied here indicate that in the reduction and 1,3-dipolar addition reactions of 4-disubstituted-2-adamantylidene or adamantan-2-one 11–14 steric hindrance is the dominating factor in determining the face selectivity. Despite the difficulties in isolating electronic effects from these steric biased probes, we found valuable information about ‘neighboring group participation’ in the carbene addition and epoxidation reactions. Finally, an enhanced zu-face attack of hydride on 15 can be achieved by complexation with b-CD which is opposite to our expectation based on previously proposed model.13a,17b Molecular dynamic calculations as well as1H NMR titration experiments support the 1:1 inclusion complexes of 15 with b-CD.
4. Experimental 4.1. General
4.1.1. The preparation of adamantane-2,4-dione (10).11a,b To a chromium oxide solution (66.7 g, 0.67 mol) in acetic anhydride (300 mL) was added dropwise a solution of 2-admantanone (3-H) (16.7 g in 200 mL of acetic anhy-dride) through addition funnel under nitrogen. The solution was vigorously stirred and the temperature was controlled at 20 8C by a circulator. After ten days, the solution was neutralized with saturated sodium bicarbonate solution and extracted several times (100 mL!6) with methylene chloride. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated. The
mixture was recrystallized in n-hexane/ethyl acetate (5/1) to give 10 (5.5 g, 33.5 mmol) and the residue from recrystal-lization was purified on a silica gel column by elution with n-hexane/ethyl acetate to give 10 (3.3 g, 20.1 mmol). The total amount of 10 is 8.8 g (an average of 37–50%).
Scheme 5. Scheme 6.
Colorless solid; mp 279–281 8C (lit.8 280–282 8C, lit.10a 282–283 8C); dH 1.75–1.80 (m, 1H), 1.99–2.18 (m, 6H), 2.41 (bs, 2H), 2.77 (bs, 2H), 3.38 (bs, 1H); dC 27.0 (CH), 30.1 (CH2), 38.3 (CH2), 44.2 (CH2), 45.2 (CH), 68.4 (CH), 208.6 (Cq); MS (EI, m/z) 164 (MC, 28), 95 (40), 79 (100), 66 (50), 55 (59), 53 (39); HRMS m/z calcd for C10H12O2
164.0838, found 164.0830. The preparation of 4-ethylene-ketaladamantan-2-one (11) followed a literature procedure.5 4.1.2. Synthesis of 4-ethylenethioketaladamantan-2-one (12).20bThe procedure for the synthesis of 12 is similar to that of 11, and the amount of reagents used is as follows: 10 (104 mg, 0.63 mmol), ethane-1,2-dithiol (64 mg, 0.68 mmol), PTSA$H2O (30 mg, 0.16 mmol) and benzene
(6 mL). The yield is 95%. Colorless liquid; (lit.20bmp 56– 59 8C); dH1.89–2.00 (m, 6H), 2.15–2.19 (m, 3H), 2.37–2.45
(m, 2H), 2.64 (bs, 1H), 3.18–3.24 (m, 4H); dC 25.7 (CH),
36.1 (CH2), 36.6 (CH2), 38.4 (CH2), 38.7 (CH2), 39.2 (CH2),
39.4 (CH2), 40.7 (CH), 45.0 (CH), 60.8 (CH), 76.3 (Cq),
213.9 (Cq); MS (EI, m/z) 240 (MC, 100), 212 (78), 184 (34);
HRMS m/z calcd for C12H16OS2240.0644, found 240.0651.
4.1.3. Synthesis of 4-ethyleneketal-2-methyleneadaman-tane (13). To a solution of methyltriphenylphosphonium bromide (941 mg, 2.51 mmol) in dried tetrahydrofuran (10 mL) at 0 8C was slowly added n-butyllithium (2.5 M in n-hexane, 2.50 mmol) via syringe under nitrogen. After the solution was stirred for 1–2 h at room temperature, 11 (253 mg, 1.21 mmol) in dried tetrahydrofuran (10 mL) was added gradually and refluxed for 24 h. After cooling, the solution was washed with water and separated into organic and water layers. The water layer was extracted several times (30 mL!4) with methylene chloride. The organic layers were combined, dried over MgSO4, filtered and
concentrated. The residue was purified on a silica gel column by elution with n-hexane/ethyl acetate to give 13 (217 mg, 87%). Colorless liquid; dH 1.72–2.18 (m, 10H), 2.41 (m, 2H), 3.94–3.98 (m, 4H), 4.61, 4.66 (AX, JZ 1.8 Hz, 2H); dC 26.8 (CH), 34.3 (CH2), 35.0 (CH2), 36.0 (CH), 37.1 (CH2), 37.6 (CH), 39.0 (CH2), 47.5 (CH), 64.2 (2!CH2), 103.7 (CH2), 111.2 (Cq), 154.5 (Cq); MS (EI, m/z) 206 (MC , 100), 91 (32), 73 (47), 57 (36); HRMS m/z calcd for C13H18O2206.1307, found 206.1297.
4.1.4. Synthesis of 4-ethylenethioketal-2-methylene-adamantane (14). The procedure for the synthesis of 14 is similar to that of 13. The amount of reagents used is as follows: 12 (900 mg, 3.75 mmol), methyltriphenylphos-phonium bromide (2.19 g, 5.77 mmol), dried tetrahydro-furan (100 mL) and n-butyllithium (2.5 M in n-hexane, 5.63 mmol). The yield is 85%. Colorless solid; mp 51– 52 8C; dH1.69–1.84 (m, 6H), 2.01 (bs, 1H), 2.14–2.26 (m, 3H), 2.40 (bs, 1H), 2.58 (bs, 1H), 3.14–3.24 (m, 4H), 4.60, 4.62 (AB, JZ2.2 Hz, 2H); dC 26.4 (CH), 35.9 (CH2), 37.1 (CH), 38.2 (CH2), 38.5 (CH2), 39.1 (CH2), 39.3 (CH2), 39.4 (CH2), 41.9 (CH), 52.6 (CH), 77.6 (Cq), 104.7 (CH2), 154.3 (Cq); MS (EI, m/z) 238 (MC , 27), 210 (32), 185 (49), 183 (100), 108 (59); HRMS m/z calcd for C13H18S2238.0851,
found 238.0854. Anal. calcd for C13H18S2: C, 65.49; H,
7.61, found: C, 65.38; H, 7.66.
4.1.5. Synthesis of 4-methyleneadamantan-2-one (15).A well-stirred solution of 13 (755 mg, 3.70 mmol) in 70%
acetone (aq) (20 mL) was added PTSA$H2O (84.5 mg,
0.44 mmol) as a catalyst and maintained at 35 8C for 22 h. The solution was washed with water and separated into two layers. The water layer was extracted several times (5 mL! 4) with methylene chloride. The organic layers were combined, dried over MgSO4, filtered and concentrated.
The residue was purified on a silica gel column by elution with n-hexane/ethyl acetate to give 15 (566 mg, 85%). Colorless solid; mp 280–281 8C (lit.23 135–138 8C; lit.24 280–282 8C); dH 1.88–2.12 (m, 9H), 2.59–2.62 (m, 2H), 3.14 (bs, 1H), 4.62, 4.66 (AB, JZ15 Hz, 2H); dC27.5 (CH), 37.6 (CH), 37.6 (CH2), 37.9 (CH2), 39.1 (CH2), 42.2 (CH2), 46.2 (CH), 58.4 (CH), 105.1 (CH2), 152.7 (Cq), 214.4 (Cq); MS (EI, m/z) 162 (MC, 69), 134 (31), 119 (29), 105 (32), 93 (79), 92 (100), 91 (80), 79 (43), 77 (39); HRMS m/z calcd for C11H14O 162.1045, found 162.1048.
4.2. General procedure for the reduction of 4-substituted-admantan-2-ol (Z-16, Z-17 and Z-, E-18) (a) Sodium borohydride reduction. The procedure for Z-16 is given as an example. To a solution of 11 (25.8 mg, 0.01 mmol) in methanol (4 mL) was added sodium borohydride (6.7 mg, 0.02 mmol) in one portion at room temperature. After stirred for 1 h, the solution was washed with saturated ammonium chloride and extracted several times (3 mL!4) with methylene chloride. The organic layers were combined, dried over MgSO4, filtered and
concentrated to give Z-16 in 98% yield. For other reduction the yields are as follows: Z-17, 98%; Z- and E-18 (1:1), 98%. (b) Lithium aluminum hydride reduction. The procedure for Z-16 is given as an example. To a well-stirred solution of lithium aluminum hydride in dried tetrahydrofuran (THF) at 0 8C under nitrogen was added 11(in THF) via syringe and stirred for 1 h. The solution was worked up with THF/water (1/1) and washed with water. The water layer was extracted several times (3 mL!4) with methylene chloride. The organic layers were combined, dried over MgSO4, filtered and concentrated. The residue
was purified on a silica gel column by elution with n-hexane/ethyl acetate and the yields are as follows: Z-16, 70%; Z-17, 71%; Z- and E-18 (1:1), 73%.
4.2.1. Data for 4-ethyleneketaladamantan-2a-ol (Z-16).24
Colorless liquid; dH1.60–2.00 (m, 12H), 2.15–2.22 (m, 1H), 3.86 (bs, 1H), 3.93–4.01 (m, 4H); dC25.5 (CH), 29.0 (CH2), 34.0 (CH2), 34.3 (CH), 34.6 (CH2), 36.07 (CH), 36.09 (CH2), 41.1 (CH), 63.7 (CH2), 64.4 (CH2), 76.2 (CH), 111.8 (Cq); MS (EI, m/z) 210 (MC , 32), 208 (100), 192 (36), 182 (31), 148 (32), 137 (36), 112 (31), 99 (61), 79 (45), 55 (35); HRMS m/z calcd for C12H18O3210.1256, found 210.1258.
4.2.2. Data for 4-ethylenethioketaladamantan-2a-ol
(Z-17). Colorless solid; mp 87–88 8C; dH 1.68–1.79 (m, 6H), 1.93 (bs, 1H), 2.08–2.18 (m, 2H), 2.22–2.32 (m, 3H), 3.18–3.31 (m, 4H), 3.85 (d, JZ7.3 Hz, OH), 3.97 (m, 1H); dC 25.4 (CH), 31.4 (CH2), 33.7 (CH), 36.6 (CH2), 36.9 (CH2), 37.3 (2!CH2), 38.2 (CH2), 41.3 (CH), 46.2 (CH), 75.1 (Cq), 77.1 (CH); MS (EI, m/z) 242 (MC, 92), 214 (91), 196 (63), 182 (65), 180 (45), 154 (49), 149 (76), 131 (43), 121 (100), 112 (45), 91 (61), 79 (68), 69 (52), 55 (25); HRMS m/z calcd for C12H18OS2242.0800, found 242.0796.
4.2.3. Data for 4-methyleneadamantan-2a-ol (Z-18).25 Colorless solid; mp 86–87 8C; dH1.67–2.01 (m, 11H), 2.43– 2.48 (m, 2H), 3.88 (bs, 1H), 4.67, 4.77 (AX, JZ2.1 Hz, 2H); dC 26.8 (CH), 33.8 (CH2), 34.5 (CH), 35.9 (CH2), 37.9 (CH2), 38.2 (CH), 38.7 (CH2), 46.1 (CH), 75.2 (CH), 106.7 (CH2), 153.4 (Cq); MS (EI, m/z) 164 (M C , 100), 94 (31), 93 (33); HRMS m/z calcd for C11H16O 164.1202, found
164.1197.
4.2.4. Data for 4-methyleneadamantan-2e-ol (E-18).25
Compound E-18 was not separated from its geometric isomers but its spectrum can be differentiated from the 1:1 mixture, because Z-18 was obtained through an independent synthesis from Z-19. Colorless solid; dH 1.51–1.96 (m,
11H), 2.12–2.25 (m, 2H), 3.84 (bs, 1H), 4.60 (s, 2H); dC
27.4 (CH), 30.6 (CH2), 32.6 (CH2), 34.3 (CH), 36.6 (CH2),
37.6 (CH), 39.1 (CH2), 45.5 (CH), 74.7 (CH), 103.3 (CH2),
155.5 (Cq); GC-MS (EI, m/z) 164 (MC, 100), 94 (61), 93
(68).
4.3. General procedures for1H NMR titration studies of 15 with b-CD
Solutions containing different proportions of guest-to-b-CD were prepared by stirring 5 mM of 15 with 0, 1, 2, 3, 4, 5, 7, 9, and 15 mM of b-CD solutions (15 mM stock solution in D2O) in 1 mL D2O for ca. 3 h before measurements. The
NMR spectra of all the b-CD complexes, b-CD and 15 in D2O and CDCl3with a coaxial external standard (CDCl3)
were recorded with a 300 MHz NMR and the results are shown inFigures 2 and 3.
4.3.1. Synthesis of 4a-hydroxyadamantan-2-one (19).The
procedure for the synthesis of 1925,26is similar to that of 15. And the amounts of reagents used are as follows: Z-16 (250 mg, 1.20 mmol), PTSA$H2O (50 mg, 0.26 mmol) and
70% acetone (aq) (17 mL). The yield for 19 is 61%. Colorless solid; mp not determined (lit.9mp 316–320 8C); dH 1.82–2.06 (m, 9H), 2.39–2.51 (m, 2H), 2.72 (bs, 1H), 2.73 (bs, 1H), 4.23 (bs, 1H); dC26.2 (CH), 33.2 (CH2), 33.5 (CH), 35.1 (CH2), 37.6 (CH2), 38.9 (CH2), 46.5 (CH), 54.3 (CH), 78.1 (CH), 217.7 (Cq); MS (EI, m/z) 166 (MC , 72), 148 (53), 138 (80), 96 (55), 79 (100), 78 (76); HRMS m/z calcd for C10H14O2166.0994, found 166.0986.
4.4. General procedure for the 1,3-dipolar reaction of 13–15
To a well-stirred solution of 13 (38.6 mg, 0.19 mmol) and benzohydroximinoyl chloride (43.5 mg, 0.28 mmol) in dried tetrahydrofuran (5 mL) under nitrogen was added triethylamine (31.9 mg, 0.32 mmol) via syringe and refluxed for 24 h. After cooled down to room temperature, the solution was washed with water and the water layer was extracted several times (3 mL!4) with methylene chloride. The organic layers were combined, dried over MgSO4,
filtered and concentrated. The residue was purified on a silica gel column by elution with n-hexane/ethyl acetate to give E-20. The yields are as follows: E-20 (from 13), 49%; E- and Z-22 (1:1) (from 15), 51%. Only recovered starting material 14 was obtained under this reaction condition. 4.4.1. Data for
(E)-4-ethyleneketalspiro[adamantane-2,50-30-phenyl-D2-isoxazoline] (E-20). Colorless liquid; dH 1.49–1.55 (m, 1H), 1.63–2.05 (m, 9H), 2.29–2.35 (m, 2H), 3.08, 3.56 (AX, JZ17.7 Hz, 2H), 3.93–3.96 (m, 4H), 7.37–7.40 (m, 3H), 7.68–7.71 (m, 2H); dC25.2 (CH), 30.8 (CH2), 31.0 (CH2), 32.7 (CH2), 34.5 (CH2), 35.7 (CH), 35.7 (CH), 44.0 (CH2), 44.4 (CH), 63.8 (CH2), 64.4 (CH2), 90.8 (Cq), 111.5 (Cq), 126.4 (CH), 128.5 (CH), 129.7 (CH), 130.3 (Cq), 157.6 (Cq); MS (EI, m/z) 325 (MC , 100), 179 (50), 99 (35), 91 (32), 77 (70), 55 (32); HRMS m/z calcd for C20H23O3N 325.1678, found 325.1680.
4.4.2. Data for (E)-spiro[adamantan-2-one-4:50-30 -phenyl-D2-isoxazoline] (E-22). Colorless solid; mp 131–132 8C; dH 1.82–1.92 (m, 3H), 2.03–2.12 (m, 5H), 2.41–2.45 (m, 1H), 2.60–2.68 (m, 3H), 2.97, 3.06 (AB, JZ 16.7 Hz, 2H), 7.36–7.40 (m, 3H), 7.60–7.63 (m, 2H); dC 25.8 (CH), 32.3 (CH2), 33.4 (CH2), 35.1 (CH2), 36.6 (CH), 38.7 (CH2), 43.6 (CH2), 45.6 (CH), 56.2 (CH), 90.4 (Cq), 126.4 (CH), 128.6 (CH), 129.4 (Cq), 130.1 (CH), 156.6 (Cq), 214.2 (Cq); MS (EI, m/z) 281 (MC, 100), 144 (31), 117 (47), 77 (36); HRMS m/z calcd for C18H19O2N
281.1416, found 281.1414. Anal. calcd for C18H19O2N: C,
76.84; H, 6.81; N, 4.98, found: C, 76.64; H, 6.83; N, 5.01. 4.4.3. Data for (Z)-spiro[adamantan-2-one-4:50-30 -phenyl-D2-isoxazoline] (Z-22). Colorless solid; mp 118– 119 8C; dH1.92–2.13 (m, 9H), 2.52–2.67 (m, 3H), 3.24, 3.34 (AB, JZ16.8 Hz, 2H), 7.41 (m, 3H), 7.66 (m, 2H); dC26.3 (CH), 33.2 (CH2), 34.6 (CH2), 36.4 (CH), 37.7 (CH2), 39.1 (CH2), 43.0 (CH2), 45.3 (CH), 55.4 (CH), 93.7 (Cq), 126.5 (CH), 128.7 (CH), 129.7 (Cq), 130.1 (CH), 155.7 (Cq), 213.6 (Cq); MS (EI, m/z) 281 (MC, 100), 146 (30), 144 (45), 117 (65), 91 (39), 77 (51); HRMS m/z calcd for C18H19O2N
281.1416, found 281.1422. Anal. calcd for C18H19O2N: C,
76.84; H, 6.81; N, 4.98, found: C, 76.55; H, 6.87; N, 5.07. 4.5. General procedure for the synthesis of 4-substituted-11-dichlorocyclopropylspiro-adamantane (E-23 and E-, Z-25)
The procedure for E-23 is given as an example. To a well-stirred solution of 13 (69.5 mg, 0.34 mmol) and triethylbenzylammonium chloride (10 mg, 0.04 mmol) in chloroform (1 mL) was added 50% NaOH (aq) (1 mL) at room temperature and stirred overnight. The solution was washed with water and extracted several times (5 mL!4) with methylene chloride. The organic layers were com-bined, dried over MgSO4, filtered and concentrated. The
residue was purified on a silica gel column by elution with n-hexane/ethyl acetate to give E-23. The yields are as follows: E-23, 98%; E- and Z-25 (1:O99), 48%.
4.5.1. Data for (E)-4-ethylketal-11-dichlorocyclopropyl-spiroadamantane (E-23). Colorless liquid; dH 1.21, 1.41
(AX, JZ7.4 Hz, 2H), 1.58–2.01 (m, 12H), 3.84–3.94 (m, 4H); dC25.7 (CH), 32.4 (CH2), 32.9 (CH2), 33.2 (CH2), 33.8 (CH2), 34.2 (CH), 35.2 (CH2), 35.5 (CH), 37.9 (Cq), 41.9 (CH), 64.1 (CH2), 64.3 (CH2), 66.0 (Cq), 111.3 (Cq); MS (EI, m/z) 292 (MC C4, 2), 290 (MC C2, 10), 288 (MC , 13), 253 (100), 99 (45); HRMS m/z calcd for C14H18O235Cl2
288.0685, found 288.0682. Anal. calcd for C14H18O2Cl2: C,
58.14; H, 6.27, found: C, 57.99; H, 6.36. For characteristic
4.5.2. Data for (Z)-11-dichlorocyclopropylspiroadaman-tan-2-one (Z-25). Colorless solid; mp 53–54 8C; dH 1.27,
1.37 (AB, JZ7.2 Hz, 2H), 1.80–2.08 (m, 9H), 2.09–2.13 (m, 1H), 2.43 (bs, 1H), 2.64 (bs, 1H); dC 26.5 (CH), 30.7 (CH2), 33.8 (CH), 34.7 (CH2), 35.7 (CH2), 38.3 (CH2), 38.8 (CH2), 41.2 (Cq), 45.6 (CH), 51.5 (CH), 65.9 (Cq), 214.4 (Cq); MS (EI, m/z) 248 (MC C4, 7), 246 (MC C2, 39), 244 (MC , 61), 209 (38), 181 (87), 178 (86), 152 (33), 145 (75), 139 (56), 138 (49), 105 (39), 91 (65), 79 (100); HRMS m/z calcd for C12H14O35Cl2 244.0423, found 244.0415. Anal.
calcd for C12H14OCl2: C, 58.79; H, 5.76, found: C, 58.65; H,
5.82.
4.5.3. Data for (E)-11-dichlorocyclopropylspiroadaman-tan-2-one (E-25). Which was obtained from the acid catalyzed hydrolysis of E-22, a colorless liquid; dH 1.15,
1.31 (AB, JZ7.3 Hz, 2H), 1.85 (bs, 1H), 1.95–2.15 (m, 8H), 2.29–2.35 (m, 2H), 2.56 (bs, 1H); dC26.3 (CH), 31.2 (CH2), 34.0 (CH), 34.4 (CH2), 35.5 (CH2), 37.6 (CH2), 38.2 (CH2), 40.5 (Cq), 45.3 (CH), 52.4 (CH), 64.4 (Cq), 214.6 (Cq); MS (EI, m/z) 248 (MC C4, 9), 246 (MC C2, 51), 244 (MC , 76), 209 (34), 181 (100), 145 (69), 91 (91), 79 (94); HRMS m/z calcd for C12H14O35Cl2244.0423, found 244.0418.
4.6. General procedure for the synthesis of 4-substituted-2-oxacyclopropyladamantane (Z-, E-26 and Z-, E-28) The procedure for Z-, E-26 is given as an example. To a well-stirred solution of 13 (40.5 mg, 0.20 mmol) in methylene chloride (2 mL) was added 70–75% mCPBA (48.2 mg, 0.28 mmol) at room temperature and kept stirred for 1.5 h. The solution was washed with water and the water layer was extracted several times (3 mL!4) with methylene chloride. The organic layers were combined, dried over MgSO4, filtered and concentrated to give Z-, E-26. The
yields are as follows: Z- and E-26 (1:1), 80%; Z- and E-28 (67:33), 71%.
4.6.1. Data for (Z)-4-ethyleneketal-2-oxacyclopropyl-adamantane (Z-26). Colorless liquid; dH 1.33 (bs, 1H),
1.41 (bs, 1H), 1.65–1.84 (m, 7H), 1.95–2.00 (m, 1H), 2.12– 2.28 (m, 2H), 2.52, 2.57 (AB, JZ4.8 Hz, 2H), 3.88–4.02 (m, 4H); dC25.9 (CH), 31.6 (CH2), 34.1 (CH2), 34.6 (CH2), 35.0 (CH), 35.4 (CH), 36.6 (CH2), 43.9 (CH), 51.5 (CH2), 64.0 (CH2CCq), 64.6 (CH2), 110.9 (Cq); MS (EI, m/z) 222 (MC, 62), 221 (MCK1, 87), 192 (53), 179 (36), 151 (39), 149 (62), 99 (100), 91 (74), 79 (66), 55 (62); HRMS m/z calcd for C13H18O3222.1256, found 222.1266.
4.6.2. Data for (E)-4-ethyleneketal-2-oxacyclopropyl-adamantane (E-26). Colorless liquid; dH 1.36 (bs, 2H),
1.62–1.84 (m, 5H), 1.96–2.02 (m, 5H), 2.66, 2.71 (AB, JZ 4.7 Hz, 2H), 3.86–3.94 (m 4H); dC25.6 (CH), 31.9 (CH2), 32.4 (CH2), 34.1 (CH2), 34.4 (CH2), 34.6 (CH), 35.7 (CH), 44.1 (CH), 55.7 (CH2), 63.2 (Cq), 64.2 (2!CH2), 111.5 (Cq); MS (EI, m/z) 222 (MC, 100), 221 (67), 193 (46), 192 (43), 149 (35), 99 (71), 91 (32); HRMS m/z calcd for C13H18O3222.1256, found 222.1261.
4.6.3. Data for (Z)-4-oxacyclopropyladamantan-2-one (Z-28). Colorless solid; mp 96–98 8C; dH 1.86–1.91 (m, 2H), 2.01–2.11 (m, 7H), 2.18–2.21 (m, 1H), 2.37–2.41 (m, 1H), 2.60 (bs, 1H), 2.64, 2.72 (AB, JZ4.5 Hz, 2H); dC26.3 (CH), 33.0 (CH2), 33.8 (CH2), 34.6 (CH), 37.4 (CH2), 38.7 (CH2), 45.3 (CH), 54.4 (CH2), 54.9 (CH), 63.9 (Cq), 214.0 (Cq); MS (EI, m/z) 178 (MC, 60), 150 (100), 105 (31), 92 (60), 91 (34); HRMS m/z calcd for C11H14O2 178.0994, found 178.0988.
4.6.4. Data for (E)-4-oxacyclopropyladamantan-2-one (E-28). Colorless solid; mp 99–100 8C; dH 1.56 (bs, 1H),
2.00–2.19 (m, 10H), 2.59 (bs, 1H), 2.63, 2.74 (AB, JZ 4.5 Hz, 2H); dC 26.4 (CH), 34.3 (CH2), 34.9 (CH), 35.7
(CH2), 38.7 (CH2), 39.0 (CH2), 45.6 (CH), 52.8 (CH2), 54.6
(CH), 66.4 (Cq), 214.2 (Cq); MS (EI, m/z) 178 (MC, 68),
150 (100), 93 (32), 92 (65), 91 (30); HRMS m/z calcd for C11H14O2 178.0994, found 178.0991. Anal. calcd for
C11H14O2: C, 74.13; H, 7.92, found: C, 73.79; H, 8.00.
Acknowledgements
We thank the National Science Council of Republic of China for financial support. We also thank National Chiao Tung University for a financial support to the Center for Interdisciplinary Molecular Science.
Supplementary Data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.tet.2004.07. 075
References and notes
1. (a) Kaselj, M.; Chung, W.-S.; le Noble, W. J. Chem. Rev. 1999, 99, 1387 and references cited therein. (b) Adcock, W.; Trout, N. A. Chem. Rev. 1999, 99, 1415. (c) Ohwada, T. Chem. Rev. 1999, 99, 1337.
2. (a) Tomoda, S.; Kaneno, D. Org. Lett. 2003, 5, 2947. (b) Knoll, W.; Bobek, M. M.; Malchhauser, H.; Rosenberg, M. G.; Brinker, U. H. Org. Lett. 2003, 5, 2943. (c) Tomoda, S.; Senju, T.; Kawamura, M.; Ikeda, T. J. Org. Chem. 1999, 64, 5396. (d) Knoll, W.; Bobek, M. M.; Giester, G.; Brinker, U. H. Tetrahedron Lett. 2001, 42, 9161.
3. Lange, H.; Gleiter, R.; Fritzsche, G. J. Am. Chem. Soc. 1998, 120, 6563.
4. (a) Mehta, F.; Uma, R. J. Org. Chem. 2000, 65, 1685. (b) Mehta, G.; Khan, F. A.; Gadre, S. R.; Shirsat, R. N.; Ganguly, B.; Chandrasekhar, J. Angew. Chem. Int. Ed. 1994, 33, 1390. (c) Mehta, G.; Gunasekaran, G.; Gadre, S. R.; Shirsat, R. N.; Ganguly, B.; Chandrasekhar, J. J. Org. Chem. 1994, 59, 1953. (d) Mehta, G.; Praveen, M. Tetrahedron Lett. 1992, 33, 1759.
5. Kaselj, M.; le Noble, W. J. J. Org. Chem. 1996, 61, 4157. 6. Cuddy, B. D.; Grant, D.; McKervey, M. A. J. Chem. Soc. (C)
1971, 3173.
7. (a) Maurin, J. K.; Lasek, W.; Gorska, A.; Switzj, T.; Wamil, M.; Mlynarczuk, I.; Kazimierczuk, Z. Anti-Cancer Drug. Design. 2001, 16, 73. (b) Chen, C. S. H.; Shen, D.-M.;
Wentzek, S. E. PCT Int. Appl. WO 94 28.885, 1994; Chem. Abstr. 1995, 122, 239227. (c) Kolocouris, N.; Foscolos, G. B.; Kolocouris, A.; Marakos, P.; Pouli, N.; Fytas, G.; Ikeda, S.; De Clercq, E. J. Med. Chem. 1994, 37, 2896. 8. Udding, A. C.; Strating, J.; Wynberg, H. Tetrahedron Lett.
1968, 1345.
9. McKervey, M. A.; Faulkun, D.; Hamill, H. Tetrahedron Lett. 1970, 1971.
10. (a) Duddeck, H.; Wiskamp, V.; Rosenbaum, D. J. Org. Chem. 1981, 46, 5332. (b) Duddeck, H.; Feuerhelm, H.-T.; Snatzke, G. Tetrahedron Lett. 1979, 829. (c) Duddeck, H.; Rosenbaum, D. J. Org. Chem. 1991, 56, 1700.
11. (a) Gilbert, E. E. Synth. Commun. 1985, 15, 53. (b) Chu, J.-H. Master Thesis, National Chiao Tung University, Hsinchu, Taiwan, 1999. Surprisingly, we later found that similar synthesis of 10 by exactly the same method was reported contemporarily, see: Bobek, M. M.; Brinker, U. H. Synth. Commun. 1999, 29, 3221.
12. (a) Lightner, D. A.; Bouman, T. D.; Wijekoon, W. M. D.; Hansen, A. E. J. Am. Chem. Soc. 1986, 108, 4484. (b) Olah, G. A.; Krishnamurthy, V. V. J. Am. Chem. Soc. 1982, 104, 3987.
13. (a) Chung, W.-S.; Wang, N.-J.; Liu, Y.-D.; Leu, Y.-J.; Chiang, M. Y. J. Chem. Soc., Perkin Trans. 2 1995, 307 and earlier references cited therein. (b) Li, W.-S.; Chung, W.-S.; Chao, I. Chem. Eur. J. 2003, 9, 951.
14. For reviews of the determination of host–guest complexation: (a) Diederich, F. Angew. Chem., Int. Ed. 1988, 27, 362 and references cited therein. (b) Connors, K. A. Binding Constants, the Measurement of Molecular Complex Stability; Wiley: New York, 1987.
15. Similar results in1H NMR spectroscopy in the studies of b-CD
complexes were observed. For example: (a) Greatbanks, D.; Pickford, R. Magn. Reson. Chem. 1987, 25, 208. (b) Reddy, G. D.; Usha, G.; Ramanathan, K. V.; Ramamurthy, V. J. Org.
Chem. 1986, 51, 3085. (c) Eaton, D. F. Tetrahedron 1987, 43, 1551.
16. (a) Hamilton, J. A.; Steinrauf, L. K.; vanEtten, R. L. Acta Crystallogr. 1968, B24, 1560. (b) Crugler, M.; Eckle, E.; Stezowski, J. J. Chem. Soc., Chem. Commun. 1981, 1291. 17. (a) Benesi, H. A.; Hilderbrand J. Am. Chem. Soc. 1949, 71,
2703. (b) Similar results have been observed in Indium mediated allylation of 3-X in b-CD: Jan, M. -D. MS Thesis, National Chiao Tung University, 1999 and Proceeding of the 11th IUPAC Symposium on OMCOS, p. 319, July 22-26, Taipei, 2001.
18. For 1,3-dipolar addition reaction on 3-X see (a) Chung, W.-S.; Tsai, T.-L.; Ho, C.-C.; Chiang, M. Y. N.; le Noble, W. J. J. Org. Chem. 1997, 62, 4672. (b) Tsai, T.-L.; Chen, W.-C.; Yu, C.-H.; le Noble, W. J.; Chung, W.-S. J. Org. Chem. 1999, 64, 1099.
19. (a) Cieplak, A. S. J. Am. Chem. Soc. 1981, 103, 4550. (b) Cieplak, A. S.; Tait, B. D.; Johnson, C. R. J. Am. Chem. Soc. 1989, 111, 8447. (c) Johnson, C. R.; Tait, B. D.; Cieplak, A. S. J. Am. Chem. Soc. 1987, 109, 5875. (d) Cieplak, A. S. Chem. Rev. 1999, 99, 1265.
20. (a) Mlinaric´-Majerski, K.; Pavlovic´, D.; Veljkovic´, J.; Sˇindler-Kulyk, M. Croat. Chem. Acta 1993, 66, 385. (b) Mlinaric´-Majerski, K.; Vinkovic´, M.; Sˇkare, D.; Marchand, A. P. Arkivoc 2002, 30 part IV.
21. Chan, J. H.; Rickborn, B. J. Am. Chem. Soc. 1968, 90, 6406. 22. Adam, W.; Wirth, T. Acc. Chem. Res. 1999, 32, 703. 23. (a) Mlinaric´-Majerski, K.; Majerski, Z. J. Am. Chem. Soc.
1980, 102, 1418. (b) Mlinaric´-Majerski, K.; Majerski, Z. J. Am. Chem. Soc. 1983, 105, 7389.
24. Henkel, J. G.; Spector, J. H. J. Org. Chem. 1983, 48, 3657. 25. Grob, C. A.; Wittwer, G.; Rao, K. R. Helv. Chim. Acta 1985,
68, 760.