1
2
Flavokawain B inhibits growth of human squamous carcinoma cells: involvement of
3
apoptosis and cell cycle dysregulation in vitro and in vivo
4
Elong Lin
a, Wen-Hsin Lin
b, Sheng-Yang Wang
c, Chih-Sheng Chen
h, Jiuun-Wang Liao
d, Hsueh-Wei Chang
e,
5Ssu-Ching Chen
f, Kai-Yuan Lin
g, Lai Wang
h, Hsin-Ling Yang
h,⁎
, You-Cheng Hseu
i,⁎
6 a
Department of Food Science and Technology, Central Taiwan University, Taichung, Taiwan
7 b
School of Pharmacy, China Medical University, Taichung, Taiwan
8 c
Department of Forestry, National Chung-Hsing University, Taichung, Taiwan 9 dGraduate Institute of Veterinary Pathology, National Chung-Hsing University, Taichung, Taiwan
10 eDepartment of Biomedical Science and Environmental Biology, Graduate Institute of Natural Products, College of Pharmacy, Center of Excellence for Environmental Medicine,
11 Kaohsiung Medical University, Kaohsiung, Taiwan
12 f
Department of Life Sciences, National Central University, Chung-Li, Taiwan
13 g
Department of Medical Research, Chi-Mei Medical Center, Tainan, Taiwan
14 h
Institute of Nutrition, China Medical University, Taichung, Taiwan
15 i
Department of Cosmeceutics, China Medical University, Taichung, Taiwan
Q1 16
17 Received 29 January 2010; received in revised form 4 November 2010; accepted 6 January 2011
18 Abstract
19 Flavokawain B is a natural chalcone isolated from the rhizomes of Alpenia pricei Hayata. In the present study, we have investigated the antiproliferative and
20 apoptotic effect of flavokawain B (5–20 μg/ml; 17.6–70.4 μM) against human squamous carcinoma (KB) cells. Exposure of KB cells with flavokawain B resulted
21 in apoptosis, evidenced by loss of cell viability, profound morphological changes, genomic DNA fragmentation and sub-G1 phase accumulation. Apoptosis
22 induced by flavokawain B results in activation of caspase-9, -3 and -8, cleavage of PARP
Q2 and Bid in KB cells. Flavokawain B also down-regulate Bcl-2 with
23 concomitant increase in Bax level, which resulted in release of cytochrome c. Taken together, the induction of apoptosis by flavokawain B involved in both
24 death receptor and mitochondrial pathway. We also observed that flavokawain B caused the G2/M phase arrest that was mediated through reductions in the
25 levels of cyclin A, cyclin B1, Cdc2 and Cdc25C and increases in p21/WAF1, Wee1 and p53 levels. Moreover, flavokawain B significantly inhibits matrix
26 metalloproteinase-9 and urokinase plasminogen activator expression, whereas tissue inhibitor of matrix metalloproteinase-1 and plasminogen activator
27 inhibitor-1 were increased, which are playing critical role in tumor metastasis. In addition, flavokawain B treatment significantly inhibited in vivo growth of
28 human KB cell-derived tumor xenografts in nude mice, which is evidenced by augmentation of apoptotic DNA fragmentation, as detected by in situ terminal
29 deoxynucleotidyl transferase-meditated dUTP nick end-labeling staining. The induction of cell cycle arrest and apoptosis by flavokawain B may provide a
30 pivotal mechanism for its cancer chemopreventive action.
31 © 2011 Published by Elsevier Inc.
32
33 Keywords: Flavokawain B; Cell cycle arrest; Apoptosis; KB cells
34
35 1. Introduction
36 The rhizomes of Zingiberaceae including ginger, turmeric and 37 cardamon plants are widely used as spices in Asian countries, eaten 38 raw, cooked as vegetables or used as flavoring[1]. Alpinia plants (shell 39 gingers, family Zingiberaceae) have been shown by several previous 40 studies to have various biological activities, including, antioxidant, 41 anti-inflammatory, anticancer, immunostimulating, hepatoprotective
42 and antinociceptive activities [2,3]. A. pricei Hayata is a perennial
43 rhizomatous plant indigenous to Taiwan. It has various traditional
44 and commercial uses, such as use of the leaves to make traditional
45 zongzi (glutinous rice dumplings) in Taiwan and use of the aromatic
46 rhizomes as a folk medicine for dispelling abdominal distension and
47 enhancing stomach secretion and peristalsis[4]. In earlier studies, we
48 demonstrated that ethanol (70%) extracts of A. pricei exhibit
49 antitumor effects by induction of cell cycle arrest/apoptosis in
50 human squamous carcinoma KB cells[2,5]. However, the
phytochem-51 istry and bioactivity of A. pricei extracts have not yet been elucidated.
52 Chemoprevention, which refers to the administration of agents to
53 prevent initiation and promotion of events associated with
carcino-54 genesis, is being increasingly considered an effective approach for the
55 management of neoplasms. Many studies investigating the use of cell
56 cycle inhibitors and apoptosis-inducing agents for the management of
Available online at www.sciencedirect.com
Journal of Nutritional Biochemistry xx (2011) xxx–xxx
⁎ Corresponding authors. Hsin-Ling Yang is to be contacted at Institute of Nutrition, China Medical University, Taichung 40402, Taiwan; You-Cheng Hseu, Department of Cosmeceutics, China Medical University, Taichung 40402, Taiwan. Tel.: +886 4 22053366x5308; fax: +886 4 22078083.
E-mail addresses:hlyang@mail.cmu.edu.tw(H.-L. Yang), ychseu@mail.cmu.edu.tw(Y.-C. Hseu).
0955-2863/$ - see front matter © 2011 Published by Elsevier Inc. doi:10.1016/j.jnutbio.2011.01.002
57 cancer have shown associations between abnormal cell cycle 58 regulation and apoptosis and cancer [6]. Eukaryotic cell cycle 59 progression involves the sequential activation of cyclin-dependent 60 kinases (CDKs), which is dependent on association with cyclins[7]. 61 Progression through the mammalian mitotic cycle is controlled by 62 multiple holoenzymes, including a catalytic CDK and a cyclin 63 regulatory subunit[7]. These cyclin–CDK complexes are activated at 64 specific intervals during the cell cycle but can be induced and 65 regulated by exogenous factors. Apoptosis is characterized by a 66 number of well-defined features, including cellular morphological 67 changes, chromatin condensation, internucleosomal DNA cleavage 68 and the activation of a family of cysteine-aspartic acid proteases 69 (caspases) [8]. Thus, agents that alter regulation of cell cycle 70 machinery, resulting in arrest in different phases, thereby reducing 71 growth and proliferation of, and even inducing apoptosis in, 72 cancerous cells, may be useful in cancer chemoprevention.
73 Found abundantly in edible plants, chalcones (1,3-diaryl-2-74 propen-1-ones) are important biological compounds and are pre-75 cursors in the biosynthesis of flavonoids and isoflavonoids. Chalcones 76 have been reported to possess many useful properties, including anti-77 inflammatory, antimicrobial, antifungal, antioxidant, cytotoxic, anti-78 tumor and anticancer activities [9,10]. It has bee shown that 79 flavokawains, chalcone derivatives in kava extracts as used by South 80 Pacific Islanders for thousands of years, are novel apoptosis inducers 81 and anticarcinogenic agents [11]. Studies have identified that 82 flavokawain A from extracts of kava (Piper methylsticum) roots can 83 induce apoptosis and cell cycle arrest in the invasive bladder cancer 84 cell line T24 and that flavokawains B and C, also from kava extract, 85 have strong antiproliferative effects against several cancer cell lines 86 (RT4, T24 and EJ cells)[11]. The rootstock of kava is commonly used to 87 prepare a beverage for ceremonial activities by the native Pacific 88 Islanders. An epidemiologic study found that cancer incidence in the 89 three highest kava-drinking countries— Vanuatu, Fiji and Western 90 Samoa— was one quarter to one third of those in non-kava-drinking 91 countries, such as New Zealand (Maoris) and the United States 92 (Hawaii and Los Angeles)[12]. These findings should encourage the 93 development of more potent chalcone derivatives for both prevention 94 and treatment of cancer, as well as epidemiologic studies of the 95 relationship between flavokawain consumption and cancer. Here, we 96 investigate the anticancer effects of flavokawain B (5–20 μg/ml; 17.6– 97 70.4μM), a chalcone purified from ethanol (70%) extracts of A. pricei 98 rhizomes, in terms of tumor regression using both in vitro cell culture 99 and in vivo athymic nude mice models of KB cells. The levels of cell 100 cycle/apoptosis/metastatic control and related molecules were 101 assayed to determine the flavokawain B anticancer mechanism. 102 2. Materials and methods
103 2.1. Reagents
104 Dulbecco's modified Eagle's medium (DMEM) contained the following: fetal
105 bovine serum (FBS), glutamine and penicillin/streptomycin/neomycin (GIBCO BRL,
106 Grand Island, NY, USA); antibodies against cytochrome c, caspase-3, caspase-8,
107 caspase-9, Bcl-2, Bax, Fas, Fas ligand (FasL), cyclin B1, Cdc2, p21/WAF1, Wee1, p53,
108 matrix metalloproteinase-9 (MMP-9), urokinase plasminogen activator (u-PA), tissue
109 inhibitor of matrix metalloproteinase-1 (TIMP-1) and plasminogen activator
inhibitor-110 1 (PAI-1) (Santa Cruz Biotechnology Inc., Heidelberg, Germany); PARP rabbit
111 polyclonal antibody (Upstate Biotechnology, Lake Placid, NY, USA); antibody against
112 β-actin (Sigma Chemical Co., St. Louis, MO, USA) and antibodies against Bid, cyclin A
113 and Cdc25C (Cell Signaling Technology Inc., Danvers, MA), which were obtained from
114 their respective suppliers. All other chemicals were of the highest grade commercially
115 available and supplied either by Merck (Darmstadt, Germany) or Sigma.
116 2.2. Identification and quantification of flavokawain B in A. pricei extracts
117 Air-dried roots (2 kg) of A. pricei were extracted with 10 L of 70% (vol/vol) ethanol
118 at room temperature as previously described[2]. We further characterized the main
119 composition of A. pricei extracts using chromatography followed by spectral analysis.
120 A. pricei extracts were separated by semipreparative high-performance liquid
121
chromatography. A Luna silica column (250×10 mm, Phenomenex Co.) was used
122
with two solvent systems: A, H2O, and B, acetonitrile. The gradient elution profile was 123
as follows: 0–3 min, 80% A to B; 3–60 min, 80–0% A to B (linear gradient) and 60–80
124
min 0% A to B. The flow rate was 2.5 ml/min, and the detector wavelength was set at
125
280 nm. The three major compounds in the A. pricei extracts were obtained at retention
126
times of (1) 32.5 min, (2) 37.0 min and (3) 46.7 min. The structures of compounds 1–3
127
were determined by spectroscopic analysis. The UV spectra of these compounds were
128
recorded with a Jasco V-550 spectrometer, and the infrared spectra were obtained with
129
a Bio-Rad FTS-40 spectrophotometer. Electron-impact mass spectrometry and
high-130
resolution electron-impact mass spectrometry data were collected with a Finnigan
131
MAT-958 mass spectrometer. The nuclear magnetic resonance (NMR) spectra were
132
recorded with Bruker Avance 500 and 300 MHz FT-NMR spectrometers, at 500 MHz
133
(1
H) and 75 MHz (13C). According to the mass and NMR analysis, compounds 1–3 were 134
identified as: (1) desmethoxyyangonin, (2) cardamonin and (3) flavokawain B[11].
135
The standard calibration curves (peak area vs. concentrations) of compounds 1–3
136
ranged from 5 to 100μg/ml. The linear regression equations were
137 139 141 desmethoxyyangonin y=13,134x+13, 147 142 144 cardamonin y=25,853x+2128.6 145 147 flavokawain B y=11,211x+14, 573 148149 150 151
Each of these equations showed good linearity (R2=0.9995–0.9998). According to 152
the results of high-performance liquid chromatography analysis, the amounts of the
153
compounds desmethoxyyangonin, cardamonin and flavokawain B in A. pricei extracts
154
were 1.1%, 8.9% and 5.7%, respectively. Stock solutions of desmethoxyyangonin (1.1
155
mg), cardamonin (1.2 mg) and flavokawain B (10 mg) were prepared in 100% dimethyl
156
sulfoxide (DMSO) at 25°C, then stored at−20°C.
157
2.3. Cell culture and assessment of cell viability
158
The human squamous carcinoma cell line KB (HeLa derivative) and the human
159
gingival fibroblast (HGF) cell line HGF were obtained from the American Type Culture
160
Collection (Rockville, MD, USA). The KB cell line was used by the National Cancer
161
Institute for some of the earliest in vitro anticancer drug-screening work[13]. KB cells
162
were once thought to be derived from an oral cancer, but in fact, they were derived
163
from a glandular cancer of the cervix[13]. KB and HGF cells were grown in a humidified
164
incubator (5% CO2in air at 37°C) in DMEM supplemented with 10% heat-inactivated 165
FBS, 2 mol/l glutamine, 1% penicillin, 1% streptomycin and 1% neomycin. Cells were
166
seeded in 6- or 12-well plates before the addition of flavokawain B. Cultures were
167
harvested, and cell number was determined by counting cell suspensions using a
168
hemocytometer. Cell viability (3.0×105
cells/12 wells) and growth (1.0×105 cells/6
169
wells) were assayed before and after treatment with flavokawain B using trypan blue
170
exclusion and phase contrast microscopy.
171
2.4. Terminal deoxynucleotidyl transferase-meditated dUTP nick end-labeling assay for
172
DNA apoptotic fragmentation
173
DNA fragmentation was detected using terminal deoxynucleotidyl
transferase-174
meditated dUTP nick end-labeling (TUNEL) with the Klenow FrgEL DNA fragmentation
175
detection kit (Calbiochem, San Diego, CA, USA). Briefly, KB cells (5×105
cells/6 wells)
176
were harvested, fixed with 4% formaldehyde and applied to glass slides. Fixed cells
177
were permeabilized with 20μg/ml of protease K in TBS, and endogenous peroxidase Q4 178
was inactivated by 3% H2O2in methanol. Apoptosis was detected by labeling 3′-OH 179
ends of fragmented DNA with biotin–dNTP using Klenow at 37°C for 1.5 h. Slides were
180
then incubated with streptavidin–horseradish peroxidase conjugate for 30 min,
181
followed by incubation with 3,3′-diaminobenzidine and H2O2for 10 min. Apoptotic 182
cells were identified by their dark brown nuclei as seen under a light microscope.
183
2.5. Flow cytometric analysis
184
Cellular DNA content was determined by flow cytometric analysis of propidium
185
iodide (PI)-labeled cells. After plates of KB cells (1×106
cells/ml) were grown to
186
semiconfluence, cell growth was arrested by washing plates with growth media
187
supplemented with 1% FBS. Growth arrest was maintained for 24 h. The cell cycle
188
synchronized cells were then washed with phosphate-buffered saline (PBS) and
189
restimulated to enter the G1 phase together by addition of growth media containing
190
flavokawain B, without FBS. After treatment with flavokawain B (5–20 μg/ml for 24, 48
191
and 72 h), cells were collected by trypsinization and fixed in 70% ethanol at−20°C
192
overnight. Cells were suspended in PBS containing 1% Triton X-100, 0.5 mg/ml RNase
193
and 4μg/ml PI at 37°C for 30 min. A FACSCalibur flow cytometer (Becton Dickinson, San
194
Jose, CA, USA) equipped with a single argon ion laser (488 nm) was used for flow
195
cytometric analysis. Forward and right-angle light scattering, correlated with cell size
196
and cytoplasmic complexity, respectively, were used to establish size gates and exclude
197
cellular debris from the analysis. The DNA content of 10,000 cells/analysis was
198
monitored using the FACSCalibur system. Apoptotic nuclei were identified as a
199
subploid DNA peak and were distinguished from cell debris on the basis of forward
200
light scattering and PI fluorescence. Cell cycle profiles were analyzed with ModFit
201
software (Verity Software House, Topsham, ME, USA).
202 2.6. Measurement of reactive oxygen species generation
203 Production of intracellular reactive oxygen species (ROS) was detected by
204 fluorescence microscopy or flow cytometry using 2′,7′-dihydrofluorescein-diacetate
205 (DCFH-DA). Cells (5×105
cells/6 wells) were cultured in DMEM supplemented with
206 10% heat-inactivated FBS, with renewal of the culture medium when the cells
207 reached 80% confluence. Samples were then incubated with 10μmol/l DCFH-DA in
208 culture medium at 37°C for 30 min. During loading, the acetate groups on DCFH-DA
209 were removed by intracellular esterase, trapping the probe inside the KB cells. After
210 loading, cells were washed with warm PBS buffer. Production of ROS species can be
211 measured by changes in fluorescence due to intracellular production of
dichloroflu-212 orescein (DCF) caused by oxidation of DCFH. Intracellular ROS, as indicated by DCF
213 fluorescence, was measured with a fluorescence microscope (Olympus 1X 71) or a
214 flow cytometer (FACSCalibur).
215 2.7. Analysis of mitochondrial membrane potential
216 The loss of mitochondrial membrane potential was assessed by flow cytometry.
217 Cells (5×105
cells/6 wells) were harvested and washed twice, suspended in 500μl of
218 DiOC6 (20μmol/l) and incubated at 37°C for 30 min. The excitation wavelength was
219 488 nm, with monitoring at 530 nm (DiOC6). Cell percentages were calculated with
220 ModFit software.
221 2.8. Western blotting
222 KB cells (3.0×106
cells/100-mm dish) were detached, washed once in cold PBS
223 and then suspended in 100μl lysis buffer (10 mmol/l Tris–HCl, pH 8, 0.32 mol/
224 l sucrose, 1% Triton X-100, 5 mmol/l EDTA, 2 mmol/l DTT, 1 mmol/l PMSF
Q5 ).
225 Suspensions were kept on ice for 20 min, then centrifuged at 13,000×g for 20 min at
226 4°C. Total protein content was determined with the Bio-Rad protein assay reagent,
227 using BSA
Q6 as the standard. Protein extracts were reconstituted in sample buffer [0.062
228 mol/l Tris–HCl, 2% sodium dodecyl sulfate (SDS), 10% glycerol, 5%
β-mercaptoetha-229 nol], and the mixture was boiled for 5 min. Equal amounts (50μg) of denatured
230 protein samples were loaded into each lane, separated by SDS–polyacrylamide gel
231 electrophoresis (PAGE) on an 8%–15% polyacrylamide gradient and then transferred
232 to polyvinylidene diflouride membranes overnight. Membranes were blocked with
233 0.1% Tween-20 in PBS containing 5% (wt/vol) nonfat dried milk for 20 min at room
234 temperature, incubated with primary antibodies for 2 h, then incubated with either
235 horseradish peroxidase-conjugated goat antirabbit or antimouse antibodies for 2
236 h before being developed using the SuperSignal ULTRA chemiluminescence substrate
237 (Pierce, Rockford, IL, USA). Band intensities were quantified by densitometry, with the
238 absorbance of the mixture at 540 nm determined using an enzyme-linked
239 immunosorbent assay plate reader. Western blot analysis, with antibodies against
240 cytochrome c, caspase-3, caspase-8, caspase-9, PARP, Bcl-2, Bax, Fas, FasL, Bid, cyclin
241 A, cyclin B1, Cdc2, Cdc25C, p21/WAF1, Wee1, p53, MMP-9, u-PA, TIMP-1 and PAI-1,
242 was done as previously described[2].
243 2.9. Determination of MMP-9 activity by zymography
244 MMP-9 activity in the medium was measured using a gelatin zymography
245 protease assay[14]. Briefly, an appropriate volume (adjusted by vital cell number) of
246 medium was collected and prepared in SDS sample buffer without boiling or
247 reduction and then subjected to SDS-PAGE (8% polyacrylamide, 0.1% gelatin).
248 Following electrophoresis, gels were washed with 2.5% Triton X-100, incubated in
249 reaction buffer (40 mmol/l Tris–HCl, pH 8.0; 10 mmol/l CaCl2; 0.01% NaN3) at 37°C 250 for 24 h and then stained with CBB
Q7 R-250.
251 2.10. Animals
252 Female athymic nude mice (BALB/c-nu), 5–7 weeks of age, were purchased from
253 GlycoNex, Inc., Taiwan, and were maintained in caged housing in a specifically
254 designed pathogen-free isolation facility with a 12/12-h light–dark cycle; mice were
255 provided rodent chow and water ad libitum. All experiments were conducted in
256 accordance with the guidelines of the China Medical University Animal Ethics
257 Research Board.
258 2.11. Tumor cell inoculation
259 KB cells were grown in DMEM medium supplemented with 10% heat-inactivated
260 FBS, 2 mmol/l glutamine and 1% penicillin–streptomycin–neomycin in a humidified
261 incubator (5% CO2in air at 37°C). Experiments were performed using cells from fewer 262 than 20 passages. Cells (1×106) were mixed in a 200-μl atrix gel including growth 263 factors and then injected subcutaneously on the right-hind flank. Tumor volume, as
264 determined by caliper measurements of tumor length, width, and depth, were
265 calculated using the formula: length×width2×0.5236, every 3 days[15]. In this study, 266 the three pretested mouse (n=1) received intraperitoneal injections of flavokawain B
267 at doses of 0, 0.35 and 0.75 mg/kg. Tumor growth and volume significantly decreased
268 with a flavokawain B dose of 0.75 mg/kg, which suggested that this dose should be used
269 in xenografted nude mice. Therefore, two groups received intraperitoneal injections of
270
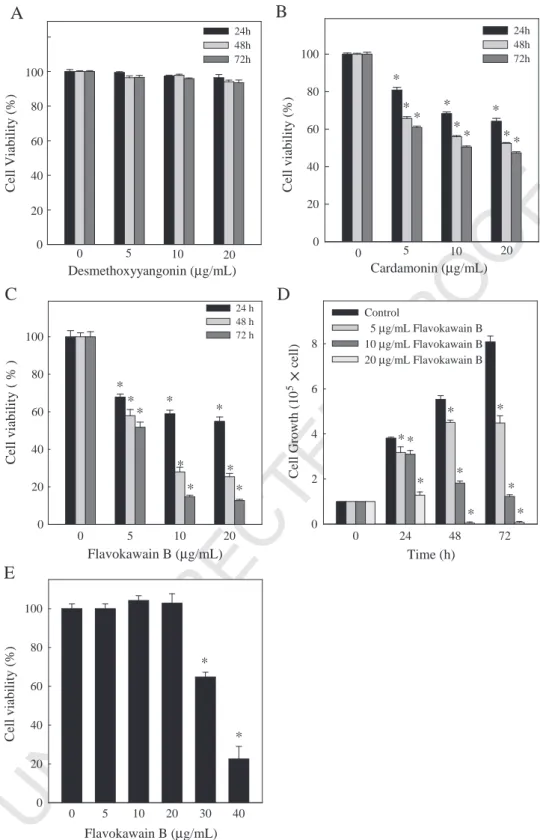
flavokawain B (0.2 ml/mouse) dissolved in 0.1% DMSO buffer at a dose of 0.75 mg/kg
271
every 2 days, while the control group received daily injections of vehicle only.
272
Following 27 days of treatment, the mice were photographed and killed. Tumors were
273
removed before fixing in 4% paraformaldehyde, sectioning, and staining with
274
hematoxylin–eosin for light microscopy. Samples tissue from each tumor tissue was
275
immediately frozen, and the rest were fixed in 10% neutral-buffered formalin and
276
embedded in paraffin. To monitor drug toxicity, the body weight of each animal was
277
measured every 3 days. In addition, a pathologist examined the mouse organs,
278
including the liver, lungs and kidneys.
279
2.12. In situ apoptosis detection
280
Apoptotic cell death in deparaffinized tissue sections was detected using TUNEL
281
with the Klenow DNA fragmentation detection kit (Calbiochem)[16]. Briefly, sections
282
were permeabilized with 20μg/ml protease K in TBS, and endogenous peroxidase was Q8 283
inactivated by 3% H2O2in methanol. Apoptosis was detected by labeling 3′-OH ends of 284
fragmented DNA with biotin–dNTP using Klenow at 37°C for 1.5 h. Slides were then
285
incubated with streptavidin–horseradish peroxidase conjugate, followed by incubation
286
with 3,3′-diaminobenzidine and H2O2. Apoptotic cells were identified by the dark 287
brown nuclei observed under light microscopy.
288
2.13. Statistics
289
In vitro results are presented as mean±standard deviation (mean±S.D.). For
290
in vivo experiments, mean data values are presented with standard error (mean±S.E.).
291
All study data were analyzed using analysis of variance, followed by Dunnett's
292
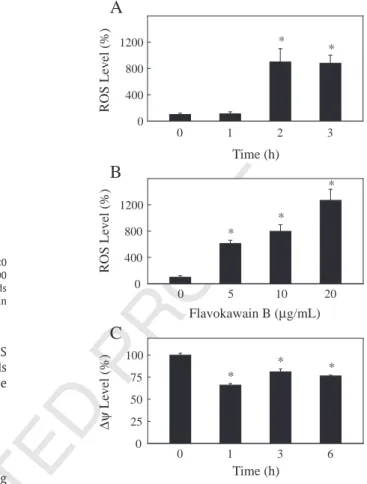
test for pairwise comparison. Statistical significance was defined as Pb.05 for
293
all tests.
294 3. Results
295 In this study, the human squamous carcinoma cell line KB was
296 used to investigate the capability of flavokawain B (5–20 μg/ml), a
297 chalcone purified from ethanol (70%) extracts of A. pricei rhizomes, to
298 induce cell cycle arrest and apoptosis, and to elucidate the molecular
299 mechanisms involved.
300 3.1. Effects of desmethoxyyangonin, cardamonin and flavokawain B on
301 KB cell death
302 To investigate the effects of A. pricei extracts on survival or
303 growth, KB cells were exposed to 5, 10 or 20 μg/ml doses of
304 desmethoxyyangonin and cardamonin for 24 h and for flavokawain
305 B for 24, 48 or 72 h. Fig. 1B–D shows that cardamonin and
306 flavokawain B induced cell death (viability or growth) in a dose- and
307 time-dependent manner, as determined by trypan blue exclusion.
308 However, desmethoxyyangonin concentrations of 5–20 μg/ml did
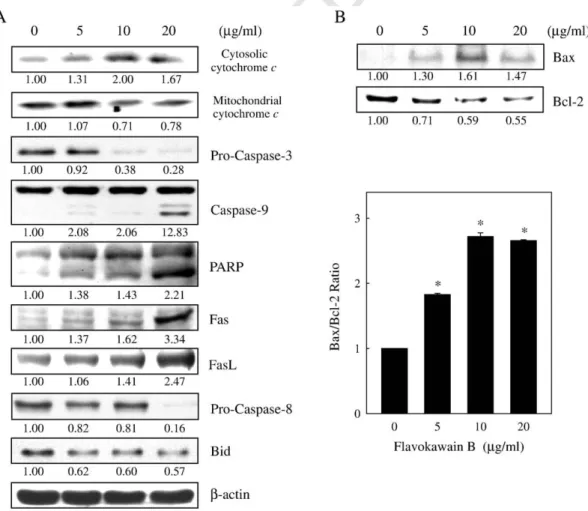
309 not affect the number of KB cells at 24 h (Fig. 1A). The
310 concentrations of flavokawain B required for 50% inhibition of KB
311 cell viability (IC50) were approximately 30.0, 5.7 and 4.3 μg/ml for
312 24, 48 and 72 h, respectively (Fig. 1D). The effect of flavokawain B
313 on human HGF cells was then investigated. At 24 h, flavokawain B
314 concentrations of 5, 10 and 20μg/ml did not affect the number of HGF
315 cells; however, flavokawain B concentrations of 30 and 40 μg/ml
316 proved to be cytotoxic (Pb.05) (Fig. 1E). Comparative experiments
317 on the responses of KB and HGF cells to treatment with flavokawain B
318 showed reduced cell viability in response to treatment in both cell
319 lines, but the reduction was more pronounced in KB cells than in
320 HGF cells.
321 3.2. Induction of apoptotic DNA fragmentation by flavokawain B
322 After incubation for 24 h, the majority of KB cells (Pb.05) treated
323 with flavokawain B (at 0, 5, 10 and 20μg/ml) contained condensed
324 nuclei (data not shown).Fig. 2showed characteristic populations of
325 flavokawain B-treated KB cells obtained using the TUNEL assay for
326 DNA apoptotic fragmentation. Apoptotic cells were identified by their
327 dark nuclei as seen under a light microscope.
328 3.3. Sub-G1 accumulation and G2/M arrest in flavokawain B-treated 329 KB cells
330 DNA content profiles of flavokawain B-treated KB cells were 331 obtained using flow cytometry to measure the fluorescence of PI–
332 DNA binding. Cells with less DNA staining relative to diploid analogs
333 were considered apoptotic. There was a remarkable (Pb.05)
accu-334 mulation of subploid cells, the so-called sub-G1 peak, in flavokawain
335 B-treated KB cells (5–20 μg/ml for 24 h) compared with the untreated
336 group (Fig. 3). Furthermore, flavokawain B-induced growth
Desmethoxyyangonin (
μg/mL)
Cell Viability
(%)
24h 48h 72h 0 5 10 20 0 40 80 60 100 20Cardamonin (
μg/mL)
Cell viability (%)
0 20 40 60 80 100 24h 48h 72h 0 5 10 20*
*
*
*
*
*
*
* *
Flavokawain B (
μg/mL)
0 5 10 20 30 40Cell v
iability (%)
0 20 40 60 80 100*
*
Time (h)
0 24 48 72Cell Growth (10
5cell)
0 2 4 6 8 Control 5 μg/mL Flavokawain B 10 μg/mL Flavokawain B 20 μg/mL Flavokawain B* *
*
*
*
*
*
*
*
Flavokawain B (
μg/mL)
0 5 10 20Cell viability
( % )
0 20 40 60 80 100 24 h 48 h 72 h*
*
*
*
*
*
*
*
*
C
D
E
B
A
Fig. 1. Effects of desmethoxyyangonin, cardamonin and flavokawain B upon cell death (viability or growth) of human squamous carcinoma KB cells and normal HGF cells. (A–D) KB cells were treated with 0, 5, 10 or 20μg/ml of (A) desmethoxyyangonin or (B) cardamonin for 24 h and (C and D) flavokawain B for 24, 48 or 72 h. Cultures were harvested and cell number determined by counting cell suspensions using a hemocytometer. (E) HGF cells were treated with 0, 5, 10, 20, 30 or 40μg/ml of flavokawain B for 24 h. Cell numbers determined by counting cell suspensions using a hemocytometer. Results are presented as mean±S.D. of three assays. An asterisk (*) indicates a significant difference in comparison with the control group (Pb.05).
337 inhibition led to increased percentages of KB cells in G2/M and S 338 phase, resulting in a progressive and sustained accumulation of cells 339 in the G2/M phase. Correspondingly, percentages of cells in G1 phase 340 decreased over time.
341 3.4. ROS generation and mitochondrial dysfunction in flavokawain 342 B-treated KB cells
343 Fluorescence microscopic or flow cytometric analysis using 344 DCFH-DA as a fluorescence probe was used for estimating the 345 generation of ROS. Basal DCFH-DA fluorescence was demonstrated 346 in the untreated KB cells (control). Incubation of cells with 347 flavokawain B (10 μg/ml for 0, 1, 2 or 3 h) caused a significant 348 increase in fluorescence, with a maximum ROS increase (Pb.05) 349 observed at 2 h after treatment (Fig. 4A). Dose-dependent increase 350 (Pb.05) in ROS generation after flavokawain B treatment (0, 5, 10 or 351 20 μg/ml for 2 h) were also observed (Fig. 4B). To determine 352 whether an early loss of mitochondrial membrane potential 353 occurred during treatment with flavokawain B, KB cells were 354 grown in the absence (control) or in the presence of flavokawain 355 B (10 μg/ml for 0, 1, 3 or 6 h). The mitochondrial membrane 356 potential was determined by flow cytometry. Fig. 4C shows that 357 treatment with flavokawain B resulted in loss of the mitochondrial 358 membrane potential in KB cells (Pb.05), indicating its ability to
359 induce mitochondrial dysfunction. 3.5. Flavokawain B induces release of cytochrome c, activation 360 361 of caspase-3 and -9 and cleavage of PARP
362 It has been reported that treatment of cells with a variety of
363 chemotherapeutic agents is accompanied by increased cytosolic
364 translocation of cytochrome c, activation of caspase-3 and
degrada-365 tion of PARP[17]. In the present study, cytosolic and mitochondrial
366 levels of cytochrome c were examined using Western blot analysis.
367 The results revealed that flavokawain B induced the release of
368 cytosolic cytochrome c from 24 h after treatment (Fig. 5A). As
369 cytochrome c is reportedly involved in the activation of the caspases
370 that trigger apoptosis[17], we investigated the roles of caspase-3 and
371 -9 in the cellular response to flavokawain B. Immunoblotting analysis
372 revealed that treatment of KB cells with flavokawain B induced
373 proteolytic cleavage of pro-caspase-3 and -9 into their active forms
374 (Fig. 5A).Fig. 5A shows the increase in levels of cleaved caspase-9, and
375 there seems to be no change in total pro-caspase-9 levels. Since
PARP-376 specific proteolytic cleavage by caspase-3 is considered to be a
377 biochemical characteristic of apoptosis, a Western blot experiment
Flavokawain B (
μg/mL)
0 5 10 20Apoptosis cells
0 100 200 300 400*
*
*
Fig. 2. TUNEL assay of KB cells exposed to flavokawain B. Cells treated with 0, 5, 10 or 20 μg/ml of flavokawain B for 24 h were examined under a light microscope (×400 magnification). The average number of apoptotic-positive cells in microscopic fields from three separate samples. An asterisk (*) indicates a significant difference in comparison with the control group (Pb.05).
Apoptotic cells Non-apoptotic cells
(μg/mL) sub-G1 G1 S G2/M
0 0.3 ± 0.1 77.8 ± 1.2 13.0 ± 1.0 9.2 ± 0.2 5 1.0 ± 0.8* 59.8 ± 2.2* 16.8 ± 0.9* 23.5 ± 2.4* 10 6.8 ± 0.6* 56.1 ± 0.4* 20.7 ± 0.7* 23.9 ± 0.7* 20 16.6 ± 2.2* 55.2 ± 3.9* 22.8 ± 5.8* 22.0 ± 2.3*
Fig. 3. Effects of flavokawain B on cell cycle distribution in KB cells. Cells were treated with 0, 5, 10 or 20μg/mll flavokawain B for 24 h, stained with PI and analyzed for sub-G1 and cell cycle phase using flow cytometry. Cellular distribution (percentage) in different phases of the cell cycle (sub-G1, G1, S and G2/M) after treatment with flavokawain B. Apoptotic nuclei were identified as a subploid DNA peak and distinguished from cell debris on the basis of forward light scattering and PI fluorescence. Results are presented as mean±S.D. of three assays. An asterisk (*) indicates a significant difference in comparison with the control group (Pb.05).
Time (h)
0 1 3 6 0 25 50 75 100 * * *Flavokawain B (
μg/mL)
0 5 10 20 0 400 800 1200 * * *Time (h)
0 1 2 3ROS Level (%)
ROS Level (%)
Δψ
Level (%)
0 400 800 1200 * *A
B
C
Fig. 4. Effects of flavokawain B on intracellular ROS levels and mitochondrial membrane potential in KB cells. (A and B) Cells were treated with 0–20 μg/ml of flavokawain B for 0, 1, 2 or 3 h. The nonfluorescent cell membrane-permeable probe DCFH-DA was added to the culture medium at a final concentration of 10μmol/l 30 min before the end of each experiment. DCFH-DA was used to penetrate cells, react with cellular esterases and ROS and be metabolized into fluorescent DCF. The intracellular ROS level (as a percentage of the control), as indicated by DCF fluorescence, was measured by fluorescence microscopy (×200 magnification) (A and B). (C) Effect of flavokawain B on the mitochondrial membrane potential of KB cells. Cells were grown in the absence (control) or presence of flavokawain B (10μg/ml) for 0, 1, 3 or 6 h; stained with DiOC6 and analyzed by flow cytometry as described in “Materials and Methods.” The mitochondrial membrane potential after treatment with flavokawain B as a percentage of the control, as indicated by DiOC6 fluorescence, is shown. Results are the mean±S.D. of three assays. An asterisk (*) indicates a significant difference in comparison with the control group (Pb.05).
378 was done using an antibody against PARP, a nuclear enzyme involved 379 in DNA repair[18].Fig. 5A demonstrates that following the addition of 380 flavokawain B, the 115-kd PARP protein is cleaved to a 85-kd 381 fragment in KB cells.
382 3.6. Activation of the Fas-mediated apoptosis pathway by flavokawain B 383 results in activation of caspase-8 and cleavage of Bid
384 To assess whether flavokawain B (5–20 μg/ml for 24 h) promoted 385 apoptosis via a receptor-mediated pathway, the levels of Fas and FasL 386 proteins in KB cells were determined by Western blot. The results show 387 that flavokawain B stimulated the expression of Fas and FasL (Fig. 5A). 388 To verify whether the activation of caspase-8 is associated with Fas and 389 FasL production in response to treatment with flavokawain B[19], 390 involvement of caspase-8 activation is further supported by immuno-391 blotting analysis, with the results suggesting tat proteolytic cleavage of 392 pro-caspase-8 is induced (Fig. 5A). Next, the expression levels of 393 proapoptosis protein Bid, which produces the truncated Bid fragment 394 upon cleavage by caspase-8, were measured. Bid fragment causes 395 mitochondrial damage and amplifies apoptotic signals by activating the 396 mitochondrial pathway[20]. The results indicate that flavokawain B 397 induced down-regulation of Bid in KB cells (Fig. 5A).
398 3.7. Flavokawain B induces dysregulation of Bcl-2 and Bax proteins 399 As shown in Fig. 5B, incubation of KB cells with flavokawain B 400 caused a dramatic reduction in the level of Bcl-2, a potent cell-death
401 inhibitor, and increased the level of Bax protein, which
hetero-402 dimerizes with and thereby inhibits Bcl-2. These results indicate that
403 flavokawain B induced dysregulation of Bcl-2 and Bax in KB cells.
404 3.8. Inhibitory effects of flavokawain B on cyclin A, cyclin B1, Cdc2
405 and Cdc25C expression
406 In order to examine the molecular mechanism(s) and underlying
407 changes in cell cycle patterns caused by flavokawain B treatment, we
408 investigated the effects upon various cyclins and CDKs involved in cell
409 cycle control in KB cells. KB cells were treated with flavokawain B (5–
410 20μg/ml) for 24 h. Dose- and time-dependent reductions in mitotic
411 cyclins A and B1, mitotic-cyclin-dependent kinase Cdc2 and mitotic
412 phosphatase Cdc25C expression were observed (Fig. 6A). These
413 results imply that flavokawain B inhibits cell cycle progression by
414 reducing levels of cyclin A, cyclin B1, Cdc2 and Cdc25C.
415 3.9. Flavokawain B increases the expression of p21/WAF1, Wee1 and p53
416 As shown in this study, treatment of KB cells with flavokawain B
417 resulted in cell cycle arrest. The effect of exposure to flavokawain B on
418 cell cycle-regulatory molecules, including p21/WAF1 (CDK
inhibi-419 tors), Wee1 (CDK relative factors) and p53, was then examined.
420
Fig. 6A shows that treatment of KB cells with flavokawain B (5–20 μg/ml 421 for 24 h) induced marked (Pb.05) dose- and time-dependent
up-422 regulation of p21/WAF1, Weel and p53 protein expression.
Fig. 5. Western blot analysis of mitochondrial and cytosolic cytochrome c, caspase-3, caspase-8, caspase-9, PARP, Fas, FasL, Bid (A) and Bcl-2 and Bax protein levels (B) in KB cells exposed to flavokawain B. Cells were treated with 0, 5, 10 or 20μg/ml flavokawain B for 24 h. Protein (50 μg) from each sample was resolved by SDS-PAGE (8%–15% polyacrylamide gel) withβ-actin as a control. Relative changes in protein bands were measured by densitometry. A typical result from three independent experiments is shown.
423 3.10. Effects of flavokawain B on levels of MMP-9, u-PA, TIMP-1 424 and PAI-1 and on activity of MMP-9
425 Western blotting was used to analyze the effects of flavokawain B 426 on the expression of the metastasis-related proteins MMP-9, u-PA, 427 TIMP-1 and PAI-1. As shown inFig. 6B, treatment of KB cells with 428 flavokawain B (5–20 μg/ml for 24 h) markedly (Pb.05) induced dose-429 dependent reduction of the expression levels of MMP-9 and u-PA. 430 Dose-dependent up-regulation of the expression of their specific 431 endogenous inhibitors, TIMP-1 and PAI-1, was found after treatment 432 with flavokawain B (Fig. 6B). Moreover, gelatin zymography assays 433 showed that flavokawain B (5–20 μg/ml for 24 h) reduced MMP-9 434 activity in a dose-dependent manner in KB cells (Fig. 6C).
435 3.11. In vivo inhibition of KB xenograft growth by flavokawain B 436 Nude mice were used to evaluate the in vivo effects of 437 flavokawain B on tumor growth. KB cells were xenografted into 438 nude mice as described in “Materials and Methods.” All animals 439 appeared healthy, with no loss of body weight noted during 440 flavokawain B treatment (Fig. 7A). In addition, no signs of toxicity 441 were observed in any of the nude mice (body weight and 442 microscopic examination of individual organs; data not shown). 443 The time course for KB xenograft growth with flavokawain B (0.75 444 mg/kg every 2 days) or with vehicle only (control) is shown inFig.
445 7B. Evaluation of tumor volume showed significant time-dependent 446 growth inhibition associated with flavokawain B treatment. Tumor 447 volume in the flavokawain B-treated mice was inhibited compared 448 with the control group (Fig. 7C). At the end of 27 days, the KB
449 xenograft tumor was excised from each animal that was killed. In
450 addition, microscopic examination of tumor sections was done to
451 distinguish differences in nucleic and cytoplasmic morphology after
452 27 days of flavokawain B treatment. As shown in Fig. 8A, the
453 histopathological findings from inoculated squamous cell carcinomas
454 in tumor control nude mice presented newly formed blood vessels
455 with massive necrosis in the area of the tumor mass. Tumor cells
456 were large, round to oval in shape with predominant nucleoli and
457 expressed high levels of cellular activity and mitotic figures. In
458 contrast, tumors in the flavokawain B-treated nude mice showed less
459 angiogenesis, had smaller cells with shrunken and had condensed
460 and pyknotic nuclei, indicating tumor cell inactivity or regression
461 (Fig. 8A). Interestingly, while abundant mitosis was observed in the
462 proliferating cells in the control group, few mitotic cells were seen in
463 sections from flavokawain B-treated animals (Fig. 8B). These results
464 demonstrate flavokawain B-related antitumor activity in nude mice
465 bearing KB epidermoid carcinoma xenografts.
466 3.12. Induction of apoptotic DNA fragmentation by flavokawain B in
467 xenograft tumors
468 The effect of flavokawain B on tumor growth (apoptosis) in the KB
469 xenograft mice was also examined using the TUNEL assay on tumor
470 sections.Fig. 9A, B shows that there were more TUNEL-positive cells
471 in tumors from flavokawain B-treated animals, compared to
untreat-472 ed controls (Pb.05), which demonstrates that flavokawain B
treat-473 ment was associated with decreased proliferation and increased
474 apoptosis in the study animals. Analysis of our data suggests that
Fig. 6. Western blot analysis of cyclin A, cyclin B1, Cdc2, Cdc25C, p21/WAF1, Wee1, p53 (A), MMP-9, u-PA, TIMP-1 and PAI-1 protein levels (B) and MMP-9 activity (C) in KB cells after exposure to flavokawain B. (A and B) Cells were treated with 0, 5, 10 or 20μg/ml flavokawain B for 24 h. Protein (50 μg) from each sample was resolved by SDS-PAGE (8%–15% polyacrylamide gel) and Western blot analysis withβ-actin as a control. (C) Cells were treated with 0, 5, 10 or 20 μg/ml flavokawain B for 24 h and then subjected to gelatin zymography to analyze MMP-9 activity. Relative changes in bands were measured by densitometry. Typical results from three independent experiments are shown. Results are presented as mean±S.D. of three assays. An asterisk (*) indicates a significant difference in comparison with the control group (Pb.05).
475 flavokawain B promoted antitumor activity in nude mice bearing KB 476 epidermoid carcinoma xenografts.
477 4. Discussion
478 This study documents the chemopreventive effects of flavoka-479 wain B, a chalcone purified from A. pricei, in vitro cell culture and 480 in vivo nude mice models of human squamous carcinoma KB cells. 481 Chalcones form an important class of naturally occurring biological 482 compounds with a widespread distribution in fruits, vegetables, 483 spices, tea and soy-based foodstuffs and have been the subject of 484 great interest for their biological activities [9]. In structure, 485 chalcones are open-chain flavonoids in which the two aromatic 486 rings are joined by a three-carbon α, β-unsaturated carbonyl 487 system. A vast number of naturally occurring chalcones are 488 polyhydroxylated on the aryl rings. The radical-quenching proper-489 ties of the phenolic groups present in many chalcones have raised 490 interest in using these compounds or chalcone-rich plant extracts 491 as food preservatives [10]. We showed that flavokawain B, a 492 chalcone derivative, directly inhibited cell viability and growth of 493 KB cells by induction of cell cycle arrest and apoptosis. Interest-494 ingly, flavokawain B has been found to show less cytotoxicity in 495 normal HGF cells. Furthermore, in vivo tumor inhibition by 496 flavokawain B was observed in the nude mice xenograft model 497 in this study. Both incidence and mean tumor volume were 498 significantly reduced by flavokawain B treatment. Immunohisto-499 chemical staining revealed increased apoptosis (TUNEL assay) in
500 tumors from flavokawain B-treated animals. Analysis of our data
501 suggests that flavokawain B could inhibit proliferation of human
502 squamous carcinoma KB cells both in vitro and in vivo. The
503 chemopreventive properties of flavokawain B combined with the
504 epidemiologic and experimental data [11,12]prompted this study
505 into the inhibitory effects of treatment with flavokawain B upon
506 human squamous carcinoma cells.
507 Apoptosis is an important homeostatic mechanism that balances
508 cell division and cell death and maintains the appropriate number of
509 cells in the body. Many studies have shown associations between
510 apoptosis and cancer, and apoptosis-inducing agents are being
511 investigated as tools for the management of cancer. Apoptosis is
512 controlled by two major pathways; a mitochondrial pathway [17]
513 and a membrane death receptor (DR) pathway [19]. The first
514 involves the participation of mitochondria and, in most forms of
515 apoptosis, is a response to cellular stress, loss of survival factors and
516 developmental cues [17]. The second pathway involves the
517 interaction of cell surface receptors, such as Fas, TNFR, DR3, DR4 Q9
518 and DR5, with their ligands. In the former, activation of DRs (Fas) by
519 cross-linking with their natural ligands (FasL) leads to receptor
520 clustering and formation of a death-inducing signaling complex,
521 which results in the activation of pro-caspase-8, which subsequently
522 promotes proteolytic processing of pro-caspase-3 and Bid [19]. In
523 the latter, the loss of mitochondrial membrane potential induces the
524 release of cytochrome c from mitochondria into the cytosol, where it
525 binds to apoptotic protease activation factor-1. Meanwhile,
pro-526 caspase-9 also binds to apoptotic protease activation factor-1, and
Fig. 7. In vivo inhibition of KB xenograft proliferation by flavokawain B. Time-course effect of flavokawain B on growth of KB xenografted nude mice was evaluated by measurements of body weight (A) and tumor volume (B) every 3 days. KB cells were implanted subcutaneously into the flanks of nude mice on day 0, and animals were subsequently treated with 0.75 mg/kg of flavokawain B or vehicle only (control). (C) On the 27th day after tumor implantation, animals were photographed. Results are presented as mean±S.E. (n=6). An asterisk (*) indicates a significant difference in comparison with control group (Pb.05).
527 this interaction activates pro-caspase-9. Activated caspase-9 acti-528 vates downstream pro-caspase-3 [17,18]. Activated caspase-3 is 529 responsible for the proteolytic degradation of PARP, which occurs at 530 the onset of apoptosis [18]. The present study demonstrates that 531 treatment of KB cells with flavokawain B can induce apoptotic cell 532 death associated with internucleosomal DNA fragmentation; sub-G1 533 phase accumulation; elevation of ROS; loss of mitochondrial 534 membrane potential; translocation of cytochrome c; activation of 535 caspase-3, -8 and -9; degradation of PARP; dysregulation of Bcl-2 536 and Bax; induction of Fas and FasL expression and down-regulation 537 of Bid. Data from the present study suggest that flavokawain B-538 induced apoptosis is controlled by both mitochondrial and mem-539 brane DR pathways.
540 It has been shown that the Bcl-2 family of proteins has an 541 important regulatory role in apoptosis, both in activation (Bax) and 542 inhibition (Bcl-2)[21]. Of the Bcl-2 family members, the Bcl-2/Bax 543 protein ratio has been recognized as a key factor in regulation of the 544 apoptotic process [21]. In the present study, the increase in 545 flavokawain B-induced apoptosis was associated with a reduction 546 in the levels of Bcl-2, a potent cell-death inhibitor, as well as an 547 increase in the levels of Bax protein, which heterodimerizes with,
548 and thereby inhibits, Bcl-2. These data indicate that flavokawain B
549 treatment disturbs the Bcl-2/Bax ratio and thereby leads to
550 apoptosis of KB cells.
551 Many of the agents that induce apoptosis are oxidants or
552 stimulators of cellular oxidative metabolism, while many inhibitors
553 of apoptosis show antioxidant activity. Indeed, factors that cause or
554 promote oxidative stress, such as ROS production, lipid peroxidation,
555 down-regulation of antioxidant defences characterized by reduced
556 glutathione levels and reduced transcription of superoxide
dismu-557 tase, catalase and thioredoxin, have been shown to be involved in
558 some apoptotic processes [22,23]. Moreover, ROS can play an
559 important role in apoptosis by regulating the activity of certain
560 enzymes involved in the cell-death pathway[22,23]. All of these
561 factors point to a significant role for intracellular oxidative
562 metabolites in the regulation of apoptosis. Earlier studies have
563 shown that many stimuli such as anticancer drugs can cause cells to
564 produce ROS, which mediate mitochondria-initiated apoptosis by
565 inducing the loss of mitochondrial membrane potential[24]. In this
566 study, we also observed that flavokawain B significantly inhibits KB
567 cell survival concomitant with partial augmentation of ROS
568 accumulation, which is playing major role in apoptosis. However,
Fig. 8. Histochemical analysis of proliferation in KB xenograft tumors. (A) Control KB xenograft tumors and KB xenograft tumors following flavokawain B (0.75 mg/kg) treatment were examined using a light microscopy. Arrows indicate mitotic (tumor control) and pyknotic tumor cells (flavokawain B). Typical results from three independent experiments are shown. (B) Percentages of living cells in microscopic fields (×400 magnification) from six tumor samples were quantified and expressed compared with tumor control (100%). An asterisk (*) indicates a significant difference in comparison with the control group (Pb.05).
569 further investigations warranted to confirm flavokawain B-induced 570 ROS generation in KB cells.
571 Disturbance of the cancer cell cycle is one of the therapeutic 572 targets for development of new anticancer agents. The results of cell 573 cycle analysis in the present study, as evaluated by flow cytometry, 574 show that treatment with flavokawain B had a profound effect on cell 575 cycle control, with squamous carcinoma cells accumulating in the G2/ 576 M phase. This cell cycle blockade was associated with reductions in 577 cyclin A, cyclin B1, Cdc2 and Cdc25C and increased CDK inhibitor p21/ 578 WAF1, Weel and p53. Eukaryotic cell cycle progression involves the 579 sequential activation of CDKs, whose activation is dependent on 580 association with cyclins. Among CDKs that regulate cell cycle 581 progression, CDK2 and Cdc2 kinases are activated primarily in 582 association with cyclin A and cyclin B1 during progression of the 583 G2/M phase[25]. The phosphorylation of Tyr15 of Cdc2 suppresses 584 activity of the Cdc2/cyclin A and B1 kinase complex. Dephosphory-585 lation of Tyr15 of Cdc2 is catalyzed by Cdc25C phosphatase, and this 586 reaction is believed to be the rate-limiting step for entry into mitosis 587 [26]. Cell cycle progression is also regulated by the relative balance 588 between the cellular concentrations of CDK inhibitors such as p21/ 589 WAF1, which may help to maintain G2/M cell cycle arrest by 590 inactivating the cyclin B1/Cdc2 complex, disrupting the interaction 591 between proliferating cell nuclear antigen and Cdc25C [27]. Wee1 592 protein kinase negatively regulates entry into mitosis by catalyzing 593 the inhibitory tyrosine phosphorylation of Cdc2-cyclin B kinase[28]. 594 p53 could act as a sensor for DNA damage that arrests the cell cycle for 595 DNA repair or up-regulates proapoptotic factors, resulting in 596 increased susceptibility to apoptosis[27]. The results imply that the 597 expression levels of cyclin A, cyclin B, Cdc2 and Cdc25C are down-598 regulated and that p21/WAF1, Weel and p53 levels are increased in 599 flavokawain B-treated KB cells, which is consistent with a G2/M block. 600 Analysis of our data suggests that the observed inhibition of KB cell
601 growth associated with flavokawain B treatment could be the result of
602 cell cycle arrest during the G2/M phase.
603 There is increasing evidence that the related processes of
604 neoplastic transformation, progression and metastasis involve
alter-605 ation of the normal apoptotic pathways. In this study, we reveal that
606 flavokawain B extracts decreased the levels of tumor
metastasis-607 related proteins, such as MMP-9 and u-PA, in KB cells. Meanwhile,
608 their endogenous inhibitors TIMP-1 and PAI-1 were increased in KB
609 cells. Metastasis is the spread of cancer cells from the primary tumor
610 to new metastatic sites via the blood or lymph vessels[29]. MMPs and
611 u-PA, which are secreted by invasive cancer cells, have important
612 roles in cancer cell invasion and metastasis because tumor cells must
613 cross the type IV collagen-rich basement membrane of vessel walls to
614 spread to other sites during cancer metastasis [14]. Therefore,
615 inhibition of invasion mediated by MMPs and u-PA may be a key
616 feature of treatments that can successfully prevent cancer metastasis.
617 The physiological activity of MMPs and u-PA was highly correlated to
618 their specific endogenous inhibitors TIMPs and PAIs, respectively.
619 TIMPs has a key role in determining the proteolytic activity of tumor
620 tissues by regulating the activity of MMPs. PAIs (serine protease
621 inhibitors) regulate u-PA and the tissue plasminogen activator (tPA)
622 to control plasmin generation. TIMPs and PAIs have been implicated
623 as mediators of invasion and metastasis in several types of tumor
624
[30,31]. It has been shown that KB cells exhibit reduced motility and 625 reflect fewer invasions without altering the MMP status [32].
626 Therefore, the inhibition of KB cell migration and invasion by
627 flavokawain B was not examined in this study.
628 The results obtained in vitro and in vivo in this study imply that
629 flavokawain B could act as a chemopreventive agent with respect to
630 inhibition of the growth of human squamous carcinoma KB cells
631 through the induction of cell cycle arrest and apoptosis. These data
632 provide an important step that might help model the effects of
Fig. 9. Immunohistochemical staining of apoptotic DNA fragmentation in KB xenograft tumors. (A) In situ apoptosis detection using TUNEL staining in tumor sections from control animals and experimental analogs treated with flavokawain B (0.75 mg/kg). Arrow indicates example apoptotic-positive cells (×400 magnification). Typical results from three independent experiments are shown. (B) The number of apoptotic-positive cells in microscopic fields from three samples was averaged. An asterisk (*) indicates a significant difference in comparison with the control group (Pb.05).
633 flavokawain B for potential future studies with animal models and 634 human patients and thereby facilitate the development of nutraceu-635 tical products using this agent.
636 Acknowledgments
637 This work was supported by grants NSC-99-2320-B-039-035-638 MY3, CMU 96-207, CMU 96-112 and CMU 97-130 from the National 639 Science Council and China Medical University of Taiwan.
640 References
641 [1] Larsen K, Ibrahim H, Khaw SH, Saw LG. In: Wong KM, editor. Natural history
642 publications, Borneo. Gingers of Peninsular Malaysia and Singapore
Q10 ; 1999. p. 135.
643 Kota Kinabalu, Sabah.
644 [2] Yang HL, Chen SC, Chen CS, Wang SY, Hseu YC. Alpinia pricei rhizome extracts
645 induce apoptosis of human carcinoma KB cells via a mitochondria-dependent
646 apoptotic pathway. Food Chem Toxicol 2008;46:3318–24.
647 [3] Lin CT, Kumar KJS, Tseng YH, Wang ZJ, Pan MY, Xiao JH. Anti-inflammatory activity
648 of flavokawain B from Alpinia pricei Hayata. J Agric Food Chem 2009;57:6060–5.
649 [4] Chen IN, Chang CC, Ng CC, Wang CY, Shyu YT, Chang TL. Antioxidant and
650 antimicrobial activity of Zingiberaceae plants in Taiwan. Plant Foods Hum Nutr
651 2008;63:15–20.
652 [5] Hseu YC, Chen CS, Wang SY. Alpinia pricei rhizome extracts induce cell cycle arrest
653 in human squamous carcinoma KB cells and suppress tumor growth in nude mice.
654 Evid Based Complement Alternat Med 2009 (accepted
Q11 ).
655 [6] Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature
656 2001;411:342–8.
657 [7] Sherr CJ. The ins and outs of RB: coupling gene expression to the cell cycle clock.
658 Trends Cell Biol 1994;4:15–8.
659 [8] Wyllie AH. Apoptosis: cell death in tissue regulation. J Pathol 1987;153:313–6.
660 [9] Go ML, Wu X, Liu XL. Chalcones: an update on cytotoxic and chemoprotective
661 properties. Curr Med Chem 2005;12:481–99.
662 [10] Dhar DN. The chemistry of chalcones and related compounds. New York: John
663 Wiley; 1981.
664 [11] Zi X, Simoneau AR. Flavokawain A, a novel chalcone from kava extract, induces
665 apoptosis in bladder cancer cells by involvement of Bax protein-dependent and
666 mitochondria-dependent apoptotic pathway and suppresses tumor growth in
667 mice. Cancer Res 2005;65:3479–86.
668 [12] Steiner GG. The correlation between cancer incidence and kava consumption.
669 Hawaii Med J 2000;59:420–2.
670 [13] Masters JR. HeLa cells 50 years on: the good, the bad and the ugly. Nat Rev Cancer
671 2002;2:315–9.
672
[14] Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in
673
tumor invasion. FASEB J 1999;13:781–92.
674
[15] Collins A, Yuan L, Kiefer TL, Cheng Q, Lai L, Hill SM. Overexpression of the MT1
675
melatonin receptor in MCF-7 human breast cancer cells inhibits mammary tumor
676
formation in nude mice. Cancer Lett 2003;89:49–57.
677
[16] Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in
678
situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992;119:
679
493–501.
680
[17] Green DR, Reed JC. Mitochondria and apoptosis. Science 1998;281:1309–12.
681
[18] Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR. Yama/CPP32
682
beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves
683
the death substrate poly(ADP-ribose) polymerase. Cell 1995;81:801–9.
684
[19] Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin
685
Cell Biol 1999;11:255–60.
686
[20] Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization
687
and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol
688
2000;20:929–35.
689
[21] Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science
690
1998;281:1322–6.
691
[22] Briehl MM, Baker AF. Modulation of the antioxidant defense as a factor in
692
apoptosis. Cell Death Differ 1996;3:63–70.
693
[23] Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A. Mitochondrial
694
permeability transition is a central coordinating event of apoptosis. J Exp Med
695
1996;184:1155–60.
696
[24] Zhuge J, Cederbaum AI. Serum deprivation-induced HepG2 cell death is
697
potentiated by CYP2E1. Free Radic Biol Med 2006;40:63–74.
698
[25] Stark GR, Taylor WR. Analyzing the G2/M checkpoint. Methods Mol Cell Biol
699
2004;280:51–82.
700
[26] Bulavin DV, Higashimoto Y, Demidenko ZN, Meek S, Graves P, Phillips C, et al. Dual
701
phosphorylation controls Cdc25 phosphatases and mitotic entry. Nat Cell Biol
702
2003;5:545–51.
703
[27] Guillot C, Falette N, Paperin MP, Courtois S, Gentil-Perret A, Treilleux I, et al.
704
p21WAF1/CIP response to genotoxic agents in wild type TP53 expression breast
705
primary tumors. Oncogene 1997;14:45–52.
706
[28] Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the
707
human WEE1 tyrosine kinase. Science 1992;257:1955–7.
708
[29] Stacker SA, Baldwin ME, Achen MG. The role of tumor lymph angiogenesis in
709
metastatic spread. FASEB J 2002;16:922–34.
710
[30] Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in
711
tumor growth, invasion, and metastasis. Cell Mol Life Sci 2000;57:25–40.
712
[31] Verstappen J, Von den Hoff JW. Tissue inhibitors of metalloproteinases (TIMPs):
713
their biological functions and involvement in oral disease. J Dent Res 2006;85:
714
1074–84.
715
[32] Khan MH, Yasuda M, Higashino F, Haque S, Kohgo T, Nakamura M, et al. nm23-H1
716
suppresses invasion of oral squamous cell carcinoma-derived cell lines without
717
modifying matrix metalloproteinase-2 and matrix metalloproteinase-9
expres-718
sion. Am J Pathol 2001;158:1785–91.