The association between chronic obstructive pulmonary disease and Parkinson’s
disease: a nationwide population-based retrospective cohort study
Chia-Hsiang Li, MDa,b; Wei-Chun Chen, MDa,b; Wei-Chih Liao, MDa, c; Chih-Yen Tu, MD a,d,e;
Cheng-Li Cheng-Lin, MScf,g; Fung-Chang Sung, PhD, MPHf,g; Chia-Hung Chen, MDa,c,h; Wu-Huei Hsu, MD, FCCPa,d a Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine,
China Medical University Hospital
b China Medical University
c Graduate institute of clinical medical science, China Medical University d School of Medicine, China Medical University
e Department of Life Science, National Chung Hsing University
f Management Office for Health Data, China Medical University Hospital g Department of Public Health, China Medical University
h Department of Respiratory Therapy, China Medical University * Dr. Li CH and Dr. Chen WC contributed equally to this work * Dr. Chen CH contributed equally as the corresponding author # Dr. Tu CY contributed equally as the co-corresponding author.
Funding/Support: This work was supported by Taiwan Department of Health Clinical Trial and
Research Center and for Excellence (DOH102-TD-B-111-004) and Taiwan Department of Health Cancer Research Center for Excellence (DOH102-TD-C-111-005). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Address correspondence to: Chia-Hung Chen, MD
Department of Internal Medicine, China Medical University Hospital,
No. 2, Yude Road, Taichung, Taiwan Telephone: +886-4-22052121, Ext: 3485 Fax number: +886-4-22038883
E-mail address: d7996@mail.cmuh.org.tw
ABSTRACT
OBJECTIVEPrevious research has shown that patients with chronic obstructive pulmonary disease (COPD) tend to have a higher risk for cognitive impairment and dementia, a neurodegenerative disorder. The goal of this study was to examine what relationship, if any, exists between COPD and Parkinson’s disease (PD), which is also a neurodegenerative disorder.
METHOD
Our study analyzed medical data from the population of Taiwan from 1998 to 2008, with a follow-up period extending to the end of 2010. We identified patients with COPD by the Taiwan National Health Insurance Research Database (NHIRD). We selected a comparison cohort from the general population that was random frequency-matched by age (in 5 year increments), sex, and index year, and further analyzed the risk of PD using Cox’s regression model, including sex, age, and comorbidities.
RESULTS
The study enrolled 20,728 COPD patients (71.1% male, mean age = 68.2 years) and 41,147 controls. The risk of developing PD was 1.37 times greater in patients with COPD compared with patients without COPD after adjusting for age, sex, and comorbidities. A significantly increased risk of PD was also found in patients with COPD who had any comorbidity other than diabetes.
CONCLUSION
This nationwide retrospective cohort study demonstrates that PD risk is significantly increased in patients with COPD compared to those of the general population.
Keywords: chronic obstructive pulmonary disease, Parkinson’s disease, hypoxia, neurodegenerative disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a disabling and progressive lung disorder caused mainly by cigarette smoking and exposure to biomass fuels. COPD is characterized by partially reversible airflow limitation and the condition often progresses into a chronic inflammation of the airway. In addition to chronic inflammation, destruction of lung parenchyma may occur, which impairs gas exchange. These two factors lead to pulmonary dysfunction and eventually hypoxemia and hypercapnia.
COPD is the major cause of mortality and morbidity in the world with increasing economic burden. WHO estimates 65 million developed moderate to severe COPD and contributed 5% of deaths globally. COPD is often accompanied by other comorbidities, associated
with poor outcomes, and at present is suspected of being related to systemic inflammation. There is growing recognition that the inflammatory state associated with COPD is not confined to the lungs, but also involves systemic circulation which can affect non-pulmonary organs. Previous studies have indicated that COPD increases risk of mild cognitive impairment (1, 2), dementia (2, 3), and other neurodegenerative disorders. (2, 3) Neurodegenerative disorders include amyotrophic lateral sclerosis, Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease,
etc. (4) Prevalence may increase in patients who present with impairment of gas exchange or
systemic inflammation.
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease, with an incidence rate in developed countries of 14 per 100,000 person-years. (5) The main pathological finding associated with the motor deficits of PD is degeneration of the dopaminergic neurons of the pars compacta of the substantia nigra, which leads to deficiency of dopamine in the striatum. The etiology of PD may be due to both genetic or environmental factors (6), and the mechanisms may include defective handling of proteins, mitochondrial dysfunction, oxidative stress, and/or inflammation. It is presumed that oxidative stress may be a main mechanism for degeneration. (7) While neurodegenerative disorders are often comorbidities of COPD, there have been no previous descriptions in the literature of a relationship, if any, existing between COPD and PD. (8, 9)
Taiwan initiated its National Health Insurance program in 1995; the data available from this program offers a unique opportunity for research. In the present study, we compiled a 13-year nationwide population-based dataset to determine the relationship between COPD and PD.
Methods and materials
Source of dataWe conducted a nationwide retrospective cohort study using the Taiwan National Health Institute Research Database (NHIRD). The NHI Bureau of Taiwan has collected claims records covering all outpatient, inpatient, emergency, and prescription medical benefit claims for almost the entire population of Taiwan from the inception of its single-payer NHI program in 1995. By 2009, the NHI covered 99% of 23.74 million people in Taiwan and is contracted with 97% of hospitals and clinics. (10) To help researchers perform studies of issues relevant to the NHI program, the Taiwan National Health Research Institute (NHRI) created and released the LHID2000 dataset to the public for research purposes. The LHID2000 comprises historical claims data for one million individuals selected randomly from the 23.74 million individuals in the NHI registry of beneficiaries. The NHRI asserts that there are no statistically significant differences in age, sex, and health care cost between the one million randomly sampled individuals and the 23.74 million enrolled beneficiaries. We confirm that all data was de-identified and analyzed anonymously. This study was approved by the Ethics Review Board of China Medical University (CMU-REC-101-012).
Study population
The International Classification of Disease, Ninth Revision (ICD-9-CM), was used to define diagnostic disease codes. For the claims data collected from 1998 to 2008, the ICD-9-CM code 496 was used to identify newly diagnosed chronic COPD or inclusion in the study cohort. The date of COPD diagnosis was defined as the index date. Patients aged less than 20 years and those with a history of PD (ICD-9-CM 332) prior to the index date, or with incomplete age or sex information, were excluded. All patients without a history of COPD were randomly selected from the registry of beneficiaries. To increase the statistical power of our analysis, the cohort was frequency-matched by age (in 5 year increments), sex, and index year for each study case and randomized twice. The same exclusion criteria were also applied to the non-COPD comparison cohort. The high degree of accuracy and validity of using the NHIRD to diagnose major diseases such as COPD has been previously demonstrated in the literature.(11-13)
Study end point and comorbidities
Each study subject was followed up until one of the following outcomes: subject was diagnosed of PD; subject was lost during follow-up; subject died; subject was withdrawn from the NHI system; or treatment of the subject continued beyond December 31, 2010. We also searched
for several comorbidities that were diagnosed before the index date that could be related to Parkinson’s disease. These comorbid conditions included coronary artery disease (CAD) (ICD-9-CM 410-414), stroke (ICD-9-CM 430–438), hyperlipidemia (ICD-9-CM 272), hypertension (ICD-9-CM 401 to 405), diabetes (ICD-9-CM 250), and head injury (ICD-9-CM 850-854, 959.01).
Statistical analysis
We compared differences in sociodemographic factors and comorbidities between the cohorts with and without COPD using the chi-square test. We compared the incidence rate of Parkinson’s disease between the two cohorts stratified by sex, age, and comorbidity. Poisson regression analysis was used to evaluate the COPD cohort to non-COPD cohort incidence rate ratio (IRR) and 95% confidence interval (CI). Cox proportional hazards analyses were used to investigate the association between COPD and the risk of developing Parkinson’s disease, and were adjusted for age, sex, and comorbidity. Kaplan-Meier analysis was used to compare the cumulative incidence of Parkinson’s disease in the COPD cohort and non-COPD cohort using the log-rank test to determine significance. A two-tailed p-value of < 0.05 was considered statistically significant. SAS software (Version 9.2, SAS Institute Inc., Carey, NC) was used for data analysis.
Results
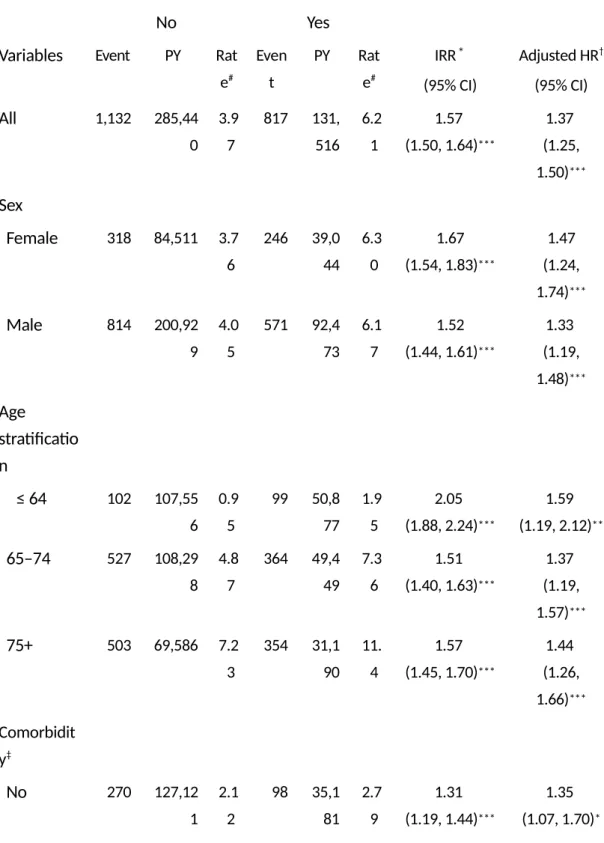
Demographic characteristics and baseline comorbidities are as shown in Table 1. The dominant gender was male (71.1%). The majority of patients were older than 65 years of age (67%). The prevalence of comorbidities was greater in the COPD cohort (all p-values <0.0001). The incidence rate and adjusted HR between the COPD cohort and non-COPD cohort are as shown in Table 2. The overall incidence rate of Parkinson’s disease was 57% higher in the COPD cohort than in the non-COPD cohort (6.21 vs. 3.97 per 1,000 person-years), with an adjusted HR of 1.37 (95% CI=1.25–1.50) after adjusting for age, sex, and comorbidities in the following 13 years. The incidence rate of Parkinson’s disease was 1.67 times greater in women with COPD than women without COPD (6.30 vs. 3.76 per 1,000 person-years), with an adjusted HR of 1.47 (95% CI=1.24– 1.74). The age-specific incidence rate of Parkinson’s disease increased with age in both cohorts. Among subjects under 65 years old, patients with COPD had a higher risk of Parkinson’s disease than the non-COPD cohort (adjusted HR = 1.59, 95% CI=1.19–2.12). The adjusted HR of Parkinson’s disease was significantly greater in COPD patients with comorbidities (adjusted HR=1.41, 95% CI=1.27–1.55) compared to non-COPD patients with comorbidities. Figure 1 shows the comparative cumulative incidence of Parkinson’s disease between the COPD cohort and
non-COPD cohort. The incidence of Parkinson’s disease (log-rank test, p <0.001) was significantly greater in patients in the COPD cohort than in subjects without COPD.
Discussion
Our study is the first nationwide population-based study evaluating the relationship between COPD and PD. In this study, the overall incidence rate of Parkinson’s disease was 57% higher in the COPD cohort than in the non-COPD cohort, with an adjusted HR of 1.37 after adjusting for age,
sex, and comorbidities in the following 13 years. We therefore postulate that chronic obstructive
pulmonary disease increases the risk of developing Parkinson’s disease.
COPD is a chronic disease and effects more than just the lungs. (14) The effects of COPD on the neuropsychiatric system have been described in recent years. We know that COPD is an inflammatory disease. The systemic inflammation effect can cause atherosclerosis, which may cause further cardiovascular diseases. (15, 16) The course of the disease causes pulmonary dysfunction which results in hypoxia, which can further exacerbate the inflammation process. (17, 18) Moreover, tissue hypoxia in COPD plays an important role in the maladaptive process and extrapulmonary comorbidities. (16) Neuronal dysfunction in COPD seems to increase in patients with gas exchange deficiency. (1) Hypoxia status causes the consumption of neurotransmitters due to dysfunction of oxygen-dependent enzymes. (16) Impairment brain perfusion can also exist in hypoxemic COPD patients (19, 20), which may lead to neuron cell damage and even death. (21)
Many studies suggest that inflammation may be related to the pathogenesis of PD, which reveals the presence of activated microglia cells and increased levels of inflammatory cytokines in substantia nigra and striatum. (22-24) Some studies have also reported altered levels of serum inflammatory markers in PD patients. (25, 26) These findings suggest that inflammation may precede neurodegeneration. Therefore, inflammation in COPD patients may be a key factor in the development of PD.
Hypoxia inducible factor-1 (HIF-1), a heterodimer consisting of a constitutively expressed β subunit and an oxygen-regulated α subunit, is a transcriptional factor responsible for cellular and tissue adaption to low oxygen tension; HIF-1 regulates a series of genes that participate in angiogenesis, iron metabolism, glucose metabolism, and cell proliferation and survival. Regulating HIF-1 might ameliorate cellular and tissue damage caused by neurodegenerative diseases, which suggests that HIF-1 may potentially have medical value for treating neurodegenerative diseases. (27) Recent studies have shown that HIF-1 can increase dopamine synthesis and dopaminergic neuron growth. Moreover, HIF-1 may protect dopaminergic neurons through the alteration of iron
homeostasis and defense against oxidative stress and mitochondrial dysfunction. (28, 29) These studies conclude that HIF-1 may have a neuroprotective effect on the brain of a PD patient. COPD patients usually have an impaired response to hypoxia, and recent studies have shown that COPD patients have lower levels of histone deacetylase 7 and HIF- 1α. (30) A lower level of HIF- 1α is associated with a decreased neuroprotective effect; hence, the higher incidence of PD may explain why COPD increases the risk of developing PD.
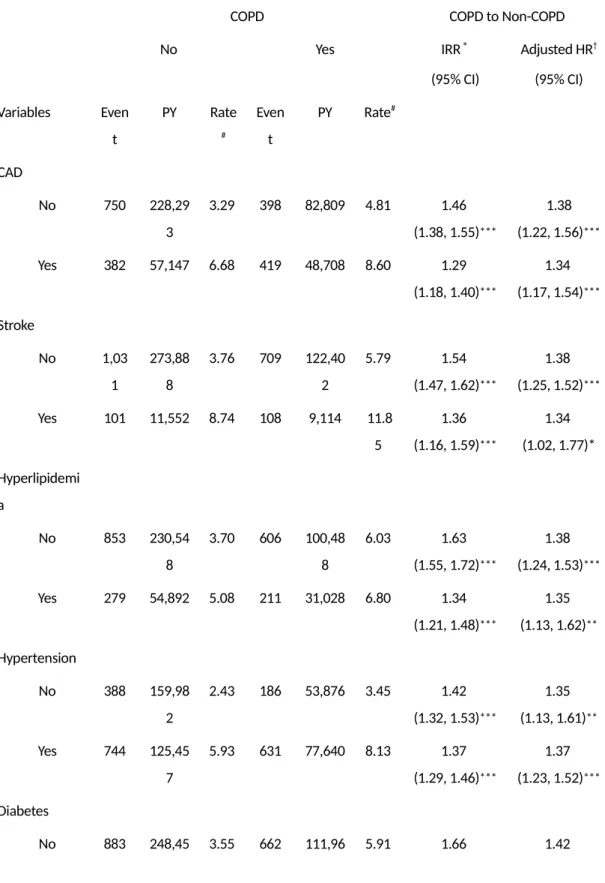
The comorbidity-specific incidence rate of Parkinson’s disease was greater with comorbidities in both COPD and non-COPD cohorts, yet a difference between the comorbidities still exists. Among subjects with a head injury, patients with COPD had a 50% greater risk of Parkinson’s disease compared to the non-COPD cohort. Patients in the COPD cohort without diabetes had a 42% greater risk of Parkinson’s disease compared to the non-COPD cohort without diabetes. However, patients in the COPD cohort with diabetes only had a 17% greater risk of Parkinson’s disease compared to the non-COPD cohort with diabetes. Pathogenic mechanisms shared by diabetes and PD are involved the inflammatory and mitochondrial dysfunction pathways. It is also generally accepted that chronic low-grade inflammation contributes to the development of insulin resistance. (31) A few recent studies have suggested the possibility that diabetic patients have an increased risk of developing Parkinson’s disease. (32) However, these findings are not consistent with previous studies. (33, 34) In our study, COPD patients with diabetes did not have an increased risk of developing PD compared to COPD patients without diabetes. Therefore, the role of diabetes in the development of PD may warrant further investigation.
On the other hand, our study found that patients with COPD had more vascular comorbidities than the control group. However, previous studies show that these comorbidities do not increase the risk of PD, which may indicate that inflammation alone does not promote the development of PD. In which case, it is possible that inflammation must be accompanied by hypoxia to facilitate the development of PD.
One major limitation of our study is that our database did not include the smoking status of subjects, which is an important factor in both COPD and PD. Smoking is one of the most extensively studied lifestyle exposures in relation to PD. An inverse association between smoking and PD has been reported in the literature.(8) From our study, we cannot accurately determine the relative effect that smoking has on COPD and PD. Another limitation of our study is that we were unable to analyze the relationship between pulmonary function and PD. A further prospective study should be conducted to confirm whether severity of COPD increases the risk of developing PD.
Conclusion
This nationwide population-based retrospective cohort study found that patients with a history of chronic obstructive pulmonary disease have an increased risk of Parkinson’s disease and more vascular comorbidities. We recommend that greater importance be placed on preventing the development of COPD as well as effectively treating COPD in order to better prevent PD.
References
1. Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. The European respiratory journal. 2010 Apr;35(4):913-22. PubMed PMID: 20356988.
2. Villeneuve S, Pepin V, Rahayel S, Bertrand JA, de Lorimier M, Rizk A, et al. Mild cognitive impairment in moderate to severe COPD: a preliminary study. Chest. 2012 Dec;142(6):1516-23. PubMed PMID: 23364388.
3. Rusanen M, Ngandu T, Laatikainen T, Tuomilehto J, Soininen H, Kivipelto M. Chronic obstructive pulmonary disease and asthma and the risk of mild cognitive impairment and dementia: a population based CAIDE study. Current Alzheimer research. 2013 Jun;10(5):549-55. PubMed PMID: 23566344.
4. Zhang Z, Yan J, Chang Y, ShiDu Yan S, Shi H. Hypoxia inducible factor-1 as a target for neurodegenerative diseases. Current medicinal chemistry. 2011;18(28):4335-43. PubMed PMID: 21861815. Pubmed Central PMCID: 3213300.
5. Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the "common" neurologic disorders? Neurology. 2007 Jan 30;68(5):326-37. PubMed PMID: 17261678.
6. Davie CA. A review of Parkinson's disease. British medical bulletin. 2008;86:109-27. PubMed PMID: 18398010.
7. Fujita K, Nakabeppu Y, Noda M. Therapeutic effects of hydrogen in animal models of Parkinson's disease. Parkinson's disease. 2011;2011:307875. PubMed PMID: 21687749. Pubmed Central PMCID: 3109337.
8. Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. European journal of epidemiology. 2011 Jun;26 Suppl 1:S1-58. PubMed PMID: 21626386.
9. Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G, Lees AJ, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Annals of
neurology. 2012 Dec;72(6):893-901. PubMed PMID: 23071076. Pubmed Central PMCID: 3556649.
10. TM. C. Taiwan’s National Health Insurance system: high value for the dollar. . 2009. 11. Shen TC, Lin CL, Chen CH, Tu CY, Hsia TC, Shih CM, et al. Increased risk of chronic obstructive pulmonary disease in patients with rheumatoid arthritis: a population-based cohort study. QJM : monthly journal of the Association of Physicians. 2014 Feb 21. PubMed PMID: 24497528.
12. Shen TC, Lin CL, Chen CH, Tu CY, Hsia TC, Shih CM, et al. Increased risk of chronic obstructive pulmonary disease in patients with systemic lupus erythematosus: a population-based cohort study. PloS one. 2014;9(3):e91821. PubMed PMID: 24622340. Pubmed Central PMCID: 3951498.
13. Shen TC, Chung WS, Lin CL, Wei CC, Chen CH, Chen HJ, et al. Does Chronic Obstructive Pulmonary Disease with or without Type 2 Diabetes Mellitus Influence the Risk of Lung Cancer? Result from a Population-Based Cohort Study. PloS one. 2014;9(5):e98290. PubMed PMID: 24854189.
14. Eisner MD, Blanc PD, Yelin EH, Sidney S, Katz PP, Ackerson L, et al. COPD as a systemic disease: impact on physical functional limitations. The American journal of medicine. 2008 Sep;121(9):789-96. PubMed PMID: 18724969. Pubmed Central PMCID: 2548403.
15. Agusti AG. Systemic effects of chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2005;2(4):367-70; discussion 71-2. PubMed PMID: 16267364. 16. Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. International journal of chronic obstructive pulmonary disease. 2011;6:199-208. PubMed PMID: 21660297. Pubmed Central PMCID: 3107696.
17. Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. The European respiratory journal. 2009 May;33(5):1195-205. PubMed PMID: 19407053.
18. Fitzpatrick SF, Tambuwala MM, Bruning U, Schaible B, Scholz CC, Byrne A, et al. An intact canonical NF-kappaB pathway is required for inflammatory gene expression in response to hypoxia. Journal of immunology. 2011 Jan 15;186(2):1091-6. PubMed PMID: 21149600.
19. Antonelli Incalzi R, Marra C, Giordano A, Calcagni ML, Cappa A, Basso S, et al.
Cognitive impairment in chronic obstructive pulmonary disease--a neuropsychological and spect study. Journal of neurology. 2003 Mar;250(3):325-32. PubMed PMID: 12638024.
20. Ortapamuk H, Naldoken S. Brain perfusion abnormalities in chronic obstructive pulmonary disease: comparison with cognitive impairment. Annals of nuclear medicine. 2006 Feb;20(2):99-106. PubMed PMID: 16615418.
21. de la Torre JC. Critical threshold cerebral hypoperfusion causes Alzheimer's disease? Acta neuropathologica. 1999 Jul;98(1):1-8. PubMed PMID: 10412794.
22. Liu B, Gao HM, Hong JS. Parkinson's disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environmental health perspectives. 2003 Jun;111(8):1065-73. PubMed PMID: 12826478. Pubmed Central PMCID: 1241555.
23. McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson's disease. Parkinsonism & related disorders. 2004 May;10 Suppl 1:S3-7. PubMed PMID: 15109580.
24. McGeer PL, Yasojima K, McGeer EG. Inflammation in Parkinson's disease. Advances in neurology. 2001;86:83-9. PubMed PMID: 11554012.
25. Dobbs RJ, Charlett A, Purkiss AG, Dobbs SM, Weller C, Peterson DW. Association of circulating TNF-alpha and IL-6 with ageing and parkinsonism. Acta neurologica Scandinavica. 1999 Jul;100(1):34-41. PubMed PMID: 10416510.
26. Chen H, O'Reilly EJ, Schwarzschild MA, Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson's disease. American journal of epidemiology. 2008 Jan 1;167(1):90-5. PubMed PMID: 17890755.
27. Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Molecular pharmacology. 2006 Nov;70(5):1469-80. PubMed PMID: 16887934.
28. Lee DW, Rajagopalan S, Siddiq A, Gwiazda R, Yang L, Beal MF, et al. Inhibition of prolyl hydroxylase protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity: model for the potential involvement of the hypoxia-inducible factor pathway in Parkinson disease. The Journal of biological chemistry. 2009 Oct 16;284(42):29065-76. PubMed PMID: 19679656. Pubmed Central PMCID: 2781452.
29. Ben-Shachar D, Eshel G, Finberg JP, Youdim MB. The iron chelator desferrioxamine (Desferal) retards 6-hydroxydopamine-induced degeneration of nigrostriatal dopamine neurons. Journal of neurochemistry. 1991 Apr;56(4):1441-4. PubMed PMID: 1900527.
30. To M, Yamamura S, Akashi K, Charron CE, Barnes PJ, Ito K. Defect of adaptation to hypoxia in patients with COPD due to reduction of histone deacetylase 7. Chest. 2012
31. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003 Dec;112(12):1821-30. PubMed PMID: 14679177. Pubmed Central PMCID: 296998.
32. Schernhammer E, Hansen J, Rugbjerg K, Wermuth L, Ritz B. Diabetes and the risk of developing Parkinson's disease in Denmark. Diabetes care. 2011 May;34(5):1102-8. PubMed PMID: 21411503. Pubmed Central PMCID: 3114482.
33. Driver JA, Smith A, Buring JE, Gaziano JM, Kurth T, Logroscino G. Prospective cohort study of type 2 diabetes and the risk of Parkinson's disease. Diabetes care. 2008 Oct;31(10):2003-5. PubMed PMID: 18599528. Pubmed Central PMCID: 2551644.
34. Palacios N, Gao X, McCullough ML, Jacobs EJ, Patel AV, Mayo T, et al. Obesity, diabetes, and risk of Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2011 Oct;26(12):2253-9. PubMed PMID: 21739472. Pubmed Central PMCID: 3627531.
Figure Legends
Figure 1: Cumulative incidence comparison of Parkinson’s disease for patients with (dashed line)
Table 1: Demographic characteristics and comorbidities in cohorts with and
without chronic obstructive pulmonary disease Variable COPD p-value No Yes N = 41147 N = 20728 Sex n (%) n (%) Female 11,972 (29.1) 5,986 (28.9) 0.57 Male 29,175 (70.9) 14,742 (71.1) Age, mean ± SD† 67.1 (12.5) 68.2 (12.5) Age stratification ≤ 64 13,674 (33.2) 6,837 (33.0) 0.43 65–74 14,710 (35.8) 7,355 (35.5) 75+ 12,763 (31.0) 6,536 (31.5) Urbanization# 1 (highest) 11,039 (26.8) 4,906 (23.7) <0.0001 2 10,875 (26.4) 5,521 (26.6) 3 6,896 (16.8) 3,538 (17.1) 4 (lowest) 12,236 (30.0) 6,763 (32.6) Comorbidity CAD 9,060 (22.0) 8,025 (38.7) <0.0001 Stroke 2,439 (5.93) 2,200 (10.6) <0.0001 Hyperlipidemia 8,239 (20.0) 4,855 (23.4) <0.0001 Hypertension 19,271 (46.8) 12,758 (61.6) <0.0001 Diabetes 6,077 (14.8) 3,474 (16.8) <0.0001 Head injury 2,668 (6.48) 1,949 (9.40) <0.0001 Chi-squared test †: Student’s t-test
#: Level of urbanization was categorized into 4 levels based on the population density of the
Table 2: Incidence of Parkinson’s disease by sex, age, and comorbidity, and hazards
ratio for patients with chronic obstructive pulmonary disease compared those without chronic obstructive pulmonary disease as measured using the Cox model
COPD COPD to Non-COPD
No Yes
Variables Event PY Rat e# Even t PY Rat e# IRR* (95% CI) Adjusted HR† (95% CI) All 1,132 285,44 0 3.9 7 817 131, 516 6.2 1 1.57 (1.50, 1.64)*** 1.37 (1.25, 1.50)*** Sex Female 318 84,511 3.7 6 246 39,0 44 6.3 0 1.67 (1.54, 1.83)*** 1.47 (1.24, 1.74)*** Male 814 200,92 9 4.0 5 571 92,4 73 6.1 7 1.52 (1.44, 1.61)*** 1.33 (1.19, 1.48)*** Age stratificatio n ≤ 64 102 107,55 6 0.9 5 99 50,8 77 1.9 5 2.05 (1.88, 2.24)*** 1.59 (1.19, 2.12)** 65–74 527 108,29 8 4.8 7 364 49,4 49 7.3 6 1.51 (1.40, 1.63)*** 1.37 (1.19, 1.57)*** 75+ 503 69,586 7.2 3 354 31,1 90 11. 4 1.57 (1.45, 1.70)*** 1.44 (1.26, 1.66)*** Comorbidit y‡ No 270 127,12 1 2.1 2 98 35,1 81 2.7 9 1.31 (1.19, 1.44)*** 1.35 (1.07, 1.70)*
Yes 862 158,31 9 5.4 4 719 96,3 35 7.4 6 1.37 (1.30, 1.45)*** 1.41 (1.27, 1.55)***
Rate#, incidence rate; PY, per 1,000 person-years; IRR*, incidence rate ratio; Adjusted HR†, multivariable
analysis including age, sex, urbanization level, and co-morbidities; *p < 0.05; **p < 0.01; ***p < 0.001 Comorbidity‡: Subjects with one or more additional disorders are classified under the comorbidity group.
Table 3: Incidence of Parkinson’s disease by comorbidity, and hazards
ratio for patients with chronic obstructive pulmonary disease compared to those without chronic obstructive pulmonary disease as measured using the Cox model
COPD COPD to Non-COPD
No Yes IRR* (95% CI) Adjusted HR† (95% CI) Variables Even t PY Rate # Even t PY Rate# CAD No 750 228,29 3 3.29 398 82,809 4.81 1.46 (1.38, 1.55)*** 1.38 (1.22, 1.56)*** Yes 382 57,147 6.68 419 48,708 8.60 1.29 (1.18, 1.40)*** 1.34 (1.17, 1.54)*** Stroke No 1,03 1 273,88 8 3.76 709 122,40 2 5.79 1.54 (1.47, 1.62)*** 1.38 (1.25, 1.52)*** Yes 101 11,552 8.74 108 9,114 11.8 5 1.36 (1.16, 1.59)*** 1.34 (1.02, 1.77)* Hyperlipidemi a No 853 230,54 8 3.70 606 100,48 8 6.03 1.63 (1.55, 1.72)*** 1.38 (1.24, 1.53)*** Yes 279 54,892 5.08 211 31,028 6.80 1.34 (1.21, 1.48)*** 1.35 (1.13, 1.62)** Hypertension No 388 159,98 2 2.43 186 53,876 3.45 1.42 (1.32, 1.53)*** 1.35 (1.13, 1.61)** Yes 744 125,45 7 5.93 631 77,640 8.13 1.37 (1.29, 1.46)*** 1.37 (1.23, 1.52)*** Diabetes No 883 248,45 3.55 662 111,96 5.91 1.66 1.42
0 1 (1.58, 1.75)*** (1.28, 1.57)*** Yes 249 36,990 6.73 155 19,555 7.93 1.18 (1.05, 1.32)** 1.17 (0.95, 1.43) Head injury No 1,05 5 270,59 2 3.90 733 121,92 3 6.01 1.54 (1.47, 1.62)*** 1.36 (1.23, 1.49)*** Yes 77 14,848 5.19 84 9,593 8.76 1.69 (1.43, 2.00)*** 1.50 (1.09, 2.05)* Rate#, incidence rate; PY, per 1,000 person-years; IRR*, incidence rate ratio; Adjusted HR†, multivariable