The hyperaggregability of platelets from
normal pregnancy is mediated through
thromboxane A
2and cyclic AMP pathways
J.R. SHEU* , G. HSIAO , M.Y. SHEN*, W.Y. LIN*, C.R. TZENGà
*Graduate Institute of Medical Sciences and Departments of Pharmacology and àObstetrics & Gynecology, Taipei Medical University, Taipei 110, Taiwan
Summary There is substantial evidence of increased platelet reactivity in vivo and in vitro during pregnancy, with the risk of developing pre-eclampsia. In this study, platelet function was studied during 28–40 weeks of gestation in a group of women who remained normotensive and in a group of nonpregnant female controls. Platelet aggregation stimulated by thrombin and adenosine diphosphate was markedly enhanced in washed platelets from pregnant subjects. Thrombin (0.04 U/ml)-evoked increases in intracellular Ca+2mobilization of Fura 2-AM-loaded platelets were also enhanced in pregnant subjects. The binding of fluorescein isothiocyanate (FITC)-triflavin (2lg/ml) to the glycoprotein IIb/IIIa complex in thrombin-activated platelets did not differ sig-nificantly between the nonpregnant and pregnant groups. Thromboxane A2(TXA2) formation in both resting and thrombin-activated platelets from pregnant subjects was significantly greater than from nonpregnant subjects. Levels of cyclic adenosine monophosphate (cAMP) in both resting and prostaglandin E1-treated platelets (10lmol/l) from pregnant subjects were significantly lower than those from nonpregnant subjects. There were no significant differences between nonpregnant and pregnant subjects in platelet cAMP levels in the presence of imidazole (600lmol/l) and indomethacin (500lmol/l). Intracellular pH values in platelets were measured spectrofluorometrically using the fluorescent probe, BCECF-AM. The increase in intracellular pH stimulated by thrombin (0.04 U/ml) in pregnant subjects was markedly greater than that in observed nonpregnant subjects. We conclude that the agonist-induced hyperaggregability of platelets in normal pregnancy may be due, at least partly, to stimulation of the Na+/H+ exchanger and subsequently to elevated intracellular Ca+2mobilization, and then to increased TXA2formation and a lowered level of cAMP, which leads to further increases in intracellular Ca+2mobilization, and finally to enhanced platelet aggregation.
Keywords Cyclic AMP, hyperaggregability, Na+/H+ exchanger, normal pregnancy, platelet, thromboxane A2
Introduction
During normal pregnancy, there is considerable evidence of platelet activation in vivo (Nicolini et al., 1994). This
contributes to the hypercoagulable state that often occurs during pregnancy (Nicolini et al., 1994), which may lead in turn to such complications as thromboembolism and disseminated intravascular coagulation (Redman, 1990; Nicolini et al., 1994). Platelet activation is thought to contribute to the pathogenesis of pre-eclampsia, which may result in haemodynamic changes, platelet aggrega-tion, endothelial injury and vasoconstricaggrega-tion, generating or amplifying the progressive damage to the maternal
Accepted for publication 16 November 2001
Correspondence: J.-R. Sheu, Graduate Institute of Medical Sciences, Taipei Medical University, no. 250, Wu-Hsing Street, Taipei 110,
vasculature associated with the latter condition (Redman, 1990). Pre-eclampsia is a common complication of pregnancy, a major cause of maternal death in developed countries and is associated with premature delivery and intrauterine growth restriction (Hall, Chang & McGillivray, 1980). The factors mediating increased platelet reactivity in normal pregnancy remain unclear. Knowledge of the mechanisms of increased platelet reactivity in healthy pregnancy may elucidate the role of platelets in pre-eclampsia.
In an effort to elucidate these mechanism(s), many studies of platelet reactivity in vitro have been carried out. Increased platelet aggregation has been demonstrated in platelet-rich plasma (PRP) and in whole blood using such agonists as adenosine 5¢-diphosphate (ADP) and arachi-donic acid (Orrison et al., 1985; Louden et al., 1990). Formation of thromboxane A2 (TXA2) in platelets is reported to increase during pregnancy (Ylikorkala & Viinikka, 1980; Fitzgerald et al., 1987). It has also been shown that the increased platelet reactivity demonstrated ex vivo during pregnancy can be prevented by adminis-tration of a low dose of aspirin, which acts as an antiplatelet agent by irreversibly inhibiting cyclooxygen-ase, a key enzyme in TXA2synthesis (Louden et al., 1992). These data raise the question of whether or not there is an increase in thromboxane formation during pregnancy. Ylikorkala & Viinikka (1980) found that platelet throm-boxane formation is increased in vitro during pregnancy; however, another report suggested that no in vitro change occurs (Louden et al., 1990). The question thus remains controversial. It has been shown that the thromboxane synthetase inhibitor dazoxiben fails to prevent arachidonic acid-induced platelet aggregation in PRP in vitro in the majority of pregnant women (Orrison et al., 1985). In contrast, this agent was generally an effective inhibitor under the same experimental conditions in PRP from nonpregnant women of childbearing age (Orrison et al., 1985). This suggests that other thromboxane-independ-ent mechanisms may be involved in the agonist-induced hyperaggregability of platelets during pregnancy. On the other hand, studies of agonist-induced hyperaggregability in washed platelets rarely provide comparisons with PRP or whole blood from normal pregnancies (Ylikorkala & Viinikka, 1980; Orrison et al., 1985; Fitzgerald et al., 1987; Louden et al., 1990). The detailed mechanisms underlying platelet hyperaggregability in normal preg-nancy thus remain obscure. We therefore systematically examined the influence of agonist-induced hyperaggrega-bility in washed platelets during pregnancy and utilized the findings to characterize the mechanisms involved in this reaction.
Materials and methods Materials
Adenosine 5¢-diphosphate (ADP), sodium citrate, EDTA (disodium salt), hydroxylamine, HEPES, indomethacin, prostaglandin E1 (PGE1), trichloroacetic acid, nigericin, sodium bicarbonate, EGTA, bovine serum albumin (BSA), apyrase, heparin, arachidonic acid and thrombin were purchased from Sigma Chemical Co. (St Louis, MO, USA). BCECF-AM, Fura 2-AM, and fluorescein isothiocyanate (FITC) were purchased from Molecular Probes Inc. (Eugene, OR, USA). Trimeresurus flavoviridis venom was purchased from Latoxan (Rosans, France). Thromboxane B2 and cAMP enzyme immunoassay (EIA) kits were purchased from Cayman Co. (Ann Arbor, MI, USA).
Subjects
The studies were approved by our hospital’s Ethics Committee, and subjects gave informed consent. All pregnant subjects were between 28 and 40 weeks of gestation and remained normotensive (118.4 ± 2.5/ 78.2 ± 2.3 mmHg) throughout their pregnancy. The nonpregnant female controls were members of the hospi-tal staff. All pregnant and nonpregnant women were aged 22–35 years, without current medical illnesses or current or past obstetric complications. All subjects were ques-tioned specifically regarding the use of medications, including non-steroidal anti-inflammatory agents or other drugs known to affect platelet function for at least 10 days prior to the studies. Nonpregnant subjects were excluded if they had taken oral contraceptives within 8 weeks of the study.
Subjects were placed in a semirecumbent position, and after 5 min of rest, blood pressure was measured. Any subject with a blood pressure in excess of 140/90 mmHg was rejected. Venepuncture was performed using a 19-gauge butterfly needle, and blood was mixed immedi-ately with acid/citrate/glucose (ACD; 9 : 1, v/v) in a polystyrene tube.
Preparation of platelet suspensions
Platelet suspensions were prepared as previously described (Huang, Sheu & Teng, 1991). After centrifugation at 120 g for 10 min at room temperature, the supernatant (platelet-rich plasma; PRP) was supplemented with PGE1 (0.5lmol/l) and heparin (6.4 IU/ml), incubated for 10 min at 37°C and centrifuged at 500 g for 10 min. The platelet pellets were suspended in 5 ml Tyrode’s
solution, pH 7.3 (containing NaCl 11.9 mmol/l, KCl 2.7 mmol/l, MgCl2 2.1 mmol/l, NaH2PO4 0.4 mmol/l, NaHCO311.9 mmol/l and glucose 11.1 mmol/l). Apyrase (1.0 U/ml), PGE1 (0.5lmol/l) and heparin (6.4 IU/ml) were then added, and the mixture was incubated for 10 min at 37°C. After centrifugation of the suspensions at 500 g for 10 min, the washing procedure was repeated. Washed platelets were finally suspended in Tyrode’s solution containing bovine serum albumin (3.5 mg/ml) and adjusted to about 3.0· 108 platelets/ml. The final concentration of Ca2+in Tyrode’s solution was 1 mmol/l.
Platelet aggregation
A turbidimetric method was applied to measure platelet aggregation (Born & Cross, 1963), using a Lumi-Aggreg-ometer (Payton, Canada). Platelet suspensions (0.4 ml) from pregnant and nonpregnant subjects were pre-warmed to 37°C for 2 min (stirring at 225 g) in a silicone-treated glass cuvette. Platelet-aggregation inducers were added and reactions allowed to proceed for at least 6 min; the extent of aggregation was expressed in light-transmission units.
Purification and fluorescence-labelling with triflavin Triflavin, a specific fibrinogen receptor (glycoprotein IIb/ IIIa complex) antagonist, was prepared as previously described (Sheu, Teng & Huang, 1992; Sheu et al., 1999). Fluorescein-conjugated triflavin was also prepared as previously described (Sheu et al., 1996). In brief, 1 mg triflavin was dissolved in 0.2 ml 0.1 mol/l sodium bicar-bonate. Twenty microliters of freshly prepared FITC solution [10 mg/ml in dimethyl sulfoxide (DMSO)] was added to the protein solution, with continuous stirring for 1 h at room temperature. The reaction was stopped by adding 20ll freshly prepared 1.5 mol/l hydroxylamine (pH 8.0–8.5) and incubating for a further 30 min. Finally, the conjugated protein was separated from the residual labelling reagent and hydroxylamine in a Sephadex G-10 column (10· 300 mm) equilibrated with phosphate-buffered saline (PBS). The conjugate was lyophilized, and the protein concentration estimated by the Lowry method (Lowry et al., 1951). The concentration of FITC-conju-gated triflavin was adjusted to 1 mg/ml.
Analysis of platelet surface glycoprotein IIb/IIIa complex by flow cytometry
Two microliters of FITC-triflavin were added to platelet suspensions (3.0· 108
/ml), followed by thrombin
(0.04 U/ml), with incubation for 6 min and final adjust-ment to 1 ml/tube with Tyrode’s solution. Fluorescein-labelled platelets were assayed in a flow cytometer (Becton Dickinson, FACScan Syst., Mountain View, CA). Data were collected from 50 000 platelets per experimental group. All experiments were repeated at least five times to ensure reproducibility.
Measurement of platelet [Ca2+]i mobilization by Fura 2-AM fluorescence
Citrated whole blood was centrifuged at 120 g for 10 min. The supernatant was protected from light and incubated with Fura 2-AM (5lmol/l) at 37°C for 1 h. Platelet suspensions were then prepared as described above. Finally, the external Ca2+ concentration of the platelet suspensions was adjusted to 1 mmol/l. The [Ca2+]i rise was measured using a fluorescence spectrophotometer (CAF 110, Jasco, Japan) with excitation at 340 nm and emission at 500 nm, at 37°C. Thrombin (0.04 U/ml) was added to the platelet suspensions to trigger a marked intracellular Ca2+mobilization. The free Ca2+ concentra-tion was calculated from the equation [Ca2+]i¼ Kd[(F – Fmin)/(Fmax – F)], where Kd represents the Ca
2+ binding dissociation constant (224 nmol/l for Fura 2), F is the 500-nm fluorescence, Fmax is the maximal fluores-cence determined after the addition of 0.1% Triton X-100 to permeabilize the cells in the presence of 1 mmol/l Ca2+, and Fminis the minimal fluorescence determined after the addition of 5 mmol/l EGTA (Grynkiewicz, Poenie & Tsien, 1985).
Measurement of TXB2formation
Washed human platelet suspensions (0.4 ml, 3.0· 108/ml) were pre-incubated with thrombin (0.04 U/ml) for 6 min, followed by addition of 2 mmol/l EDTA and 50lmol/l indomethacin. The vials were then centrifuged in an Eppendorf centrifuge (Model 5414) for 3 min at 19700 g. The TXB2 levels of the supernatants were measured using an EIA kit (Cayman), according to the instructions of the manufacturer.
Estimation of platelet cAMP levels
The method of Karniguian, Legrand & Caen (1982) was followed. In brief, platelet suspensions (3.0· 108/ml) were warmed to 37°C for 2 min, then PGE1(10lmol/l) was added, followed by incubation for 4 min. The incubation was stopped, and the solution immediately heated at 100°C for 5 min. After cooling to 4 °C, the
precipitated protein was collected by centrifugation. In other experiments, platelet suspensions (1.0· 108
/ml) were pre-incubated with imidazole (600lmol/l) and indomethacin (500lmol/l) for 3 min at 37°C followed by the addition of PGE1 (10lmol/l) and incubation for 4 min as described above. Fifty microliters of supernatant were removed for determination of the cAMP content, using an EIA kit, following acetylation of the samples as described by the manufacturer.
Platelet pHi measurement
Platelet pHi was measured with the fluorescent probe, BCECF-AM, as previously described (Touyz & Schiffrin, 1993). Washed platelets were incubated with 5lmol/l BCECF-AM at 37°C for 30 min in HEPES-buffer solution (HBS; NaCl 145 mmol/l, KCl 5 mmol/l, MgSO41 mmol/l, HEPES 10 mmol/l, glucose 5 mmol/l and CaCl21 mmol/l, pH 7.4) and then centrifuged at 450 g for 8 min. The washed pellet was finally suspended in buffer and adjusted
to 3.0· 108
/ml. Leucocyte contamination was less than 0.01%. Aliquots of this platelet suspension (50ll) were transferred to a cuvette containing 2 ml HBS (pH 7.4, 37°C) in a dual-excitation wavelength spectrofluorometer (CAF 110, Jasco). Fluorescence signals for BCECF-AM were recorded at 430- and 490-nm excitation wavelengths with an emission wavelength of 530 nm (5-nm slit). The background fluorescence of platelets was subtracted from each reading. Calibration was carried out after diluting the BCECF-loaded platelets in a high-K+buffer (KCl 120 mmol/ l, NaCl 30 mmol/l, MgSO41 mmol/l and glucose 5 mmol/l) in the presence of nigericin (0.2 mg/ml), as described by Horne et al. (1981). In all experiments, platelets were stimulated by thrombin (0.04 U/ml) to trigger the Na+/H+ exchanger.
Statistical analysis
Experimental results are expressed as the mean ± SEM and are accompanied by the number of observations. Data
Figure 1. (a) Tracing curves of thrombin (0.04 U/ml) and ADP (20lmol/l)-induced aggregation and (b) histograms of thrombin (0.04 U/ml), ADP (20lmol/l) and arachidonic acid (AA, 100lmol/l)-induced aggregation in washed platelets from nonpregnant and pregnant subjects. Platelets (3.0· 108/ml) were stirred at 37°C for 2 min, then thrombin (0.04 U/ml), ADP (20lmol/l) and arachidonic acid (100lmol/l) were added to trigger aggregation. Data are expressed as per cent increase in aggregation compared with nonpregnant subjects and are presented as the mean ± SEM (n¼ 7).
were assessed by the analysis of variance (ANOVA). If this analysis indicated significant differences among group means, then each group was compared by the Newman– Keuls method. A P-value less than 0.05 was considered statistically significant.
Results
Agonist-induced hyperaggregability of washed platelets from normal pregnancy
The extent of maximal platelet aggregation induced by thrombin (0.04 U/ml) in pregnant subjects was signifi-cantly greater than that seen in nonpregnant subjects (Figure 1). Similar hyperaggregability was also observed in pregnant subjects in response to ADP stimulation (20lmol/l) in the presence of fibrinogen (200 lg/ml; Figure 1). Increases in platelet aggregation in response to thrombin (0.04 U/ml), ADP (20lmol/l) and arachidonic acid (100lmol/l) in pregnant subjects were 12.0 ± 1.5%, 25.8 ± 2.3% and 24.7 ± 1.9%, respectively, compared with nonpregnant subjects (Figure 1b). These results indicate significant differences between pregnant and nonpregnant women with respect to the aggregation of washed platelets stimulated by various agonists.
Glycoprotein IIb/IIIa complex exposure in activated platelets from pregnant and nonpregnant subjects Triflavin is an Arg-Gly-Asp-containing antiplatelet pep-tide, purified from T. flavoviridis snake venom (Sheu, Teng & Huang, 1992). It inhibits platelet aggregation through direct interference with fibrinogen binding to the glycoprotein (GP) IIb/IIIa complex (Sheu, Teng & Huang, 1992; Sheu et al., 1999). There is now a large body of evidence suggesting that the binding of fibrinogen to the GP IIb/IIIa complex is the final common pathway for agonist-induced platelet aggregation. Therefore, we deci-ded to evaluate further whether or not platelets from pregnant subjects increase the platelet GP IIb/IIIa complex exposure, leading to enhanced agonist-stimula-ted platelet aggregation.
In this study, the relative fluorescence intensities of FITC-triflavin (2lg/ml) bound directly to thrombin-acti-vated platelets from nonpregnant and pregnant subjects were 274.5 ± 19.7 and 265.1 ± 21.4, respectively (n¼ 6, data not shown). This was markedly reduced in the presence of 5 mmol/l EDTA in pregnant (negative control, 31.9 ± 2.3, n ¼ 5) and nonpregnant subjects (27.4 ± 6.8, n ¼ 5) (data not shown). This result indicates that agonist-induced hyperaggregability in
washed platelets from pregnant subjects is not mediated through increased exposure to the GP IIb/IIIa complex.
[Ca2+]i mobilization in washed platelets from pregnant and nonpregnant subjects
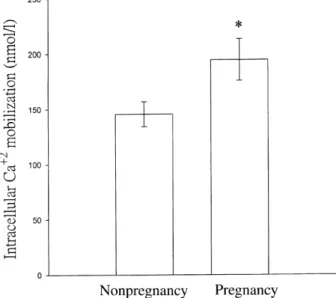
Free cytoplasmic Ca+2 concentrations in platelets were measured by the Fura 2-AM-loaded method. Figure 2 shows that thrombin (0.04 U/ml) evoked greater [Ca+2]i mobilization in pregnant subjects than in nonpregnant subjects (194.5 ± 18.7 nmol/l, 145.6 ± 11.3 nmol/l; n ¼ 7, P < 0.05). This result suggests that agonist-induced [Ca+2]i mobilization in washed platelets is markedly enhanced in pregnant subjects.
Thrombin-stimulated TXB2formation in washed platelets from pregnant and nonpregnant subjects As shown in Table 1, resting platelets produced relatively little TXB2compared with thrombin-activated platelets in both groups. However, in nonpregnant subjects 1077.9 ± 118.6 pg/ml TXB2 was produced in resting platelets. The corresponding value of resting platelets from pregnant subjects was higher at 1500.8 ± 133.5 pg/ml. A similar result was observed in thrombin-activated platelets: TXB2 formation in the pregnant group was markedly greater than that in the nonpregnant group (2138.5 ± 226.5 vs. 1541.3 ± 168.2 pg/ml; Table 1).
Figure 2. Thrombin-induced intracellular Ca+2mobilization in Fura 2-AM-loaded platelets from pregnant and nonpregnant subjects. Platelet suspensions (3.0· 108/ml) were stirred at 37°C for 2 min, followed by the addition of thrombin (0.04 U/ml) to trigger intracellular Ca+2mobilization. Data are presented as the mean ± SEM (n¼ 7). *P < 0.05 as compared with nonpregnant subjects.
These results suggest that agonist-induced platelet hyper-aggregability in normal pregnancy may be due, at least in part, to increased TXB2formation.
cAMP formation in pregnant and nonpregnant subjects
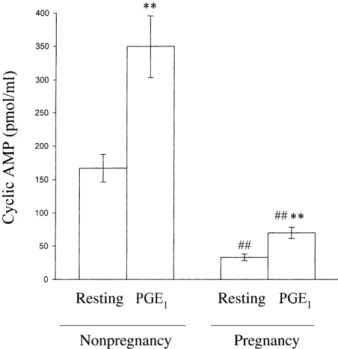
The level of cAMP in resting platelets from nonpregnant subjects was significantly higher than that observed in pregnant subjects (167.2 ± 20.4 vs. 33.2 ± 5.0 pmol/ml;
Figure 3). We also found that cAMP formation induced by PGE1 (10lmol/l) in nonpregnant subjects was markedly greater than in pregnant subjects (349.6 ± 46.1 vs. 69.8 ± 8.3 pmol/ml; Figure 3). There were no significant differences between the pregnant and nonpregnant groups with respect to cAMP levels in the presence of the thromboxane synthetase inhibitor, imidazole (600lmol/ l), and the cyclooxygenase inhibitor, indomethacin (500lmol/l), in both resting and PGE1-treated (10lmol/l) platelets (Table 2). These results suggest that the lower platelet cAMP levels in pregnant subjects may result from increased TXA2formation.
We performed similar studies, measuring cyclic guano-sine monophosphate (cGMP) responses. The level of cGMP in resting platelets was very low in both pregnant and nonpregnant subjects. However, when nitroglycerin (10lmol/l) was added to platelet suspensions from the pregnant and nonpregnant groups, cGMP levels increased 2.5-fold and 2.3-fold, respectively, as compared with individual resting platelets (data not shown). There were no significant differences in cGMP formation between these two groups in either resting or nitroglycerin-treated platelets. These results indicate that cAMP but not cGMP is involved in agonist-induced hyperaggregability in normal pregnancy.
pH in pregnant and nonpregnant subjects
Figure 4 shows pH changes triggered by thrombin (0.04 U/ml) in BCECF-AM-loaded platelets from nonpreg-nant and pregnonpreg-nant subjects. Resting platelet pH values were 7.10 ± 0.03 and 7.14 ± 0.04, respectively, in nonpregnant and pregnant subjects (n¼ 6). Addition of thrombin (0.04 U/ml) resulted in an increase in BCECF Table 1. Effect of thrombin-stimulated TXB2 formation in
washed platelets from pregnant and nonpregnant subjects Thromboxane B2(pg/ml)
Nonpregnancy Pregnancy Resting 1077.9 ± 118.6 1500.8 ± 133.5 Thrombin 1541.3 ± 168.2* 2138.5 ± 226.5* Platelet suspensions (3.0· 108/ml) were pre-incubated with thrombin (0.04 U/ml) for 6 min at 37°C to trigger TXB2 for-mation. Data are presented as the mean ± SEM (n¼ 8). *P < 0.05 as compared with the individual resting group. P < 0.05 as compared with individual nonpregnant groups.
Figure 3. Cyclic AMP formation in platelets from pregnant and nonpregnant subjects. Platelet suspensions (3.0· 108/ml) were preincubated with PGE1 (10lmol/l) for 4 min as a positive control. The supernatant of platelet suspensions was collected for the determination of cAMP levels after centrifugation. Data are presented as the mean ± SEM (n¼ 8). *P < 0.01 and **P < 0.001 as compared with the individual resting group. ##P < 0.001 as compared with the idividual nonpregnant group.
Table 2. Effect of cyclic AMP formation in the presence of indomethacin and imidazole in washed platelets from pregnant and nonpregnant subjects
Cyclic AMP (pmol/ml)
Non-pregnancy Pregnancy Resting 27.4 ± 1.1 29.7 ± 0.9 PGE1 47.3 ± 0.7** 43.4 ± 2.2** Platelet suspensions (1.0· 108/ml) were preincubated with imidazole (600lmol/l) and indomethacin (500 lmol/l) for 3 min at 37°C. The reaction was terminated 3 min after the addition of PGE1(10lmol/l). The supernatants of platelet sus-pensions were collected for the determination of cAMP levels after centrifugation. Data are presented as the mean ± SEM (n¼ 6). **P < 0.001 as compared with the individual resting group.
fluorescence equivalent to a pH increase of 0.03 ± 0.001 and 0.08 ± 0.01, respectively, in nonpregnant and preg-nant subjects (n¼ 6, Table 2). The increase in pH in the pregnant group was markedly greater than that observed in the nonpregnant group. This result suggests that the hyperaggregability of platelets in normal pregnancy may be mediated by an increased in pH.
Discussion
The principal objective of this study was to investigate in detail the mechanisms of agonist-induced hyperaggrega-bility in washed platelets from pregnant subjects. Previous studies have demonstrated increases in agonist-induced hyperaggregability in both PRP and whole blood in normal pregnancy (Orrison et al., 1985; Louden et al., 1990; Nicolini et al., 1994). The most important finding in this study was that hyperaggregability in response to agonists is also observed in washed platelets in normal pregnancy, a result which has not been reported previ-ously. This platelet hyperaggregability was demonstrable using various agonists, including thrombin, ADP and arachidonic acid. In this study, platelet aggregation induced by an agonist (i.e. thrombin) appeared to be enhanced in normal pregnancy. Therefore, we can infer that enhanced platelet aggregation may increase [Ca+2]i release from intracellular Ca2+ storage sites (i.e. dense
tubular systems or dense bodies; Figure 2) which is in accord with the concept that [Ca+2]i release is responsible for platelet aggregation (Charo, Feinman & Detwiler, 1976).
Although the mechanisms of action of various platelet aggregation agonists, such as thrombin and ADP, differ, platelet aggregation in pregnant subjects was enhanced by all of them. This implies that agonist-induced hyperag-gregability in platelets from pregnant subjects involve a common step, shared by all these inducers. These results also indicate that basis of agonist-stimulated hyperaggre-gability does not involve receptors for individual agonists on the platelet surface membrane. Triflavin acts by binding to the GP IIb/IIIa complex on the platelet surface membrane, interfering with the interaction of fibrinogen with its specific receptor (Sheu, Teng & Huang, 1992). In this study, we found that FITC-triflavin binding to the GP IIb/IIIa complex was not significantly increased in thrombin-activated platelets from pregnant subjects, indi-cating that enhanced platelet aggregation in normal pregnancy might not be due directly to increased exposure of the GP IIb/IIIa complex on the platelet membrane surface.
Fitzgerald et al. (1987) reported that thromboxane biosynthesis is increased in pregnancy. This increase is mainly platelet-derived and is consistent with increased platelet activation throughout pregnancy (Orrison et al., 1985; Fitzgerald et al., 1987). In this study, TXB2 formation in resting and thrombin-stimulated platelets from pregnant subjects was markedly greater than in the nonpregnant controls. TXA2is an important mediator of platelet aggregation. Thus, increased TXB2formation may play an important role in enhanced platelet aggregation in normal pregnancy. However, what causes increased TXB2 formation in washed platelets from pregnant subjects remains unclear and must be investigated further.
The importance of cAMP in modulating platelet reac-tivity is well established (Karniguian, Legrand & Caen, 1982). There is evidence that inhibition of platelet activation by thromboxane synthetase inhibitors is medi-ated through cAMP in response to PGD2, produced by a diversion pathway from cyclic endoperoxides when thromboxane synthetase is blocked (Bertele et al., 1984). Horn et al. (1991) also reported that platelets from pregnant women produce less of the inhibitory second messenger cAMP in response to PGD2than do those from nonpregnant women. The present study also shows a reduction in cAMP accumulation in washed platelets, both in resting and PGE1-treated platelets, in a group of pregnant women, as compared with a nonpregnant group (Figure 3). In addition to inhibition of most platelet Figure 4. Thrombin-triggered intracellular pH change in
BCECF-AM loaded platelets from pregnant and nonpregnant subjects. Platelet suspensions (3.0· 108/ml) were preincubated with BCECF-AM (5lmol/l) at 37°C for 30 min, followed by the addition of thrombin (0.04 U/ml) to trigger intracellular pH change. Data are presented as the mean ± SEM (n¼ 6). **P < 0.001 as compared with nonpregnant subjects.
responses, elevated levels of cAMP decreased [Ca+2]i with uptake of Ca+2into the dense tubular system (Zavoico & Feinstein, 1984). Therefore, reductions in intracellular concentrations of cAMP might in part reflect enhanced platelet aggregation in normal pregnancy. It has been reported that elevated platelet TXA2 levels should inhibit platelet membrane-associated adenylate cyclase, which lowers cAMP levels in platelets (Chen et al., 1990). We also found that the lower cAMP formation in washed platelets from pregnant subjects may result from increased TXA2 formation (Table 2). Therefore, increased TXA2 formation and a lower cAMP level are likely mechanisms underlying agonist-induced hyperaggregability in platelets from pregnant women.
Activation of platelets by a variety of agonists (i.e. thrombin and ADP) is associated with stimulation of the Na+/H+exchanger (Sweatt et al., 1985; Kimura, Lasker & Avir, 1992). This mode of activation of the Na+/H+ exchanger usually induces a rise in cytosolic Ca+2, granule secretion, stimulation of shape change and aggregation (Kimura, Lasker & Avir, 1992). Basal pH is normally maintained within a narrow range, and even small changes in pH may have significant effects on platelet activity. In many cell types, including fibroblasts, hepatocytes and smooth muscle cells, Na+/H+ exchange activity is regulated by [Ca+2]i (Nieuwland, Van Willigen & Akkerman, 1994). Ca+2 may not affect the exchanger directly, but may act via Ca+2/calmodulin-dependent processes (Nieuwland, Van Willigen & Akkerman, 1994). Kimura, Lasker and Avir (1992) reported that cyclic nucleotides (i.e. cAMP) modulate Na+/H+exchange in human platelets. Inhibition of Na+/H+ exchange by cAMP has been demonstrated in other cells, such as urinary epithelia (Felder et al., 1990) and osteoblast-like cells (Reid et al., 1988). Thus, agents known to stimulate adenylate cyclase in these cells (i.e. parathyroid hormone or dopamine) can also inhibit the Na+/H+exchanger (Reid et al., 1988; Felder et al., 1990). The relationships between [Ca+2]i, TXA2, cyclic nucleotides and the Na
+ / H+exchanger may play an important role in mediation of agonist-induced hyperaggregability in pregnant subjects.
In conclusion, the most important observations in this study are that agonist-induced hyperaggregability was also observable in washed platelets from normal pregnancy. Based on the above observations, we suggest that the hyperaggregability of platelets from normal pregnancy may be due to stimulation of the Na+/H+exchanger. This leads to intracellular alkalinization and elevated [Ca+2]i, activation of membrane-bound phospholipase A2, subse-quent to increased TXA2 formation and then reduced cAMP formation, followed by a further increase in the Na+/
H+ exchanger, finally resulting in increased [Ca+2]i and enhanced platelet aggregation stimulated by agonists. In normal pregnancy, the enhanced platelet reactivity may have both useful and harmful effects. On the one hand, it may confer some protection against haemorrhage, while on the other hand it may contribute to the increased risk of thromboembolism that exists in pregnancy.
Acknowledgements
This work was supported by a grant from the National Science Council of Taiwan (NSC 89–2320-B-038–002-M53).
References
Bertele V., Falanga A., Tomasiak M., Cerletti C. & De Gaetano G. (1984) SQ22536, an adenylate cyclase inhibitor prevents the antiplatelet effect of dazoxiben, a thromboxane synthetase in-hibitor. Thrombosis and Haemostasis 51, 125–128.
Born G.V.R. & Cross M.J. (1963) The aggregation of blood platelets. Journal of Physiology 168, 178–195.
Charo I.F., Feinman R.D. & Detwiler T.C. (1976) Inhibition of platelet secretion by an antagonist of intracellular calcium. Biochemical and Biophysical Research Communications 72, 1462–1467.
Chen S.Y., Yu B.J., Liang Y.Q. & Lin W.D. (1990) Platelet ag-gregation, platelet cAMP levels and thromboxane B2synthesis in patients with diabetes millitus. Chinese Medical Journal 103, 312–318.
Felder C.C., Campbell T., Albrecht F. & Jose P.A. (1990) Dop-amine inhibits Na+-H+exchanger activity in renal BBMW by stimulation of adenylate cyclase. American Journal of Physiology 259, F297–F303.
Fitzgerald D.J., Mayo G., Catella F., Entman S.S. & Fitzgerald G.A. (1987) Increased thromboxane biosynthesis in normal preg-nancy is mainly derived from platelets. American Journal of Obstetrics and Gynecology 157, 325–330.
Grynkiewicz G., Poenie M. & Tsien R.Y. (1985) A new generation of Ca2+induces with greatly improved fluorescence properties. Journal of Biological Chemistry 260, 3440–3450.
Hall M.H., Chang P.K. & McGillivray I. (1980) Is routine ante-natal care worthwhile? Lancet ii, 78–80.
Horn E.H., Cooper J., Hardy E., Heptinstall S. & Rubin P.C. (1991) A cross-sectional study of platelet cyclic AMP in healthy and hypertensive pregnancy. Clinical Science 80, 549–558. Horne W.C., Norman N.E., Schwartz D.B. & Simons E.R. (1981)
Changes in cytoplasmic pH and in membrane potential in thrombin-stimulated platelets. European Journal of Biochemistry 120, 295–302.
Huang T.F., Sheu J.R. & Teng C.M. (1991) A potent antiplatelet peptide, triflavin, from Trimeresurus flavoviridis snake venom. Biochemical Journal 277, 351–357.
Karniguian A., Legrand Y.J. & Caen J.P. (1982) Prostaglandin: specific inhibition of platelet adhesion to collagen and relationship with cAMP level. Prostaglandin 23, 437–457.
Kimura M., Lasker N. & Avir A. (1992) Cyclic nucleotides attenuate thrombin-evoked alterations in parameters of platelet Na/H antiport. The role of cytosolic Ca+2. Journal of Clinical Investigation 89, 1121–1127.
Louden K.A., Broughton P.F., Heptinstall S., Fox S.C., Mitchell J.R.A. & Symonds F.M. (1990) A longitudinal study of platelet behaviour and thromboxane production in whole blood in normal pregnancy and the puerperium. British Journal of Obstetrics and Gynaecology 97, 1108–1114. Louden K.A., Broughton P.F., Symonds E.M., Tuohy P., O’Callaghan C., Heptinstall S., Fox S. & Mitchell J.R.A. (1992) A randomized placebo-controlled study of the effect of low-dose aspirin on platelet reactivity and serum thromboxane B2 pro-duction in nonpregnant women, in normal pregnancy, and gestational hypertension. British Journal of Obstetrics and Gynaecology 99, 371–376.
Lowry D.H., Rosebrough N.J., Farr A.L. & Randall R.J. (1951) Protein measurement with the folin phenol reagent. Journal of Biological Chemistry 193, 265–275.
Nicolini U., Guarneri D., Gianotti G.A., Campagnoli C. & Cro-signani P.G. (1994) Maternal and fetal platelet activation in normal pregnancy. Obstetrics and Gynecology 83, 65–69. Nieuwland R., Van Willigen G. & Akkerman J.N. (1994) Different
pathways for control of Na+/H+exchange via activation of the thrombin receptor. Biochemical Journal 297, 47–52.
Orrison R., Crawford J., Macpherson M. & Heptinstall S. (1985) Platelet behavior in normal pregnancy, pregnancy complicated by essential hypertension and pregnancy-induced hyperten-sion. Thrombosis and Haemostasis 54, 607–611.
Redman C.W.G. (1990) Platelets and the beginnings of pre-eclampsia. Lancet ii, 478–480.
Reid I.R., Civitelli R., Avioli L.V. & Hruska K.A. (1988) Parathyroid hormone depresses cytosolic pH and DNA synthesis in osteoblast-like cells. American Journal of Physiology 255, E9–E15. Sheu J.R., Hung W.C., Wu C.H., Ma M.C., Kan Y.C., Lin C.H., Luk H.N. & Yen M.H. (1999) Reduction in lipopolysaccharide-induced thrombocytopenia by triflavin in a rat model of sep-ticemia. Circulation 99, 3056–3062.
Sheu J.R., Lin C.H., Peng C.H. & Huang T.F. (1996) Triflavin, an Arg-Gly-Asp-containing peptide, inhibits the adhesion of tumor cells to matrix protein via binding to multiple integrin receptors expressed on human hepatoma cells. Proceedings of the Society for Experimental Biology and Medicine 213, 71–79. Sheu J.R., Teng C.M. & Huang T.F. (1992) Triflavin, an
RGD-containing antiplatelet peptide, binds to GP IIIa of ADP-stimulated platelets. Biochemical and Biophysical Research Communications 189, 1236–1242.
Sweatt J.D., Johnson S.L., Cargoe E.J. & Limbird L.E. (1985) Inhibition of Na+/H+ exchange block stimulus-provoked arachidonic acid release in human platelets. Journal of Biolo-gical Chemistry 260, 12910–12919.
Touyz R.M. & Schiffrin E.L. (1993) Effect of angiotensin II and endothelin-1 on platelet aggregation and cytosolic pH and free Ca+2 concentrations in essential hypertension. Hypertension 22, 853–862.
Ylikorkala O. & Viinikka L. (1980) Thromboxane A2 in preg-nancy and puerperium. British Medical Journal 281, 1601–1602.
Zavoico G.B. & Feinstein M.B. (1984) Cytoplasmic Ca2+ in platelets is controlled by cyclic AMP: antagonism between stimulators and inhibitors of adenylate cyclase. Biochemical and Biophysical Research Communications 120, 579–585.