INHIBITION OF HUMAN VASCULAR ENDOTHELIAL CELLS PROLIFERATION
BY TERBINAFINE
Pei-Yin HO1, Yu-Chih LIANG2, Yuan-Soon HO3, Chiung-Tong CHEN4and Wen-Sen LEE5,6* 1Graduate Institute of Cellular and Molecular Biology, Taipei Medical University, Taipei, Taiwan 2Department of Internal Medicine, School of Medicine, Taipei Medical University, Taipei, Taiwan 3Graduate Institute of Biomedical Technology, Taipei Medical University, Taipei, Taiwan
4Division of Biotechnology and Pharmaceutical Research, National Health Research Institutes, Taipei, Taiwan 5Graduate Institute of Medical Sciences, Taipei Medical University, Taipei, Taiwan
6Department of Physiology, School of Medicine, Taipei Medical University, Taipei, Taiwan
We have demonstrated previously that terbinafine (TB), an oral antifungal agent used in the treatment of superficial mycosis, suppresses proliferation of various cultured human cancer cells in vitro and in vivo by inhibiting DNA synthesis and activating apoptosis. In our study, we further demon-strated that TB at a range of concentrations (0 –120 M) dose-dependently decreased cell number in cultured human umbilical vascular endothelial cells (HUVEC). Terbinafine was not cytotoxic at a concentration of 120M, indicating that it may have an inhibitory effect on the cell proliferation in HUVEC. The TB-induced inhibition of cell growth rate is reversible. [3H]thymidine incorporation revealed that TB
reduced the [3H]thymidine incorporation into HUVEC
dur-ing the S-phase of the cell-cycle. Western blot analysis dem-onstrated that the protein levels of cyclin A, but not cyclins B, D1, D3, E, CDK2 and CDK4, decreased after TB treatment. The TB-induced cell-cycle arrest in HUVEC occurred when the cyclin-dependent kinase 2 (CDK2) activity was inhibited just as the protein level of p21 was increased and cyclin A was decreased. Pretreatment of HUVEC with a p21 specific an-tisense oligonucleotide reversed the TB-induced inhibition of [3H]thymidine incorporation. Taken together, these results
suggest an involvement of the p21-associated signaling path-way in the TB-induced antiproliferation in HUVEC. Capillary-like tube formation and chick embryo chorioallantoic
mem-brane (CAM) assays further demonstrated the
anti-angiogenic effect of TB. These findings demonstrate for the first time that TB can inhibit the angiogenesis.
© 2004 Wiley-Liss, Inc.
Key words: terbinafine; angiogenesis; G0/G1 cell-cycle arrest; p21
Angiogenesis, the formation of new blood vessels from the preexisting vessels, is a complex process required in many phys-iological and pathological conditions.1,2 Normally, vascular pro-liferation occurs only during embryonic development and is a very slow process in the adult with few exceptions (e.g., female repro-ductive system and wound healing). In contrast, many pathological conditions (e.g., atherosclerosis, diabetic retinopathy and cancer) are characterized by persistent, unregulated angiogenesis.3 Al-though the exact sequence of events involved and the regulation of angiogenesis remain unclear, it is believed that major steps in angiogenesis include: local degradation of the basement membrane of the parent vessel, allowing protrusion of endothelial cells; outward migration of endothelial cells in tandem to form a capil-lary sprout; proliferation of endothelial cells within the sprout; and the formation of a lumen with subsequent branching. Control of vascular development could permit new therapeutic approaches to these disorders. The potential pharmacological regulation of an-giogenesis is now an area of intensive interest.
The occurrence of cancers has been increased strikingly during the last decade. Although intensive research during the past few years has led to considerable progress in our ability to diagnose and treat cancer diseases at an early stage, the prognoses for patients are still not satisfactory. Recently, the dependence of angiogenesis in the tumor growth and metastasis of solid tumors is well recognized4,5The data supporting the notion that progressive tumor growth and metastasis are angiogenesis-dependent has led
to a growing interest in anti-angiogenic therapy as a potential therapeutic strategy against cancer development and metastasis. Therefore, experimental and clinical investigators continue to seek to identify medicinal agents capable of inhibiting the process of angiogenesis. One approach, as pursued in our study, seeks to identify medicinal agents capable of retarding the cell-cycle in the vascular endothelial cells.
Terbinafine (TB), a newly synthesized oral antimycotic drug, inhibits ergosterol synthesis at the stage of squalene epoxidation.6 It has been reported that TB has relative few drug interaction and is safe for clinical uses.7The cream form and oral tablet of TB have been approved for clinical uses in the United States,8and the oral formulation has been on the market in various countries.9 Recently, it has been demonstrated that a number of antifungal agents exert antiproliferative or apoptotic activities in various malignant cells in vitro and in vivo.10 –12 The anti-proliferation effect of TB has not been reported until our recent studies dem-onstrating that TB suppresses proliferation of various tumor cells in vitro and in vivo by inhibiting DNA synthesis and activating apoptosis.13Because the application of anti-angiogenic therapy in the cancer treatment has been suggested, we attempt to test whether TB also exerts anti-angiogenic activity. We show that TB inhibited the growth of human vascular endothelial cells. Our experimental findings highlight the molecular mechanisms of TB-induced anti-angiogenesis activity.
MATERIAL AND METHODS Cell lines and cell culture
HUVEC were grown in M199 (GIBCO, Grand Island, NY) containing 10% FBS (HyClone, UT), endothelial cell growth
sup-Abbreviations: AS, antisense oligonucleotide; CAM, chick embryo
cho-rioallantoic membrane; CDK, cyclin-dependent kinase; CKIs, CDK inhib-itors; DTT, dithiothreitol; ECGS, endothelial cell growth supplement; FACS, fluorescence-activated cell sorter; HUVEC, human umbilical vein endothelial cells; I.P., immunoprecipitation; M199, medium 199; MTT, 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide; NBT, ni-tro blue tetrazolium; TB, terbinafine; PMSF, phenylmethyl sulfonyl fluo-ride.
Grant sponsor: National Science Council; Grant number: NSC 91-2320-B-038-045, NSC 90-2320-B-038-032, NSC 89-2314-B-038-036.
*Correspondence to: Graduate Institute of Medical Sciences, Taipei Medical University, 250 Wu-Hsing Street, Taipei 110, Taiwan. Fax:⫹886-2-27391775. E-mail: wslee@tmu.edu.tw
Received 15 May 2003; Revised 23 September 2003; Accepted 12 November 2003
DOI 10.1002/ijc.20039
Published online 12 April 2004 in Wiley InterScience (www.interscience. wiley.com).
plement (Biomedical Technologies, Stoughton, MA) (ECGS, 0.03 mg ml⫺1) and kanamycin (GIBCO) (50 U ml⫺1) in a humidified 37°C incubator. After the cells had grown to confluence, they were disaggregated in trypsin solution (GIBCO), washed with M199 containing 10% FBS, centrifuged at 125g for 5 min, resuspended, and then subcultured according to standard protocols. Cells from passages 5–9 were used.
Determination of cell growth curve
As a measurement of cell proliferation, the cells were seeded onto 6-well 1% gelatin (Sigma, St. Louis, MO)-coated plates and grown in M199 supplemented with 10% FBS and ECGS. Terbin-afine was added at the indicated doses in 0.05% DMSO (Sigma). For control specimens, the same volume of the 0.05% DMSO without TB was added. Media without (control) and with TB were changed daily until cell counting. At various times of incubation, cultures were treated with trypsin-EDTA and the released cells were counted in a Coulter apparatus.
Viability assay
Cell viability was estimated by a modified MTT [3-(4,5-di-methyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide] (Sigma)
assay as described previously.14Four samples were analyzed in each experiment.
[3
H]thymidine incorporation
[3H]thymidine (Amersham Biosciences, UK) incorporation was carried out as described previously.15Briefly, HUVEC were ap-plied to 24-well plates in growth medium (M199 plus 10% FBS and ECGS). After the cells had grown to 70 – 80% confluence, they were rendered quiescent by incubation for 24 hr in M199 contain-ing 2% FBS. M199 supplemented with 10% FBS and 0.05% DMSO (control) or various concentrations of TB was added to the cells and the cultures were allowed to incubate for 24 hr. During the last 2 hr of the incubation without or with TB, [3H]thymidine was added at 1 Ci ml⫺1 (1 Ci ⫽ 37 kBq). Incorporated [3H]thymidine was extracted in 0.2 N NaOH and measured in a liquid scintillation counter.
Flow cytometry
As described previously,16 the cells were seeded onto 10-cm petri dishes and grown in M199 supplemented with 10% FBS and ECGS. After the cells had grown to subconfluence, they were rendered quiescent and challenged with 10% FBS. Then, after
FIGURE1 – Effects of TB on cell growth rate in subcultured HUVEC. (a) Dose-dependent growth inhibition of HUVEC by TB treatment. (b)
There was no significant difference in viability between control and TB (120M)-treated HUVEC. (c) TB-induced growth inhibition of HUVEC was reversed by removal of the TB treatment. Open column, HUVEC were treated with 0.05% DMSO for 6 days; striped column, HUVEC were treated with 60M TB for 6 days; solid column, HUVEC were treated with 60 M TB for 3 days and then the medium was replaced with 0.05% DMSO without TB for an additional 3 days. (d) Time-dependent inhibition of cell-cycle in HUVEC by TB. To study the time-dependent of TB on the cell-cycle, [3H]thymidine incorporation was conducted after HUVEC release from quiescence by incubation in culture media
supplemented with 10% FBS and 0.05% DMSO (control) or 60M TB in 0.05% DMSO. Three to four samples were analyzed in each group, and values represent the means⫾ SEM. Significance was accepted at p ⬍ 0.05. *p ⬍ 0.0001 vs. control group. #p ⬍ 0.0008 vs. 6d 60 M TB-treated group. §p⬍ 0.05 vs. control group.
release using trypsin-EDTA, they were washed twice with PBS and fixed in 70% ethanol at 4°C. Nuclear DNA was stained with a reagent containing propidium iodine (Sigma) (8g ml⫺1) and DNase-free RNase (Sigma) (100g ml⫺1) and measured using a fluorescence-activated cell sorter (FACS).
Protein extraction and Western blot analysis
To determine the expression levels of cyclins, CDKs, cyclin-dependent kinase inhibitors (CKIs), and G3PDH in HUVEC, the total proteins were extracted and Western blot analyses were carried out as previously described.17Briefly, HUVEC were cul-tured in 10 cm petri dishes. After reaching subconfluence, the cells were rendered quiescent and then treated with various concentra-tions of TB for 24 hr, and then incubated in a humidified incubator at 37°C. After incubation, the cells were washed with PBS (pH 7.4), incubated with extraction buffer (Tris 50 mM, pH 7.5, NaCl 150 mM, PMSF 1 mM, NP-40 1%, 0.1% SDS) on ice, and then centrifuged at 12,000g for 30 min. The cell extract was then boiled in a ratio of 3:1 with sample buffer (Tris-HCl 250 mM, pH 6.8, glycerol 40%, dithiothreitol 400 mM, SDS 8% and bromophenol blue 0.2%). Electrophoresis was carried out using 12% SDS-polyacrylamide gel (2 hr, 110 V, 40 mA, 50g protein per lane). Separated proteins were transferred to PVDF membranes (1 hr, 400 mA), treated with 5% fat-free milk powder (Anchor, NZ) to block the nonspecific IgGs, and incubated for 1 hr with specific antibody for cyclins (Santa Cruz Biotechnology, Santa Cruz, CA), CDKs (BD Transduction Laboratories, CA), CKIs (BD Bioscience Pharmingen, CA), or G3PDH (Jackson ImmunoResearch Labora-tories, West Grove, PA). The blot was then incubated with anti-mouse or anti-rabbit IgG (Jackson ImmunoResearch Laboratories) linked to alkaline phosphatase (1:1,000) for 1 hr. Subsequently, the membrane was developed with NBT/BCIP (Kirkegaard & Perry Laboratories, Gaithersburg, MD) as a substrate.
Immunoprecipitation and kinase activity assay
As described previously,15the TB-treated cells were lysed in Rb lysis buffer (137 mM NaCl, 20 mM Tris, PH 7.9, 10 mM NaF, 5 mM EDTA, 1 mM EGTA, 10% (v/v) glycerol, 1% Triton X-100, 1 mM sodium orthovanadate, 1 mM sodium pyrophosphate, 100 M -glycerophosphate, 1 mM PMSF, 10 g/ml aprotinin, 10g/ml leupeptin), and immunoprecipitated with anti-CDK2 or anti-CDK4 antibody (2g/ml). The protein complexes in beads were washed twice with Rb lysis buffer and then once with Rb kinase assay buffer. The level of phosphorylated of Rb (for pRb), histone H1 (for CDK2) and glutathione S-transferase-Rb fusion protein (for CDK4) were measured by incubating the beads with 40l of hot Rb kinase solution (0.25 l [2 g] of Rb-GST fusion protein [Santa Cruz Biotechnology], 0.5l of [␥-32P] ATP [Am-ersham], 0.5l of 0.1 mM ATP and 38.75 l of Rb kinase buffer) at 37°C for 30 min, and then stopped by boiling the samples in SDS sample buffer for 5 min. The samples were analyzed by 12% SDS-PAGE, and the gel was then dried and subjected to autora-diography.
Antisense oligonucleotide
The antisense oligonucleotide was designed based on the coding sequence, which is complementary to the region of the initiation codon (5⬘-TCCCCAGCCGGTTCTGACAT-3⬘ for AS p21 and 5⬘-ACCTGTGCTCCGACACGTCT-3⬘ for AS p27). The sense oligonucleotide of p21 or p27 was used for the control. The oligonucleotides (Sigma-Genosys, Inc., Houston, TX), were added to the cells at a final concentration up to 20 nM at 16 hr before the cell was challenged with 10% FBS.
Protein measurement and densitometric analyses
The protein concentrations were determined by using Bio-Rad protein assay with BSA as a standard. The signals of Western blot analysis were developed with NBT/BCIP as a substrate and standardized with glyceraldehyde-3-phosphate dehydrogenase (G3PDH), one kind of housekeeping genes. To quantify the inten-sity of the protein expression levels, the PVDF membranes were
scanned using an HP ScanJet scanner (HP ScanJet 5470C) and HP Precision ScanPro software, and the band densities were deter-mined as arbitrary absorption units using the Image-Pro Plus(2) software program.
Capillary-like tube formation assay
Capillary-like tube formation assay was carried out as previ-ously described18 with minor modifications. The 96-well plates were coated with 50 l Matrigel (10 mg/ml) (BD Bioscience Pharmingen) by incubating at 37°C for 1 hr. HUVEC were sus-pended in M200 (Cascade Biologics, Portland, OR) supplemented with 10% FBS and endothelial cell growth supplement, and plated onto a layer of Matrigel at a density of 4⫻ 104cells/well without or with TB (30 –120M). The plates were then incubated for a further 3 hr at 37°C and capillary-like tube formation was observed under microscope.
Chick embryo chorioallantoic membrane assay
Angiogenesis was assessed by using the chick embryo cho-rioallantoic membrane (CAM) assay method as described previ-ously.19,20The eggs were cracked on Day 4 of incubation and each was cultured in a 10-cm dish at 37°C with 5% CO2 in air and saturated humidity. Ten microliters of TB (120M) mixed with 1% methylcellulose are placed on Teflon plates and dried under laminar flow. The methylcellulose disks were placed on the CAM on Day 7 and the response was assayed microscopically after 72 hr. The density of blood vessel formation in each case is assessed by comparing with that of a control set. Images were photographed digitally at 10⫻.
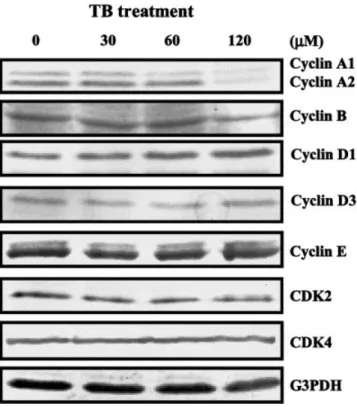
FIGURE2 – Effect of TB on the protein levels of cyclins and CDKs.
Western blot analysis was carried out to examine the changes of protein levels of cyclins and CDKs in the TB-treated HUVEC. Pro-teins were extracted from the cultured HUVEC at 24 hr after TB treatment and probed with proper dilutions of specific antibodies. TB (0 –120M) dose-dependently decreased the protein levels of cyclin A, but not cyclins B, D1, D3, E, CDK2 and CDK4. Results from a representative experiment are shown. Membrane was probed with anti-G3PHD antibody to verify equivalent loading. CDK, cyclin-de-pendent kinase.
Statistics
All data were expressed as the mean value⫾ SEM. Compari-sons were subjected to ANOVA followed by Fisher’s least signif-icant difference test. Significance was accepted at p⬍ 0.05.
RESULTS
Inhibition of cell proliferation in TB-treated HUVEC
To study the anti-angiogenic effect of TB, we examined the effect of TB on the growth of HUVEC. The cells were cultured for 5 days without or with TB (30 –120M). Cells were then har-vested and counted. As illustrated in the Figure 1a, TB decreased cell number in cultured HUVEC in a dose-dependent manner. To confirm that the TB-induced decrease in cell number of HUVEC was not due to cell death, we conducted viability assay by treating the cells with TB for 24 hr at the highest dose (120M) used in the study of cell growth inhibition. MTT assays indicated that there was no significant difference in cell viability between control and TB-treated HUVEC (Fig. 1b), suggesting that there was an inhib-itory effect of TB on the mechanisms for cell division in the subcultured HUVEC.
Reversibility of TB-induced inhibition of HUVEC proliferation We also examined the reversibility of the TB-induced growth inhibition of HUVEC. Treatment of HUVEC with TB (60M) for 6 days induced a 61% reduction of cell number as compared to the cells treated with 0.05% DMSO (Fig. 1c). Treatment of the cells with TB (60M) for 3 days followed by 0.05% DMSO without TB for an additional 3 days, however, induced only 49% inhibition as compared to the cells treated with 0.05% DMSO for 6 days. These results suggest that the TB-induced inhibition of cell growth rate is reversible.
Arrest of cell-cycle in G0/G1 phase
To demonstrate more sharply the actions of TB on a specific phase of the cell-cycle, the cells were switched to media with 2% FBS for 24 hr to render them quiescent and to synchronize their cell-cycle activities. Then they were returned to media with 10% FBS and, at various times thereafter, they were treated with [3H]thymidine. Figure 1d showed a reduction of the thymidine incorporation into HUVEC during the S-phase of the cell-cycle. The TB-induced accumulation of HUVEC at the G0/G1 phase of the cell-cycle was confirmed by flow cytometry analysis (data not shown).
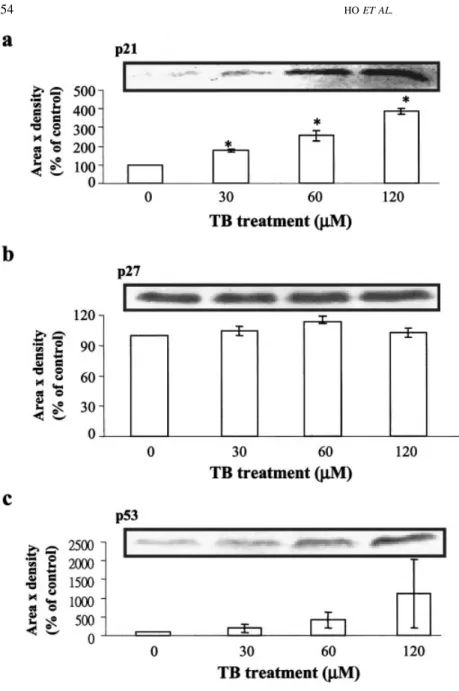
FIGURE3 – Effect of TB on the protein levels
of p21, p27 and p53. Western blot analysis was carried out to examine the changes of protein levels of p21, p27 and p53 in the TB-treated HUVEC. Proteins were extracted from the cul-tured HUVEC at 24 hr after TB treatment and probed with proper dilutions of specific antibod-ies. Treatment of HUVEC with TB (0 –120M) for 24 hr dose-dependently increased the protein levels of p21 (a) and p53 (c), but not p27 (b). Three samples were analyzed in each group, and values represent the means⫾ SEM. Membrane was probed with anti-G3PHD antibody to verify equivalent loading. *p⬍ 0.005 vs. control group.
Alterations in cell-cycle activity
To investigate the molecular mechanisms underlying of TB-induced G0/G1 arrest, the cells were switched to media with 2% FBS for 24 hr to render them quiescent at the G0/G1 phase. They were then returned to culture media supplemented with 10% FBS and 0.05% DMSO without or with TB (0 –120M), and at 24 hr thereafter, they were harvested for protein extraction and Western blot analysis to examine the effects of TB on the expression of cell-cycle regulatory proteins. It has been generally believed that progression of cell-cycle activity is regulated by coordinated suc-cessive activation of certain CDKs, which occurs late in the G1 phase and is instrumental in the transition from the G1 to the S-phase.21,22This CDK activation is in turn modulated by associ-ation with a number of regulatory subunits called cyclins, and with a group of CDK-inhibitory proteins designated CKIs.23 Cyclins have been identified as cyclins A, D1, D3 and E, whereas the most common CDKs are CDK2 and CDK4. As shown in the Figure 2, the protein levels of cyclin A, but not cyclins B, D1, D3 and E, CDK2 and CDK4, decreased in the TB-treated HUVEC. Because the CDK activity can be controlled by a group of CKIs, we examined the protein levels of p21 and p27, two known CKIs, in the TB-treated HUVEC. The protein level of p21 (Fig. 3a), but not p27 (Fig. 3b), increased in the TB-treated HUVEC as compared to
the DMSO-treated cells (control). We also examined the change of the protein level of p53 (Fig. 3c), which has been suggested to be involved in the regulation of p21 expression, in the TB-treated HUVEC. As illustrated in the Figure 3c, TB increased the level of p53 protein in a dose-dependent manner. The CKI exerts its inhibitory effect on the kinase activity through binding to cyclin-CDK complex. Accordingly, we further conducted immunopre-cipitation assay to examine the effect of TB on the formation of CDK-CKI complex. In the TB-treated cells, the formation of the CDK2-p21 complex, but not p21, CDK2-p27 and CDK4-p27 complex, was increased (Fig. 4a,b, left panels). The formation of the CDK2-cyclin A complex was decreased by TB treatment in a dose-dependent manner (Fig. 4a, left panel). To demonstrate that the increased p21 protein is associated with inhibition of CDK activation, which is necessary for cell-cycle progression from G1 to S-phase, we examined the CDK kinase activity. In the TB-treated HUVEC, the assayable kinase activity of CDK2 (Fig. 4a, right panel), but not CDK4 (Fig. 4b, right panel), was decreased significantly in a dose-dependent manner. To further demonstrate that the increased p21 expression observed in the TB-treated HUVEC correlated with G0/G1 arrest, the experiment illustrated in Figure 5 was conducted. Thus, in the sample labeled TB (for 60 M TB-treated alone), the [3H]thymidine incorporation was
de-FIGURE4 – Effect of TB on the formations of CKI-CDK complex and CDK kinase activity. (a) TB dose-dependently decreased the formation
of CDK2-cyclin A, and increased the formation of CDK2-p21, but not CDK2-p27 complex in HUVEC. The assayable CDK2, but not CDK4, kinase activity was decreased by TB treatment in a dose-dependent manner. CDK2 was immunoprecipitated by anti-CDK2 antibody, and CDK2-cyclin A association was detected by anti-cyclin A antibody, whereas CDK2-p21 and CDK2-p27 association was detected by anti-p21 antibody and anti-p27 antibody, respectively. **p⬍ 0.01 vs. control. (b) The formations of CDK4-p21 and CDK4-p27 complex and the assayable CDK4 kinase activity were not changed significantly by TB treatment. CDK4 was immunoprecipitated by anti-CDK4 antibody, and CDK4-p21 association was detected by anti-p21 antibody, whereas CDK4-p27 association was detected by anti-p27 antibody. Results from a representative experiment are shown. The CDK2 and CDK4 kinase activities were determined as described in the Material and Methods. IP, immunoprecipitation; CKI, cyclin-dependent kinase inhibitor; CDK, cyclin-dependent kinase.
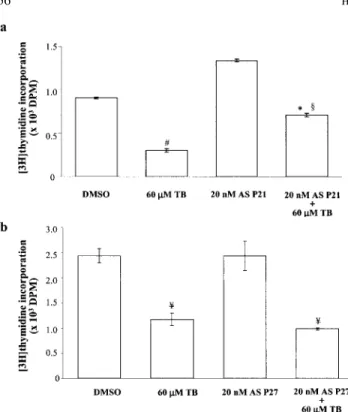
creased. Sample TB⫹ AS p21 or TB ⫹ AS p27 was pretreated with a p21 or p27 antisense oligonucleotide (AS), which blocked the expression of p21 or p27 protein respectively. Treatment of HUVEC with AS p21 or AS p27 alone did not cause any signif-icant change in [3H]thymidine incorporation into HUVEC. Con-sequently, pretreatment of HUVEC with AS p21 dose-dependently reversed the TB-induced decrease in [3H]thymidine incorporation (Fig. 5a). In contrast, pretreatment of HUVEC with sense p21 (data not shown) or AS p27 failed to prevent the TB-induced decrease in [3H]thymidine incorporation (Fig. 5b).
Anti-angiogenic effect of TB
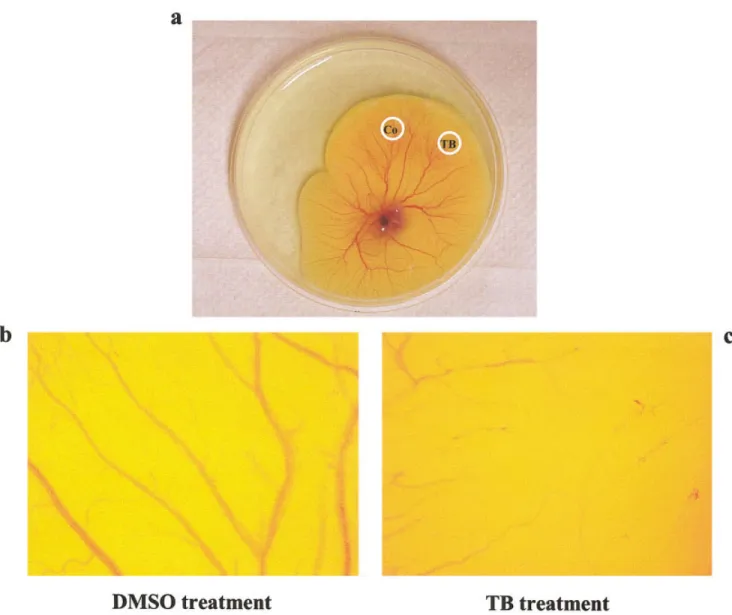
To confirm the anti-angiogenic effect of TB, we conducted the capillary-like tube formation assay. As illustrated in Figure 6, TB dose-dependently inhibited the capillary-like tube formation. The anti-angiogenic effect of TB was further confirmed by using the chick embryo chorioallantoic membrane (CAM) assay. Figure 7 demonstrated that TB (120M) inhibited the growth of capillary.
DISCUSSION
Our present study was undertaken to investigate the anti-angio-genic effect of TB and its mechanisms underlying. Our in vitro studies demonstrated that TB inhibited DNA synthesis, cellular proliferation and capillary-like tube formation in cultured HUVEC in a dose-dependent manner. The results of DNA synthesis and cellular proliferation in HUVEC were not due to cell death caused by TB treatment, indicating an inhibitory effect of TB on the mechanisms for cell division in subcultured HUVEC. The CAM assay demonstrated that TB dose-dependently inhibited the
sprout-ing angiogenesis. To our knowledge, this is the first demonstration of the anti-angiogenic effect of TB.
Historically, TB has been used in the treatment of superficial mycosis. Our recent studies have demonstrated that TB can inhibit the proliferation of various human malignant cells in vitro and in vivo, suggesting the anti-cancer effect of TB.10In cancer cell lines, treatment with TB at a range of concentrations (0 – 60M) dose-dependently inhibited DNA synthesis and decreased cell number. At these concentrations, TB did not inhibit the growth of the cultured human gingival fibroblasts. When TB concentration was increased to 120 M, a 50% and 100% growth inhibition was observed in human gingival fibroblasts and cancer cells respec-tively. In fact, TB at a concentration of 120 M induced the occurrence of apoptosis in the cancer cells, but not human fibro-blasts. These findings suggest the specificity of TB-induced growth inhibition and apoptosis in cancer cells. In our present study, however, we showed that TB at concentrations of 0 –120 M inhibited the growth of HUVEC in a dose-dependent manner (Fig. 1). Terbinafine was not cytotoxic at these concentrations. The anti-proliferation effect of TB in HUVEC further supports the potential applications of TB in the treatment of human cancer.
Treatment of HUVEC with TB resulted in an increase in the protein levels of p21 and p53 and a decrease in the protein levels of cyclin A, but not p27, cyclins B, D1, D3, E, CDK2 and CDK4 (Fig. 2). Among these changes, an increase in p21 protein level and a decrease in cyclin A protein level seem to have a major contribution to the TB-induced G0/G1 arrest in HUVEC. Our previous study in the COLO-205 cells exhibited that the binding between p53 protein and p53 consensus binding site in p21 promoter DNA probe was in-creased by TB treatment. In the COLO-205 cells, TB treatment caused an increase in the level of p53 protein, which in turn upregu-lated the p21 protein, and finally induced a decrease in the CDK4 kinase activity.10In our study, we demonstrated that the process of G0/G1 cell-cycle arrest induced by TB in HUVEC is correlated with the activation of the p21-associated signaling pathway, as evidenced by the p21 specific antisense oligonucleotide experiment, showing that pretreatment of HUVEC with AS p21 reversed the TB-induced decrease in [3H]thymidine incorporation (Fig. 5a). An increased ex-pression of p21 protein (Fig. 3a) and a decreased of the assayable CDK2 kinase activity (Fig. 4a) in the TB-treated HUVEC suggest that TB treatment caused an increase in p53 protein level, which in turn upregulated the p21 protein level, and finally induced a decrease in the CDK2 kinase activity. The consequent reduction of CDK2 kinase activity by p21 is most likely responsible for the TB-induced G0/G1 arrest in HUVEC. A decrease in cyclin A protein level might also have a contribution to the TB-induced G0/G1 arrest. In the TB-treated HUVEC, the formation of CDK2-cyclin A complex was decreased in a dose-dependent manner (Fig. 4a, left panel). This decrease might contribute to the TB-induced decrease in CDK2 kinase activity and explain why p21 antisense oligonucleotide treatment could not com-pletely reverse the TB-induced decrease in [3H]thymidine incorpora-tion (Fig. 5a). Interestingly, TB caused the growth inhibiincorpora-tion in both COLO-205 and HUVEC through a similar but not exact the same molecular mechanism. In COLO-205, the TB-induced cell-cycle ar-rest occurred when CDK4 kinase activity was inhibited just as the protein levels of p53 and p21 were increased. In HUVEC, the con-sequent reduction of CDK2 kinase activity by p21 is most likely responsible for the TB-induced G0/G1 arrest. Moreover, in our present study we found that the TB-mediated growth inhibition in HUVEC can be reversed by removal of the TB treatment (Fig. 1c), whereas our previous study showed that TB induced an irreversible growth inhibition in COLO-205 cell line.17Although we do not have a satisfactory answer for these discrepancies, one possible explanation is the different cell types used in these 2 experiments (untransformed vs. transformed cells). To address these issues, more investigations needed to be carried out.
The role of p21 in the regulation of cell-cycle progress has been well documented. P21 arrests the cell-cycle through binding and inactivating the CDK system.24,25A p21 mutation, which
specif-FIGURE 5 – Involvement of p21 in the TB-induced decrease of
[3H]thymidine incorporation in HUVEC. Antisense p21 or p27 oligo-nucleotide was added to HUVEC at a final concentration up to 20 nM at 16 hr before the cell was challenged with 10% FBS and 60M TB for additional 21 hr. Pretreatment of HUVEC with AS p21 (a), but not AS p27 (b), reversed the TB-induced decrease of [3H]thymidine
in-corporation. Values represent the means⫾ SEM. #p ⬍ 0.0008; *p ⬍ 0.05; ¥p⬍ 0.003 vs. control. §p⬍0.005 vs. 60 mM TB-treated. AS p21, antisense p21 oligonucleotide; AS p27, antisense p27 oligonu-cleotide.
ically abrogates its binding to CDKs, was identified in a primary breast tumor,26suggesting that p21 exerts tumor suppressor prop-erties. P27, on the other hand, is thought to mediate growth arrest and to play a critical role in negative regulation of cell division in vivo.27It has been demonstrated that p27 expression was associ-ated with spontaneous apoptosis and overexpression of p27 protein can lead to apoptosis in human cancer cells.28,29 Our previous study in the TB-treated cancer cells suggested that TB-induced upregulation of p27 protein in cancer cells might play an important role in TB-induced apoptosis in cancer cells but not in the induc-tion of cell growth arrest.10Consistent with this hypothesis, our present studies showed that the occurrence of apoptosis and a significant change of the protein level of p27 were not observed in the TB (0 –120M)-treated HUVEC (Fig. 3b).
It has been shown previously that after an oral administration of TB at a dose of 250 mg approximately 70% of TB was absorbed30 and the maximal plasma concentrations of 0.5–1.5 g/ml were
reached within 2 hr.31,32Moreover, a human study showed that the plasma level of TB was 5.83M after daily oral doses of 250 mg TB for 4 weeks.32 Because TB is highly lipophilic and kerato-philic, it extensively accumulates in adipose tissues, keratin-rich tissues and other tissues,33followed by slow redistribution of the drug into blood. It has been reported that TB remained in the toenails for a long time and exceeded the minimum inhibitory concentration for most dermatophytes and fungi.34Accordingly, the plasma levels of TB after oral administration in the patients with mycotic infections were much lower than the concentrations observed in our study for evaluating the anti-angiogenic effect. Evidently, a higher dose of TB or a different treatment regimen is required for anti-angiogenic therapy purposes. An in vitro study showed that TB at a range of concentrations (15–150M) dose-dependently inhibited the growth of wild-type yeast (W303-1B).35 It seems that the growth inhibition observed in yeast occurs in a similar dose-response range as with endothelial cells. We showed
FIGURE6 – TB inhibits capillary-like tube formation of HUVEC in Matrigel. HUVEC were suspended in M200 media supplemented with 10%
FBS and endothelial cell growth supplement, and plated onto a layer of Matrigel at a density of 4⫻ 104cells/well without (a) or with terbinafine
that administration of TB at a concentration as low as 60M for 24 hr arrested the HUVEC at the G0/G1 phase of the cell-cycle (Fig. 1d). Importantly, the flow cytometry assay from our previous study and cell viability assay from our study showed that at the doses used in our in vitro studies, TB was not cytotoxic for the cultured untransformed human fibroblasts and HUVEC, and the dose (50 mg/Kg body weight) used in our previous in vivo studies was not cytotoxic for the vital organs.
Our previous in vitro and in vivo studies showed that TB suppresses proliferation of cultured human cancer cells by inhib-iting DNA synthesis and activating apoptosis. These results led us to suggest the potential applications of TB in the treatment of human cancer. We further demonstrated the anti-proliferation,
anti-capillary-like tube formation, and anti-sprouting angiogenesis effects of TB in HUVEC, suggesting the anti-angiogenic activity of TB. In conclusion, the findings from our present in vitro studies in TB anti-angiogenic effect and from our previous in vitro and in vivo studies in TB anti-cancer effect strongly suggest the potential applications of TB in the treatment of human cancer.
ACKNOWLEDGEMENTS
Our study was supported by the National Science Council grant NSC 91-2320-B-038-045 and NSC 90-2320-B-038-032 to Dr. Lee and NSC 89-2314-B-038-036 to Dr. Ho.
REFERENCES
1. Risau W. Mechanisms of angiogenesis. Nature 1997;386:671– 4. 2. Bussolino F, Mantovani A, Persico G. Molecular mechanisms of
blood vessel formation. Trends Biochem Sci 1997;22:251– 6. 3. Folkman J. Clinical applications of research on angiogenesis. N Engl
J Med 1995;235:1757– 63.
4. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182– 6.
5. O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994;79:315–28.
6. Petranyi G, Ryder NS, Stutz A. Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Sci-ence 1984;224:1239 – 41.
FIGURE7 – Effect of TB on sprouting angiogenesis. Angiogenesis was assessed by using the CAM assay. (a) Gross appearance of the chick
embryo chorioallantoic membrane treated with methylcellulose disks containing DMSO (Co) or 120 M terbinafine (TB). Sprouting angiogenesis is observed after DMSO treatment (b) and inhibited after 72 hr addition of TB (c). Images were photographed digitally at 10⫻.
7. Abdel-Rahman SM, Nahata MC. Oral terbinafine: a new antifungal agent. Ann Pharmacother 1997;31:445–56.
8. Gupta AK, Shear NH. Terbinafine: an update. J Am Acad Dermatol 1997;37:979 – 88.
9. Gupta AK, del Rosso JQ, Lynde CW, Brown GH, Shear NH. Hepatitis associated with terbinafine therapy: three case reports and a review of the literature. Clin Exp Dermatol 1998;23:64 –7.
10. Chen RJ, Lee WS, Liang YC, Lin JK, Wang YJ, Lin CH, Hsieh JY, Chaing CC, Ho YS. Ketoconazole induces G0/G1 arrest in human colorectal and hepatocellular carcinoma cell lines. Toxicol Appl Phar-macol 2000;169:132– 41.
11. Rochlitz CF, Damon LE, Russi MB, Geddes A, Cadman EC. Cyto-toxicity of ketoconazole in malignant cell lines. Cancer Chemother Pharmacol 1988;21:319 –22.
12. Tzanakakis GN, Agarwal KC, Vezeridis MP. Inhibition of hepatic metastasis from a human pancreatic adenocarcinoma (RWP-2) in the nude mouse by prostacyclin, forskolin, and ketoconazole. Cancer 1990;65:446 –51.
13. Lee WS, Chen RJ, Wang YJ, Tseng H, Jeng JH, Lin SY, Liang YC, Chen CH, Lin CH, Lin JK, Ho PY, Chu JS, et al. In vitro and in vivo studies of the anticancer action of terbinafine in human cancer cell lines: G0/G1 p53-associated cell-cycle arrest. Int J Cancer 2003;106: 125–37.
14. Lee WS, Harder JA, Yoshizumi M, Lee ME, Haber E. Progesterone inhibits arterial smooth muscle cell proliferation. Nat Med 1997;3: 1005– 8.
15. Lin SY, Liu JD, Chang HC, Yeh SD, Lin CH, Lee WS. Magnolol suppresses proliferation of cultured human colon and liver cancer cells by inhibiting DNA synthesis and activating apoptosis. J Cell Biochem 2002;84:532– 44.
16. Lin SY, Chang YT, Liu JD, Yu CH, Ho YS, Lee WS. Molecular mechanisms of apoptosis induced by magnolol in colon and liver cancer cells. Mol Carcinog 2001;32:73– 83.
17. Lin SY, Liang YC, Ho YS, Tsai SH, Pan S, Lee WS. Involvement of both extracellular signal-regulated kinase and c-jun n-terminal kinase pathways in the 12-O-tetradecanoylphorbol-13-acetate-induced up-regulation of p21Cip1in colon cancer cells. Mol Carcinog 2002;35:
21– 8.
18. Grant DS, Kinsella JL, Fridman R, Auerbach R, Piasecki BA, Yamada Y, Zain M, Kleinman HK. Interaction of endothelial cells with a laminin A chain peptide (SIKVAV) in vitro and induction of angio-genic behavior in vivo. J Cell Physiol 1992;153:614 –25.
19. Knighton D, Asuprunk D, Tapper D, Folkman J. Avascular and vascular phases of tumour growth in the chick embryo. Br J Cancer 1977;35:347–56.
20. Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, Riordan JF, Vallee BL. Isolation and characterization of angiogenin, an an-giogenic protein from human carcinoma cells. Biochemistry 1985;20: 5480 – 6.
21. Hunter T, Pines J. Cyclins and cancer. II: Cyclin D and CDK inhib-itors come of age. Cell 1994;79:573– 82.
22. Morgan DO. Principles of CDK regulation. Nature 1995;374:131– 4. 23. Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent
kinases. Genes Dev 1995;9:1149 – 63.
24. el-Deiry WS, Harper JW, O’Connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill DE, Wang Y. WAF1/ CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 1994;54:1169 –74.
25. el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a poten-tial mediator of p53 tumor suppression. Cell 1993;75:817–25. 26. Balbin M, Hannon GJ, Pendas AM, Ferrando AA, Vizoso F, Fueyo A,
L´opez-Otı´n C. Functional analysis of a p21WAF1,CIP1,SDI1 mutant (Arg943Trp) identified in a human breast carcinoma. Evidence that the mutation impairs the ability of p21 to inhibit cyclin-dependent kinases. J Biol Chem 1996;271:15782– 6.
27. Naumann U, Weit S, Rieger L, Meyermann R, Weller M. p27 mod-ulates cell-cycle progression and chemosensitivity in human malig-nant glioma. Biochem Biophys Res Comm 1999;261:890 – 6. 28. Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak
K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 1996;85:733– 44.
29. Katayose Y, Kim M, Rakkar AN, Li Z, Cowan KH, Seth P. Promoting apoptosis: a novel activity associated with the cyclin-dependent ki-nase inhibitor p27. Cancer Res 1997;57:5441–5.
30. Jensen JC. Clinical pharmacokinetics of terbinafine (Lamisil). Clin Exp Dermatol 1989;14:110 –3.
31. Humbert H, Cabiac MD, Denouel J, Kirkesseli S. Pharmacokinetics of terbinafine and of its five main metabolites in plasma and urine, following a single oral dose in healthy subjects. Biopharm Drug Dispos 1995;16:685–94.
32. Kovarik JM, Mueller EA, Zehender H, Denouel J, Caplain H, Mille-rioux L. Multiple-dose pharmacokinetics and distribution in tissue of terbinafine and metabolites. Antimicrobial Agents Chemother 1995; 39:2738 – 41.
33. Faergemann J, Zehender H, Jones T, Maibach I. Terbinafine levels in serum, stratum corneum, dermis-epidermis (without stratum cor-neum), hair, sebum, and eccrine sweat. Acta Derm Venereol 1991; 71:322– 6.
34. Schatz F, Brautigam M, Dobrowolski E, Effendy I, Haberl H, Mensing H, Weidinger G, Stutz A. Nail incorporation kinetics of terbinafine in onychomycosis patients. Clin Exp Dermatol 1995;20: 377– 83.
35. Klobunˇcnı´kov´a V, Koh´ut P, Leber R, Fuchsbichler S, Schweighofer N, Turnowsky F, Hapala I. Terbinafine resistance in a pleiotropic yeast mutant is caused by a single point mutation in the ERG1 gene. Biochem Biophys Res Comm 2003;309:666 –71.