The Analysis of Parkinson’s Disease and Subsequent Cancer Risk in Taiwan - A Nationwide Population-Based Cohort Study
Running title: Parkinson’s Disease and Cancer Risk in Taiwan
Li-Min Suna, Ji-An Liangb,c, Shih-Ni Changd,e,f, Fung-Chang Sunge,g, Chih-Hsin Muoe,g, and Chia-Hung Kaoc,h
a
Department of Radiation Oncology, Zuoying Armed Forces General Hospital, Kaohsiung, Taiwan
b
Department of Radiation Oncology, gManagement Office for Health Data, and
h
Department of Nuclear Medicine and PET Center, China Medical University Hospital, Taichung, Taiwan
c
School of Medicine, College of Medicine, dThe Ph.D. Program for Cancer Biology and Drug Discovery, and eInstitute of Environmental Health, College of Public Health, China Medical University, Taichung, Taiwan
f
Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan
Li-Min Sun and Ji-An Liang contributed equally to this work.
Corresponding author: Dr. Chia-Hung Kao, Department of Nuclear Medicine and PET Center, China Medical University Hospital, No. 2, Yuh-Der Road, Taichung 404, Taiwan. Tel.: +886 4 22052121x7412; Fax.: +886 4 22336174. E-mail: d10040@mail.cmuh.org.tw
Co-corresponding author: Chih-Hsin Muo, Management Office for Health Data, China Medical University Hospital, No. 2, Yuh-Der Road, Taichung 404, Taiwan. Tel.:
Abstract:
Background: Patients with Parkinson’s disease (PD) are suggested to have a lower risk for the development of certain cancers and a higher risk for melanoma. The aim of this study is to evaluate the possible association between PD and malignancy in Taiwan. Materials and Methods: We used the data of the National Health Insurance System of Taiwan to assess this issue. The PD cohort contained 4,957 patients, and each patient was randomly frequency-matched by age and sex with four people from the general population without PD. A Cox’s proportional hazard regression analysis was conducted to estimate the effects of PD on the cancer risk. Results: In patients with PD, the risk of developing overall cancer was marginally significantly lower than subjects without PD [adjusted Hazard ratio (HR) = 0.88, 95% confidence interval = 0.78-0.99]. For individual cancers, the risks for developing colorectal and lung cancers among patients with PD were marginally significantly lower than in subjects without PD. In contrast, despite the higher HR for the development of melanoma, it did not reach statistical significance because of the relatively small sample size. Conclusion: Our study found that Taiwanese patients with PD have a lower risk of developing colorectal and lung cancers. The findings of this study are compatible with those of prior studies from other countries.
Introduction
Parkinson’s disease (PD) is the most common neurodegenerative disorder after Alzheimer's disease. Its prevalence is estimated at 0.3% of the population in industrialized countries, rising to 1% for individuals over 60 years of age and to 4% for those over 80 [1]. The prevalence of PD in Asian countries is slightly lower than in Western countries [2-4]. In Taiwan, the prevalence and incidence rates of PD are much higher than those reported in China and are closer to those in Western countries [5]. Since 1954, when Dorsey mentioned that, for unknown reasons, cancer is rare in paralyses agitans (Parkinsonism) [6], researchers have been exploring the possible linkage between PD and malignancy. They have found that patients with PD have a lower risk of most kinds of cancers than the general population. Only melanoma, and possibly other non-melanoma skin cancers, show a possible positive relationship with PD [7-10].
Cancer has been the leading cause of death among the general population of Taiwan since 1982, and the cancer registry data from Taiwan showed that more than 61% of cancer patients were diagnosed at or after the age of 60, and more than a quarter of these were in people 75 and over [11]. PD is also age-related with the incidence rapidly increasing over the age of 60 [12]. As the elderly population of the world increases, the incidence and prevalence of cancer, as well as PD, will also continue to increase.To the best of our knowledge, no large, population-based studies, which evaluate the association between malignancy and PD, have been done in Taiwan. The aim of this study is to determine whether the same pattern of PD cancer risk exists for patients in Taiwan. The results, presented in this paper, were from a retrospective cohort study which assessed the possibility of a lower risk of developing malignancy in patients with PD.
Methods Data sources
The present study used the reimbursement claims data of the universal NHI, implemented in Taiwan in March 1995 with the coverage rate of more than 96% of the country’s population and contracted with 97% of hospitals and clinics since the end of 1996.
We obtained the claims data from the National Health Research Institute (NHRI), Department of Health in the year of 1996-2008, consisting of registries and claims reported from contracted health care facilities. For confidentiality, the NHI program in Taiwan has registered all medical claims with insured scrambled identification numbers. With approval from NHRI, we were able to use the scrambled patient identification to link files, including the registry of medical facilities, details of inpatients orders, ambulatory cares, dental services, and prescriptions.
We used the electronic claims sub-database, and the NHRI established with a randomly selected sample from the insured population of one million representative reimbursement claims dataset in 2000. The International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) was used to identify patient’s disease. Study population
Our study extracted newly diagnosed patients with PD in the period of 2000–2005 from both ambulatory care and inpatient care as the exposure cohort (ICD-9-CM 332). A comparison cohort was randomly selected from insured people without history of PD by the ratio of 1:4 frequency-matched with age, sex and index year of the cases in the corresponding PD cohort. The age of each study subject was measured by the difference in time between the index date and the date of birth. Subjects with the
history of malignant cancer (ICD-9-CM 140–208) diagnosed before index date or with missing information on age or sex were excluded. We finally included 24,785 subjects in this study.
Study end-point
We linked the study subjects to the registry for Catastrophic Illness Patient Database (CIPD) to identify the newly diagnosed of cancer by the unique patient’s identification number. The diagnosis of cancer in National Health Insurance Research Database (NHIRD) needs histological confirmation and report in the CIPD. Each study subject was followed until a diagnosis of cancer was made, or until the time the subject was censored for loss to follow-up, death, or termination of insurance, or up to 31 December 2008, the end of the follow-up.
Statistical analysis
Demographic factors, including age, sex, urbanization, occupation, and co-morbidities were compared between each PD patients and non-PD patients using Chi-square tests. Cox proportional hazard regressions were used to measure the risk of developing cancer associated with PD for each study set. The sex-specific and cancer type-specific hazard ratios of cancer were also conducted by Cox’s proportional hazard regression analysis. The hazard ratio (HR) was presented with 95% CI, controlling for sex, age, occupation, hypertension, diabetes mellitus, hyperlipidemia and heart disease for adjustment in the multivariable Cox analyses.
The p-value < 0.05 was considered statistically significant. All analyses were performed with SAS 9.1 (SAS institute Inc., Cary, NC)
Results
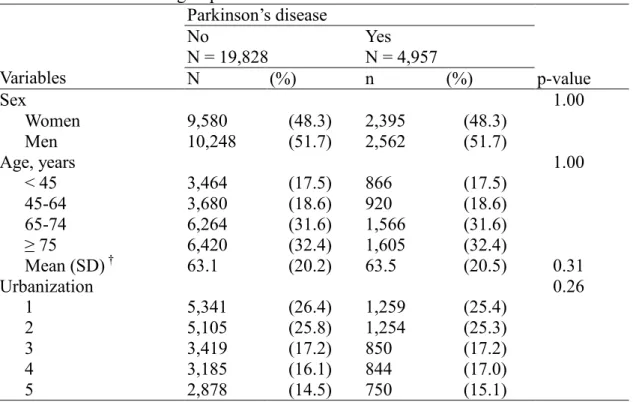
19,828 non-PD controls during the period of 2000-2005 (Table1). There were more
men subjects than women (51.7% vs. 48.3%). The average age of non-PD cohort and PD cohort were 63.1 years and 63.5 years, respectively.
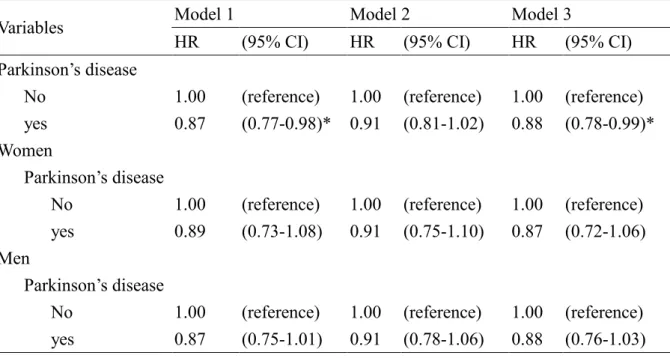
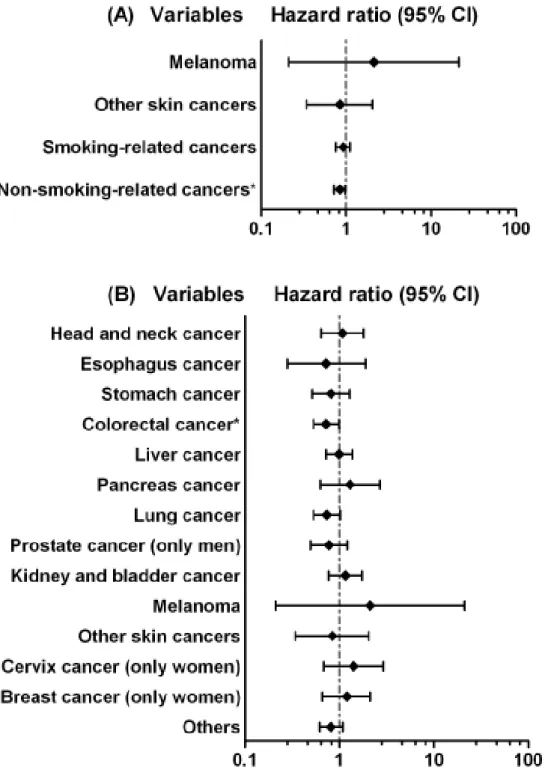
Table 2 presents the sex-specific HRs of cancer associated with PD controlling for the socio-demographic variables. The results indicate that PD patients had lower risk of developing cancer (HR = 0.88, 95% CI = 0.78-0.99). However, there was no significant relationship between cancer and PD after stratification analysis by sex (HR = 0.87, 95% CI = 0.72-1.06 in women; HR = 0.88, 95% CI = 0.76-1.03 in men). Figure 1A shows the results of HR and 95% CI of PD in skin cancers, smoking-related cancers and non-smoking-related cancers. Patients with PD had a higher HR to develop melanoma; however, the 95 % CI included 1. Both smoking-related and non-smoking-related cancers had a lower HR in PD cohort, but only non-smoking-related cancers came with a statistical significance. The lower HR became significant again when we combined smoking-related and non-smoking-related cancers together (data not shown).
The further cancer type-stratification analysis shows that the adjusted HR of developing colorectal cancer was significantly lower in PD cohort (HR = 0.72, 95% CI = 0.53-0.99), and a marginally significant lower HR was observed in lung cancer (HR = 0.73, 95% CI = 0.53-1.02) (Figure 1B).
Discussion
The results from the adjusted model show that patients with PD in Taiwan have a significantly lower overall cancer occurrence, except for melanoma and other skin cancers. Our results are partially compatible with other studies. Significantly reduced
risks were found for colorectal and non-smoking-related cancers. However, no significantly higher risk for melanoma and other skin cancers were found.
To the best of our knowledge, this is the first population-based study done on PD patients in Taiwan. The data were from the NHI system which covers more than 96% of the population and is contracted by 97% of the hospitals, thus, the results can be generalized. Patients in the PD group were randomly frequency-matched, according to age, sex, and index year, with 4 people from the general population without PD.
Our data show that patients with PD generally tended to have co-morbidity. Previous studies showed some mixed results regarding the association between PD and co-morbidity [13-15]. Our PD patients were mostly unemployed or retired. The distribution o f the urbanization level showed no significant difference between the two groups. Rural living has long been debated as a risk factor for PD [16,17]. A prior study done in Taiwan suggested that urban living was a more important risk factor for developing PD than rural living [18].
Bajaj et al. [10] conducted a meta-analysis and categorized cancer types into
smoking-related and non-smoking related cancers. They found that PD was associated with lower risks in both groups, although the decreased risk of cancer was more substantial for smoking-related cancers than for non-smoking-related cancers. We used a similar approach and pooled the data together for all smoking-related cancers and non-smoking-related cancers. For smoking-related cancers, we used the definition provided in the most recent Surgeon General’s Report and pooled these cancers together to estimate the cancer risk for PD [19]. These smoking related cancers included lung, head and neck, esophagus, kidney, bladder, pancreas, stomach, and cervix cancers. Our results revealed a lower HR for the smoking-related cancers; however, the difference did not attain statistical significance. This result is similar to
that from a study by Driver et al. [20]. For non-smoking-related cancers, the pooled data reflected a statistically significantly lower risk among patients with PD, which may be attributable to the relatively larger sample size.
For the site-specific cancer, we found Taiwanese PD patients had a significantly lower risk of developing colorectal cancer, and a marginally statistically significant lower risk of developing lung cancer. Lung cancer is definitely smoking-related and most studies have shown a negative relationship between the PD and lung cancer [9,10]. In fact, a common hypothesis for the decreased incidence of cancer in patients with PD is the well-know negative association between PD and smoking [21]. In contrast, some researchers found that cigarette smoking increased the risk of colorectal cancer and concluded that colorectal cancer was also a smoking-related cancer [22,23]. However, the Surgeon General’s Report did not include colorectal cancer as a smoking-related cancer [19], and we followed the report’s
recommendation to exclude colorectal cancer as a smoking-related cancer. The prior meta-analysis also classified colorectal cancer as a non-smoking-related cancer and found a significantly lower risk of developing it among patients with PD [10]. In Taiwan, colorectal cancer and lung cancer are the most and third most common malignancy, respectively. The mortality rates are the second and third highest for lung and colorectal cancer, respectively [11].
Melanoma is more common among patients with PD [8-10], and a shared genetic component between melanoma and PD has been proposed [24]. For the other skin cancers, no consensus exists regarding the positive association between them and PD [9,10]. Our study shows a higher HR for melanoma and a lower HR for other skin cancers among patients with PD. However, both failed to attain statistical significance. The main reason for this is that skin cancer (including melanoma) is much less
common in Taiwan than in Western countries [25], and the limited number of cases may have prevented us from finding any statistically significant results.
This study’s strength is its population-based design and its generalizability. However, one major limitation needs to be addressed. No information regarding patient life style or behavior is in the NHI database. Thus, we could not adjust for behavioral factors related to health, such as smoking and alcohol consumption.
Hernán et al. [21] found strong epidemiological evidence to suggest that smokers have a lower risk of PD, which is compatible with other research [26,27]. This may account for much of the risk reduction for smoking-related cancer. However, if we want to analyze the risk for smoking-related cancers, data unadjusted for smoking status may reflect the possible relationship more appropriately. For the interaction between alcohol consumption and PD, the results were more controversial [27,28], and adjustment for this factor is not necessary.
In conclusion, our results partially agree with previous studies and suggest that patients with PD have a lower risk of developing overall cancer, colorectal cancer, and possibly lung cancer. However, no significant linkage was found between PD and melanoma because of the relatively small case numbers.
Disclosure of Potential Conflicts of Interest No potential conflicts of interest exist.
Acknowledgement
We want to thank the grant support of the study project (DMR-97-103 and 97-104) in our hospital, Taiwan Department of Health Clinical Trial and Research Center and
for Excellence (DOH100-TD-B-111-004), and Taiwan Department of Health Cancer Research Center for Excellence (DOH100-TD-C-111-005).
References
1. de Lau LM, Breteler MM: Epidemiology of Parkinson's disease. Lancet Neurol 2006;5:525-535.
2. Muangpaisan W, Hori H, Brayne C: Systematic review of the prevalence and incidence of Parkinson's disease in Asia. J Epidemiol 2009;19:281-293.
3. Rajput AH, Birdi S: Epidemiology of Parkinson's disease. Parkinsonism Relat Disord 1997;3:175-186.
4. von Campenhausen S, Bornschein B, Wick R, Botzel K, Sampaio C, Poewe W, Oertel W, Siebert U, Berger K, Dodel R: Prevalence and incidence of Parkinson's disease in Europe. Eur Neuropsychopharmacol 2005;15:473-490.
5. Chen RC, Chang SF, Su CL, Chen TH, Yen MF, Wu HM, Chen ZY, Liou HH: Prevalence, incidence, and mortality of PD: a door-to-door survey in Ilan county, Taiwan. Neurology 2001;57:1679-1686.
6. DOSHAY LJ: Problem situations in the treatment of paralysis agitans. J Am Med Assoc 1954;156:680-684.
7. Inzelberg R, Jankovic J: Are Parkinson disease patients protected from some but not all cancers? Neurology 2007;69:1542-1550.
8. Ferreira JJ, Neutel D, Mestre T, Coelho M, Rosa MM, Rascol O, Sampaio C: Skin cancer and Parkinson's disease. Mov Disord 2010;25:139-148.
9. Olsen JH, Friis S, Frederiksen K, McLaughlin JK, Mellemkjaer L, Moller H: Atypical cancer pattern in patients with Parkinson's disease. Br J Cancer
2005;92:201-205.
10. Bajaj A, Driver JA, Schernhammer ES: Parkinson's disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control 2010;21:697-707.
11. Taiwan Cancer Registry(2007). Taipei: Department of Health, The Executive Yuan, Taiwan. Available at:
http://www.bhp.doh.gov.tw/BHPnet/Portal/StatisticsShow.aspx?No=201002050001
12. Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM: Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 2003;157:1015-1022.
13. Simon KC, Chen H, Schwarzschild M, Ascherio A: Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology 2007;69:1688-1695.
14. Hu G, Antikainen R, Jousilahti P, Kivipelto M, Tuomilehto J: Total cholesterol and the risk of Parkinson disease. Neurology 2008;70:1972-1979.
15. Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J: Type 2 diabetes and the risk of Parkinson's disease. Diabetes Care 2007;30:842-847.
16. Koller W, Vetere-Overfield B, Gray C, Alexander C, Chin T, Dolezal J,
Hassanein R, Tanner C: Environmental risk factors in Parkinson's disease. Neurology 1990;40:1218-1221.
17. Schrag A, Ben-Shlomo Y, Quinn NP: Cross sectional prevalence survey of idiopathic Parkinson's disease and Parkinsonism in London. BMJ 2000;321:21-22.
18. Chen CC, Chen TF, Hwang YC, Wen YR, Chiu YH, Wu CY, Chen RC, Tai JJ, Chen TH, Liou HH: Different prevalence rates of Parkinson's disease in urban and rural areas: a population-based study in Taiwan. Neuroepidemiology
2009;33:350-357.
19. Office of the Surgeon General (US), Office on Smoking and Health (US): The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US), 2004.
20. Driver JA, Logroscino G, Buring JE, Gaziano JM, Kurth T: A prospective cohort study of cancer incidence following the diagnosis of Parkinson's disease. Cancer Epidemiol Biomarkers Prev 2007;16:1260-1265.
21. Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ: A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol 2002;52:276-284.
22. Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P: Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765-2778.
23. Giovannucci E: An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 2001;10:725-731.
24. Gao X, Simon KC, Han J, Schwarzschild MA, Ascherio A: Family history of melanoma and Parkinson disease risk. Neurology 2009;73:1286-1291.
25. Smith DR, Sheu HM, Hsieh FS, Lee YL, Chang SJ, Guo YL: Prevalence of skin disease among nursing home patients in southern Taiwan. Int J Dermatol
2002;41:754-759.
26. Elbaz A, Moisan F: Update in the epidemiology of Parkinson's disease. Curr Opin Neurol 2008;21:454-460.
27. Wirdefeldt K, Gatz M, Pawitan Y, Pedersen NL: Risk and protective factors for Parkinson's disease: a study in Swedish twins. Ann Neurol 2005;57:27-33.
28. Ragonese P, Salemi G, Morgante L, Aridon P, Epifanio A, Buffa D, Scoppa F, Savettieri G: A case-control study on cigarette, alcohol, and coffee consumption preceding Parkinson's disease. Neuroepidemiology 2003;22:297-304.
Table legends
Table 1. Baseline characteristics between Parkinson’s disease group and non-Parkinson’s disease group in 2000-2005
Table 2. Hazard ratios and 95% confidence interval of cancer associated with Parkinson’s disease in Cox’s regression analysis
Figure 1. Hazard ratios and 95% confidence interval of cancer associated with Parkinson’s disease in multivariable Cox’s regression analysis (A) categorize skin cancers and smoking-related cancers (B) different cancers
Table 1. Baseline characteristics between Parkinson’s disease group and non-Parkinson’s disease group in 2000-2005
Parkinson’s disease No N = 19,828 Yes N = 4,957 Variables N (%) n (%) p-value Sex 1.00 Women 9,580 (48.3) 2,395 (48.3) Men 10,248 (51.7) 2,562 (51.7) Age, years 1.00 < 45 3,464 (17.5) 866 (17.5) 45-64 3,680 (18.6) 920 (18.6) 65-74 6,264 (31.6) 1,566 (31.6) ≥ 75 6,420 (32.4) 1,605 (32.4) Mean (SD) † 63.1 (20.2) 63.5 (20.5) 0.31 Urbanization 0.26 1 5,341 (26.4) 1,259 (25.4) 2 5,105 (25.8) 1,254 (25.3) 3 3,419 (17.2) 850 (17.2) 4 3,185 (16.1) 844 (17.0) 5 2,878 (14.5) 750 (15.1)
Urbanization level: 1 indicate the highest level of urbanization and 5 the lowest Chi-square test, † student T-test
Hypertension ICD-9-CM: 401-405, admission more than three times
Diabetes mellitus ICD-9-CM: 250, admission more than twice in the first year Hyperlipidemia ICD-9-CM): 272, admission more than three times
Table 2. Hazard ratios and 95% confidence interval of cancer associated with Parkinson’s disease in Cox’s regression analysis
Model 1 Model 2 Model 3
Variables
HR (95% CI) HR (95% CI) HR (95% CI) Parkinson’s disease
No 1.00 (reference) 1.00 (reference) 1.00 (reference) yes 0.87 (0.77-0.98)* 0.91 (0.81-1.02) 0.88 (0.78-0.99)* Women
Parkinson’s disease
No 1.00 (reference) 1.00 (reference) 1.00 (reference) yes 0.89 (0.73-1.08) 0.91 (0.75-1.10) 0.87 (0.72-1.06) Men
Parkinson’s disease
No 1.00 (reference) 1.00 (reference) 1.00 (reference) yes 0.87 (0.75-1.01) 0.91 (0.78-1.06) 0.88 (0.76-1.03) Model 1: unadjusted
Model 2: adjusted for age, sex and occupation
Model 3: adjusted for age, sex, occupation, hypertension, diabetes mellitus, hyperlipidemia and heart disease
Figure 1. Hazard ratios and 95% confidence interval of cancer associated with Parkinson’s disease in multivariable Cox’s regression analysis (A) categorize skin cancers and smoking-related cancers (B) different cancers
ICD-9-CM: head and neck cancer, 140.xx-149.xx; esophagus cancer, 150.xx; Stomach cancer, 151.xx; colorectal cancer, 153.xx and 154.xx; liver cancer, 155.xx; pancreas cancer, 157.xx; lung cancer, 162.xx; melanoma, 172.xx; skin cancer, 173.xx;
breast cancer, 174.xx; cervical cancer, 180.xx; prostate cancer, 185.xx; kidney and bladder cancer, 188.xx and 189.xx; smoking-related cancer, 146.xx, 148.xx, 150.xx, 151.xx, 157.xx, 162xx, 180.xx, 188.xx and 189.xx