Reactions of [Et
4N]
3[Sb
{
Fe(CO)
4}
4] with Alkyl Halides and Dihalides: Formation of the
Alkyl- and the Dialkylantimony
-
Iron Carbonyl Complexes
Minghuey Shieh,* Chii-maw Sheu, Li-Fang Ho, Jiann-Jang Cherng, Li-Fing Jang, and Chuen-Her Ueng
Department of Chemistry, National Taiwan Normal University, Taipei 11718, Taiwan, Republic of China
Shie-Ming Peng and Gene-Hsiang Lee
Department of Chemistry, National Taiwan University, Taipei 10764, Taiwan, Republic of China
ReceiVed October 18, 1995X
The reactions of [Et4N]3[Sb{Fe(CO)4}4] (1) with RX (R)Me, Et, n-Pr; X)I) in MeCN form the monoalkylated antimony complexes [Et4N]2[RSb{Fe(CO)4}3] (R)Me, 2; R)Et, 4; R)n-Pr, 6) and the dialkylated antimony clusters [Et4N][R2Sb{Fe(CO)4}2] (R) Me, 3; R ) Et, 5; R )n-Pr, 7), respectively. When [Et4N]3 [Sb{Fe-(CO)4}4] reacts with i-PrI, only the monoalkylated antimony complex [Et4N]2[i-PrSb{Fe(CO)4}3] (8) is obtained.
The mixed dialkylantimony complex [Et4N][MeEtSb{Fe(CO)4}2] (9) also can be synthesized from the reaction
of 2 with EtI. While the reaction with Br(CH2)2Br produces [Et4N]2[BrSb{Fe(CO)4}3] (10), treatment with
Cl-(CH2)3Br forms the monoalkylated product [Et4N]2[Cl(CH2)3Sb{Fe(CO)4}3] (11) and a dialkylated novel antimony -iron complex [Et4N][{µ-(CH2)3}Sb{Fe(CO)4}3] (12). On the other hand, the reaction with Br(CH2)4Br forms the
monoalkylated antimony product and the dialkylated antimony complex [Et4N][{µ-(CH2)4}Sb{Fe(CO)4}2] (13).
Complexes 2-13 are characterized by spectroscopic methods or/and X-ray analyses. On the basis of these analyses, the core of the monoalkyl clusters consists of a central antimony atom tetrahedrally bonded to one alkyl group and three Fe(CO)4fragments and the dialkyl products are structurally similar to the monoalkyl clusters, with the
central antimony bonded to two alkyl groups and two Fe(CO)4moieties in each case. The dialkyl complex 3
crystallizes in the monoclinic space group P21/c with a)13.014(8) Å, b)11.527(8) Å, c)17.085(5) Å,β) 105.04(3)°, V)2475(2) Å
3, and Z
)4. Crystals of 12 are orthorhombic, of space group Pbca, with a) 14.791-(4) Å, b)15.555(4) Å, c)27.118(8) Å, V)6239(3) Å3, and Z)8. The anion of cluster 12 exhibits a central antimony atom bonded to three Fe(CO)4fragments with a-(CH2)3-group bridging between the Sb atom and one Fe(CO)4fragment. This paper discusses the details of the reactions of [Et4N]3[Sb{Fe(CO)4}4] with a series
of alkyl halides and dihalides. These reactions basically proceed via a novel double-alkylation pathway, and this facile methodology can as well provide a convenient route to a series of alkylated antimony-iron carbonyl clusters.
Introduction
Main group-transition metal carbonyl clusters attract exten-sive attention because of their interesting bonding modes and reactivity patterns.1 The role of the main group elements has
been increasingly probed because of their significant influence on the reactivity of the mixed main group-transition metal clusters. Regarding the effects of main group elements in the catalysts,2it is of great importance to understand how the main
group element affects the interaction of main group-transition metal clusters with organic substrates.
The alkylation at the main group centers has been observed for group-16-element-containing complexes such as [S2Fe2
-(CO)6]2 -,3 [S 2Fe2(NO)4]2 -,4 [Te 6Fe8(CO)24]2 -,5 and [Se 6Fe6
-(CO)12]2-.6 The recent study of the reactivity of the
group-15-element-containing cluster [Et4N]3[Bi{Fe(CO)4}4] toward a
series of alkyl halides shows that bismuth alkyl products of the type [RBi{Fe(CO)4}3]2
-are isolated from the reactions.7 These
alkylations clearly demonstrate that the main group elements are active toward the incoming organo ligands. Nevertheless, the effects of the main group elements of the mixed main group-transition metal carbonyl clusters on the alkylation reactions have been little explored. To study this aspect, we investigate the alkylation reactions of the lighter analog [Et4N]3
-[Sb{Fe(CO)4}4] (1)8with a series of alkyl halides and dihalides.
* To whom all correspondence should be addressed.
XAbstract published in AdVance ACS Abstracts, August 1, 1996. (1) For recent reviews of transition-metal/main-group compounds, see:
(a) Herrmann, W. A. Angew. Chem., Int. Ed. Engl. 1986, 25, 56. (b) Vaira, M. D.; Stoppioni, P.; Peruzzini, M. Polyhedron 1987, 6, 351. (c) Scherer, O. J. Comments Inorg. Chem. 1987, 6, 1. (d) Scherer, O. J. Angew. Chem., Int. Ed. Engl. 1985, 24, 924. (e) Whitmire, K. H. J. Coord. Chem. 1988, 17, 95. (f) Huttner, G.; Knoll, K. Angew. Chem., Int. Ed. Ed. Engl. 1987, 26, 743. (g) Huttner, G.; Evertz, K. Acc. Chem. Res. 1986, 19, 406. (h) Vahrenkamp, H. AdV. Organomet. Chem. 1983, 22, 169. (i) Scherer, O. J. Angew. Chem. 1990, 102, 1137. (j) Cowley, A. H. J. Organomet. Chem. 1990, 400, 71. (k) Dimaio, A.-J.; Rheingold, A. L. Chem. ReV. 1990, 90, 169. (l) Whitmire, K. H. J. Cluster Sci. 1991, 2, 231. (m) Shriver, D. F.; Kasez, H. D.; Adams, R. D. The Chemistry of Metal Cluster Complexes; VCH Publishers: New York, 1990.
(2) (a) Grasselli, R. K.; Burrington, J. D. AdV. Catal. 1981, 30, 133. (b) Grasselli, R. K. J. Chem. Educ. 1986, 63, 216. (c) Sposito, G. The Surface Chemistry of Soil; Oxford University Press: New York, 1984.
(3) (a) Seyferth, D.; Henderson, R. S. J. Am. Chem. Soc. 1979, 101, 508. (b) Seyferth, D.; Henderson, R. S.; Song, L.-C. Organometallics 1982, 1, 125.
(4) (a) Seyferth, D.; Gallagher, M. K.; Cowie, M. Organometallics 1986, 5, 539. (b) Seyferth, D.; Gallagher, M. K. J. Organomet. Chem. 1981, 218, C5.
(5) (a) Shieh, M.; Shieh, M.-H. Organometallics 1994, 13, 920. (b) Shieh, M.; Chen, P.-F.; Tsai, Y.-C.; Shieh, M.-H.; Peng, S.-M.; Lee, G.-H. Inorg. Chem. 1995, 34, 2251.
(6) Shieh, M.; Shieh, M.-H. Unpublished results.
(7) (a) Shieh, M.; Liou, Y.; Peng, S.-M.; Lee, G.-H. Inorg. Chem. 1993, 32, 2212. (b) Shieh, M.; Liou, Y.; Jeng, B.-W. Organometallics 1993, 12, 4926.
(8) Luo, S.; Whitmire, K. H. Inorg. Chem. 1989, 28, 1424. S0020-1669(95)01339-5 CCC: $12.00 © 1996 American Chemical Society
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
The reactions of 1 with alkyl halides mainly proceed through a double-alkylation mechanism to form the alkylated antimony complexes of the type [Et4N]2[RSb{Fe(CO)4}3] and the
dialky-lated antimony complexes [Et4N][R2Sb{Fe(CO)4}2]. In
addi-tion, a novel dialkylated antimony-iron complex [Et4N][{ µ-(CH2)3}Sb{Fe(CO)4}3] (12) is obtained from the reaction with
Cl(CH2)3Br.
Experimental Section
All reactions were performed under an atmosphere of pure nitrogen using standard Schlenk techniques.9 Solvents were purified, dried, and distilled under nitrogen prior to use. The compound [Et4N]3[Sb{ Fe-(CO)4}4] (1) was prepared according to the published method.8 Infrared spectra were recorded on a JASCO 5300 IR spectrometer for solutions in CaF2 cells. ESI mass spectra were obtained on a Fision (VG platform) mass spectrometer. 1H NMR spectra were taken on a JEOL 400 (400 MHz) instrument. Elemental analyses were performed on a Perkin-Elmer 2400 analyzer at the NSC Regional Instrumental Center at National Taiwan University, Taipei, Taiwan.
Reaction of 1 with MeI. To a solution of 1.60 g (1.35 mmol) of 1 in 20 mL of MeCN was added 1.80 mL (28.9 mmol) of MeI. The resulting solution was stirred in an ice/water bath for 20 min to give a reddish-brown solution. The latter was filtered, and the solvent was removed under vacuum. The residue was washed with hexane and ether and then extracted with THF. The product in the THF extract was recrystallized with CH2Cl2/MeOH to give 1.00 g (1.11 mmol) of [Et4N]2[MeSb{Fe(CO)4}3] (2) (82% based on Sb). IR (νCO, CH2Cl2): 2017 w, 1986 s, 1901 vs, br cm-1. 1H NMR (400 MHz, DMSO-d6,
298 K): δ 1.71 (s) (chemical shifts not given for [Et4N]+). Negative
ion m/z (FABMS): 640. Anal. Calcd for [Et4N]2[MeSb{Fe(CO)4}3]: C, 38.66; H, 4.81; N, 3.11. Found: C, 37.10; H, 4.54; N, 2.95.
In a similar procedure, 0.50 g (0.42 mmol) of 1 and 0.56 mL (9.0 mmol) of MeI in 20 mL of MeCN were stirred at room temperature for 4.5 h to give a brown solution. The solution was filtered and solvent was removed under vacuum. The residue was washed with hexanes and extracted with ether to give 0.15 g (0.24 mmol) of [Et4N][Me2-Sb{Fe(CO)4}2] (3) (57% based on Sb). Crystals suitable for X-ray analysis were grown from ether solution. IR (νCO, ether): 2027 w, 2004 s, 1915 vs, br cm-1. 1H NMR (400 MHz, DMSO-d6, 298 K):
δ
1.36 (s) (chemical shifts not given for [Et4N]+). Negative ion m/z
(ESIMS): 487. Anal. Calcd for [Et4N][Me2Sb{Fe(CO)4}2]: C, 34.99; H, 4.24; N, 2.27. Found: C, 35.02; H, 4.21; N, 2.31.
In a similar reaction, a mixture of 1.00 g (0.84 mmol) of 1 with 1.12 mL (18.0 mmol) of MeI in 20 mL of MeCN stirred at room temperature for 10 min gave 0.15 g (0.24 mmol) of 3 (29% based on Sb) and 0.15 g (0.17 mmol) of 2 (20% based on Sb).
Reaction of 1 with EtI. To a solution of 0.80 g (0.68 mmol) of 1 in 20 mL of MeCN was added 0.64 mL (7.92 mmol) of EtI. The resulting solution was stirred at room temperature for 6 days to give a reddish-brown solution. The latter was filtered, and the solvent was removed under vacuum. The residue was washed with hexane and extracted with ether to give 0.14 g (0.22 mmol) of [Et4N][Et2Sb{Fe-(CO)4}2] (5) (32% based on Sb). IR (νCO, ether): 2025 w, 2002 s, 1925 vs, 1911 vs cm-1. 1H NMR (400 MHz, DMSO-d6, 298 K):
δ
1.89 (q, 4H, J)7.9 Hz), 1.28 (t, 6H, J)7.9 Hz) (chemical shifts not given for [Et4N]+). Anal. Calcd for [Et4N][Et2Sb{Fe(CO)4}2]: C,
37.19; H, 4.69; N, 2.17. Found: C, 36.80; H, 4.65; N, 2.19. The residue was then extracted with THF, and product in the THF extract was recrystallized with THF/ether/hexane and then with CH2Cl2/MeOH to give 0.35 g (0.38 mmol) of [Et4N]2[EtSb{Fe(CO)4}3] (4) (56% based on Sb). IR (νCO, MeCN): 2017 w, 1984 s, 1898 vs cm-1. 1H NMR
(400 MHz, DMSO-d6, 298 K): δ 2.32 (q, 2H, J)7.3 Hz), 1.42 (t, 3H, J)7.3 Hz) (chemical shifts not given for [Et4N]
+). Anal. Calcd
for [Et4N]2[EtSb{Fe(CO)4}3]: C, 39.38; H, 4.96; N, 3.06. Found: C, 39.13; H, 4.93; N, 3.04.
Reaction of 1 with n-PrI. To a solution of 0.80 g (0.68 mmol) of 1 in 25 mL of MeCN was added 0.80 mL (8.0 mmol) of n-PrI. The mixed solution was stirred at room temperature for 36 h to give a
reddish-brown solution, which was then filtered. The solvent was removed under vacuum, and Fe(CO)5was collected and detected by IR. The residue was washed with hexane and ether and extracted with CH2Cl2. The product in the CH2Cl2extract was recrystallized with CH2Cl2/MeOH to give 0.57 g (0.61 mmol) of [Et4N]2[n-PrSb{ Fe-(CO)4}3] (6) (90% based on Sb). IR (νCO, MeCN): 2015 w, 1984 s, 1898 vs, br cm-1. 1H NMR (400 MHz, DMSO-d6, 298 K):
δ 2.31 (t,
2H, J)8.5 Hz), 1.84 (m, 2H), 0.91 (t, 3H, J)7.3 Hz) (chemical shifts not given for [Et4N]+). Negative ion m/z (FABMS): 668. Anal.
Calcd for [Et4N]2[n-PrSb{Fe(CO)4}3]: C, 40.06; H, 5.10; N, 3.02. Found: C, 38.30; H, 4.95; N, 2.99.
In a similar procedure, the reaction mixture was refluxed overnight and the residue was extracted into ether. IR (νCO, ether): 2025 w, 2002 s, 1921 vs, br cm-1
. The IR spectrum of the ether extract showed it to contain [Et4N][(n-Pr)2Sb{Fe(CO)4}2] (7). However, this product formed an oil and further characterization was difficult.
Reaction of 1 with i-PrI. To a solution of 0.84 g (0.71 mmol) of 1 in 20 mL of MeCN was added 1.00 mL (10.0 mmol) of i-PrI. The mixed solution was stirred at room temperature for 22 h to give a reddish-brown solution, which was then filtered, and the solvent was removed under vacuum. The residue was washed with hexane and ether and extracted with THF. The product in the THF extract was recrystallized with hexane/ether/THF and then with CH2Cl2/MeOH to give 0.35 g (0.38 mmol) of [Et4N]2[i-PrSb{Fe(CO)4}3] (8) (54% based on Sb). IR (νCO, MeCN): 2027 w, 1994 s, 1911 s cm-1. 1H NMR
(400 MHz, DMSO-d6, 298 K): δ 2.80 (m, 1H), 1.51 (d, 6H, J)6.8 Hz) (chemical shifts not given for [Et4N]+). Anal. Calcd for
[Et4N]2-[i-PrSb{Fe(CO)4}3]: C, 40.06; H, 5.10; N, 3.02. Found: C, 39.19; H, 4.80; N, 2.87.
Reaction of 2 with EtI. To a solution of 0.91 g (1.0 mmol) of 2 in 20 mL of MeCN was added 0.87 mL (11 mmol) of EtI. The mixed solution was stirred and heated at 45°C for 54 h to give a reddish-brown solution, which was then filtered, and the solvent was removed under vacuum. The residue was washed with hexane and extracted with ether. The ether extract was cooled at-20°C to give 0.52 g (0.82 mmol) of [Et4N][MeEtSb{Fe(CO)4}2] (9) (81% based on Sb). IR (νCO, MeCN): 2027 w, 2004 s, 1913 vs, br cm-1
. 1H NMR (400 MHz, DMSO-d6, 298 K): δ 1.89 (q, 2H, J)6.8 Hz), 1.29 (m, 6H) (chemical shifts not given for [Et4N]+
). Anal. Calcd for [Et4N]-[MeEtSb{Fe(CO)4}2]: C, 36.12; H, 4.47; N, 2.22. Found: C, 36.21; H, 4.34; N, 2.13.
Reaction of 1 with Br(CH2)2Br. To a solution of 0.50 g (0.42 mmol) of 1 in 20 mL of MeCN was added 0.11 mL (1.3 mmol) of Br(CH2)2Br. The mixed solution was stirred at room temperature for 30 h to give a reddish-brown solution, which was then filtered, and the solvent was removed under vacuum. The residue was washed with hexane and ether and extracted with THF. The product in the THF extract was recrystallized with hexane/ether/THF and then with MeOH at-20°C to give 0.31 g (0.32 mmol) of [Et4N]2[BrSb{Fe(CO)4}3] (10) (76% based on Sb). IR (νCO, DMSO-d6): 1990 s, 1913 vs cm-1.
1H NMR (400 MHz, DMSO-d6, 298 K): δ 3.19 (q, 16 H, J
)7.3 Hz), 1.15 (t, 24H, J)7.3 Hz). Anal. Calcd for [Et4N]2[BrSb{Fe(CO)4}3]:
C, 34.82; H, 4.17; N, 2.90. Found: C, 35.05; H, 4.13; N, 2.90. Reaction of 1 with Cl(CH2)3Br. To a solution of 1.0 g (0.84 mmol) of 1 in 20 mL of MeCN was added 0.25 mL (2.53 mmol) of Cl(CH2)3-Br. The resulting solution was stirred and heated at 45°C for 42 h to give a reddish-brown solution. The latter was filtered, and the solvent was removed under vacuum. The residue was washed with hexane and extracted with ether to give 0.06 g (0.08 mmol) of [Et4N][{
µ-(CH2)3}Sb{Fe(CO)4}3] (12) (10% based on Sb). The crystals of 12 suitable for X-ray analysis were grown from ether solution. IR (νCO, MeCN): 2087 w, 2019 s, 2004 sh, 1915 s cm-1
. 1H NMR (400 MHz, CD2Cl2, 298 K): δ 1.99 (2H, t, J)5.8 Hz), 2.68 (4H, m) (chemical shifts not given for [Et4N]+). Anal. Calcd for [Et4N][{
µ-(CH
2)3}-Sb{Fe(CO)4}3]: C, 34.63; H, 3.29; N, 1.76. Found: C, 34.42; H, 3.32; N, 1.79. The residue was then extracted with THF, and the THF extract was recrystallized with THF/ether/hexane and then with ether/MeOH to give 0.63 g (0.65 mmol) of [Et4N]2[Cl(CH2)3Sb{Fe(CO)4}3] (11) (78% based on Sb). IR (νCO, MeCN): 2017 w, 1986 s, 1901 vs cm-1.
1H NMR (400 MHz, DMSO-d6, 298 K): δ 3.55 (t, 2H, J
)6.4 Hz), 2.29 (m, 4H) (chemical shifts not given for [Et4N]+). Anal. Calcd for
[Et4N]2[Cl(CH2)3Sb{Fe(CO)4}3]: C, 38.65; H, 4.81; N, 2.91. Found: C, 36.69; H, 4.71; N, 2.92.
(9) Shriver, D. F.; Drezdon, M. A. The Manipulation of Air SensitiVe Compounds; Wiley: New York, 1986.
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
Reaction of 1 with Br(CH2)4Br. To a solution of 1.00 g (0.84 mmol) of 1 in 20 mL of MeCN was added 0.30 mL (2.5 mmol) of Br(CH2)4Br. The resulting solution was stirred and heated at 55°C for 70 h to give a reddish-brown solution. The latter was filtered, and the solvent was removed under vacuum. The residue was washed with hexane and extracted with ether to give 0.11 g (0.17 mmol) of [Et4N]-[{µ-(CH2)4}Sb{Fe(CO)4}2] (13) (20% based on Sb). IR (νCO, CH2-Cl2): 2025 w, 2002 s, 1913 s, br cm-1. 1H NMR (400 MHz,
DMSO-d6, 298 K): δ 1.81 (4H, m), 2.15 (4H, t, J)5.4 Hz) (chemical shifts not given for [Et4N]+). Anal. Calcd for [Et4N][{µ-(CH2)4}
Sb-{Fe(CO)4}2]: C, 37.31; H, 4.38; N, 2.18. Found: C, 37.34; H, 4.42; N, 2.08. The residue was then extracted with THF to give about 0.50 g (0.49 mmol) of [Et4N]2[Br(CH2)4Sb{Fe(CO)4}3] (58% based on Sb). IR (νCO, THF): 1990 s, 1913 s, br cm-1
. This compound formed an oil, and further characterization was not carried out.
X-ray Structural Characterization of Complexes 3 and 12. A summary of selected crystallographic data for 3 and 12 is given in Table 1. Data collection was carried out on a Nonius CAD-4 diffractometer using graphite-monochromated Mo KRradiation at 25 °C. All crystals were mounted on glass fibers with epoxy cement. Data reduction and structural refinement were performed using the NRCC-SDP-VAX packages,10and atomic scattering factors were taken from ref 11.
Structures of 3 and 12. The deep brown crystal of 3 chosen for diffraction measurements was ca. 0.20× 0.50 × 0.50 mm, and the deep brown crystal of 12 had dimensions 0.20× 0.40 × 0.50 mm. Cell parameters were obtained from 25 reflections with 2θ in the ranges
16.12-28.46° for 3 and 15.46-23.00° for 12. A total of 3286 reflections with I>2.0σ(I) for 3 (1626 reflections with I>2.0σ(I) for 12) were used in the refinement. The structures were solved by the heavy-atom method and refined by least-squares cycles. All the non-hydrogen atoms were refined with anisotropic temperature factors. Full-matrix least-squares refinement led to convergence with R)4.4% and Rw)4.5% for 3 and with R)5.5% and Rw)5.8% for 12.
The selected distances and angles of 3 and 12 are given in Tables 2 and 3, respectively. Additional crystallographic data for 3 and 12 and the crystallographic data for 2, 9, 10, and 13 are available as supporting information.
Results
Reactions with MeI, EtI, n-PrI, and i-PrI. The alkylation
products [Et4N]2[MeSb{Fe(CO)4}3] (2) and [Et4N][Me2Sb{
Fe-(CO)4}2] (3) are produced in almost equal amounts from the
reaction of [Et4N]3[Sb{Fe(CO)4}4] (1) with an excess of MeI
in MeCN at room temperature for 10 min. Under milder
conditions, the monoalkyl derivative 2 is produced as the only product from the reaction with MeI. When monitored by infrared spectroscopy, the reaction proceeds to form cluster 2 first, and continued stirring gives the dialkyl complex 3. It clearly indicates that the dialkyl cluster 3 results from the further alkylation of the alkyl complex 2. In support, we found that 2 reacts readily with MeI to form 3.
As the reactivity of the reagent decreases, the reaction proceeds in a similar manner but under an elevated temperature. The reaction with EtI forms the monoalkylated complex [Et4N]2
-[EtSb{Fe(CO)4}3] (4) and the dialkylated product [Et4N][Et2
-Sb{Fe(CO)4}2] (5). Similarly, the treatment with n-PrI forms
[Et4N]2[n-Prb{Fe(CO)4}3] (6) and an undentified species
pro-posed to be [Et4N][(n-Pr)2Sb{Fe(CO)4}2] (7) on the basis of its
IR spectrum. As expected, only the monoalkylated cluster [Et4N]2[i-Prb{Fe(CO)4}3] (8) is produced in the reaction with
i-PrI and no dialkylated product is observed because of the steric effect.
Synthesis of the Mixed Dialkylantimony Cluster.
Note-worthy is that different alkyl groups can be introduced into 1. The synthesis of dialkyl complexes of the type [RR'Sb{ Fe-(CO)4}2]
-can be accomplished under suitable conditions. We have successfully isolated a mixed dialkylantimony complex [Et4N][MeEtSb{Fe(CO)4}2] (9) by treatment of the
methylan-timony cluster 2 with EtI. However, when the ethylanmethylan-timony cluster 4 reacted with MeI, a mixture of 9 and the oxidized products was obtained. It is believed that MeI acts not only as an alkylation agent but also as an oxidizing agent in the latter case due to the reactive nature of MeI and the steric hindrance of the Et group in cluster 4. The less steric effect of the methyl group in complex 2 compared to the incoming agent EtI favors the formation of cluster 9. Other mixed dialkylated antimony clusters also can be designed and synthesized along these lines.
Reactions with Br(CH2)2Br, Cl(CH2)3Br, and Br(CH2)4Br. The extended reactions with dihalides were also investigated. The reaction with Br(CH2)2Br forms a bromoantimony complex
[Et4N]2[BrSb{Fe(CO)4}3] (10). Notably, the treatment with
Cl-(CH2)3Br forms the monoalkylated product [Et4N][Cl(CH2)3
-Sb{Fe(CO)4}3] (11) and a novel antimony-iron dialkylated complex [Et4N][{µ-(CH2)3}Sb{Fe(CO)4}3] (12). The formation
of 12 indicates that the alkylation of 1 with Cl(CH2)3Br occurs
at both the central antimony and one iron center. On the other hand, the reaction with Br(CH2)4Br forms the monoalkylated
antimony product and the dialkylated antimony complex [Et4
N]-[{µ-(CH2)4}Sb{Fe(CO)4}2] (13). Cluster 13 results from the
double alkylation at the central antimony.
The reactions of 1 with alkyl halides and dihalides are summarized in Scheme 1.
(10) Gabe, E. J.; Lepage, Y.; Charland, J. P.; Lee, F. L.; White, P. S. J. Appl. Crystallogr. 1989, 22, 384.
(11) International Tables for X-ray Crystallography; Kynoch press: Bir-mingham, England, 1974; Vol. IV.
Table 1. Crystallographic Data for [Et4N][Me2Sb{Fe(CO))4}2] (3) and [Et4N][{µ-(CH2)3}Sb{Fe(CO)4}3] (12)
3 12
empirical formula SbFe2C18H26O8N SbFe3C23H26O12N
fw 617.85 797.74
crystal system monoclinic orthorhombic
space group P21/c Pbca
a, Å 13.014(8) 14.791(4) b, Å 11.527(8) 15.555(4) c, Å 17.085(5) 27.118(8) β, deg 105.04(3) V, Å3 2475(2) 6239(3) Z 4 8 D(calc), Mg m-3 1.658 1.699 abs coeff, cm-1 22.86 22.848
diffractometer Nonius (CAD-4) Nonius (CAD-4) radiation,λ(Mo KR), Å 0.7107 0.7107
temp,°C 25 25
Tmin/Tmax 0.71/1.00 0.77/1.00
residuals: R, Rwa 0.044, 0.045 0.055, 0.058 aThe functions minimized during least squares cycles were R
) ∑|Fo-Fc|/∑Foand Rw)[∑w(Fo-Fc)
2/∑w(Fo)2]1/2.
Table 2. Selected Bond Distances (Å) and Bond Angles (deg) for [Et4N][Me2Sb{Fe(CO)4}2] (3)
(A) Distances
Sb-Fe(1) 2.522(2) Sb-Fe(2) 2.538(1)
Sb-C(9) 2.150(7) Sb-C(10) 2.142(6)
(B) Angles
Fe(1)-Sb-Fe(2) 123.95(4) Fe(1)-Sb-C(9) 106.0(2) Fe(2)-Sb-C(9) 110.1(2) Fe(2)-Sb-C(10) 107.1(2)
Table 3. Selected Bond Distances (Å) and Bond Angles (deg) for [Et4N][{µ-(CH2)3}Sb{Fe(CO)4}3] (12)
(A) Distances
Sb-Fe(1) 2.671(3) Sb-Fe(2) 2.585(3)
Sb-Fe(3) 2.595(4) Sb-C(13) 2.12(2)
(B) Angles
Fe(1)-Sb-Fe(2) 114.8(1) Fe(1)-Sb-Fe(3) 115.2(1) Fe(1)-Sb-C(13) 93.5(8) Fe(2)-Sb-Fe(3) 115.0(1) Fe(2)-Sb-C(13) 109.8(6) Fe(3)-Sb-C(13) 105.7(7)
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
Structures of 2-13. Clusters 2-13 are characterized by spectroscopic methods and/or X-ray analysis. On the basis of the spectroscopic data and X-ray analyses, the dianions of the monoalkylated clusters 2, 4, 6, 8, and 11 are isostructural species and each bears a central antimony tetrahedrally coordinated to one alkyl group and three Fe(CO)4 groups; the dialkylated
clusters 3 (Figure 1), 5, 7, and 9 are structurally similar to the monoalkylated cluster except that one Fe(CO)4 fragment is
further replaced by another alkyl group in each case. The dianion of cluster 10 is isostructural with that of cluster 2 with the central antimony bonded to one Br and three Fe(CO)4
groups. Cluster 13 is a dialkylated product in which the antimony atom and the-(CH2)4-fragment form a cyclic ring with the Sb atom bonded to two Fe(CO)4moieties. The metal
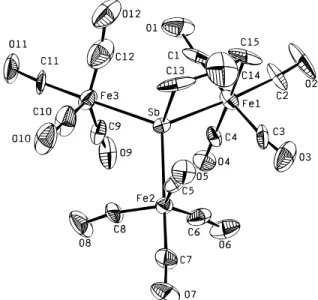
core of the novel dialkylated cluster 12 consists of the central antimony and one Fe(CO)4bridged by a-(CH2)3-group, and the coordination of the Sb atom is completed by bonding to the other two Fe(CO)4fragments (Figure 2).
Discussion
Alkylations of 1 with Alkyl Halides. Our results show that
the reactions of 1 with alkyl halides basically undergo a two-step alkylation to give the monoalkylated products of the type [Et4N]2[RSb{Fe(CO)4}3] and the dialkylated antimony clusters
[Et4N][R2Sb{Fe(CO)4}2]. The alkylation of 1 can be considered
to occur via substitution of the Fe(CO)4 moiety with the
incoming alkyl group in two successive processes. This
reactivity pattern represents an unprecedented reaction type in the heavier group-15-element-containing metal carbonyl chemistry and can be compared to that found for the bismuth
system. In the bismuth case, only the monoalkylated products [RBi{Fe(CO)4}3]2-were isolated in the analogous alkylations
and no dialkylated bismuth complexes were observed.7 The
greater thermal stability for the dialkylantimony clusters of the type [R2Sb{Fe(CO)4}2]
-may be ascribed to the increased basicity of the antimony and the stronger antimony-carbon bond.
Alkylations of 1 with Dihalides. Some unexpected
out-comes were found in the reactions with dihalides. Instead of the alkylated products, the reaction with Br(CH2)2Br produces
the bromoantimony [Et4N]2[BrSb{Fe(CO)4}3] (10) probably due
to the reactive nature of Br(CH2)2Br. Cluster 10 may be
considered as a result of substitution of Fe(CO)4by Br. In the
analogous bismuth system, the oxidized product is found in the reaction with Br(CH2)2Br.7b Cluster 10 decomposes slowly in
the solution to form the known cluster [Sb2Fe6(CO)20]2
-,12
owing to the weak bond between Sb and Br.
Of particular interest, the treatment with Cl(CH2)3Br forms
the monoalkylated product 11 and a novel dialkylated complex [Et4N][{µ-(CH2)3}Sb{Fe(CO)4}3] (12), in which the-(CH2)3 -group bridges between the Sb and Fe atoms, as a result of dialkylation at the central Sb and one Fe atom. The absence of the dialkylated antimony product containing an SbC3cyclic
ring may be attributed to the high strained energy of the four-membered ring. It is curious to know if cluster 12 arises from the further alkylation of 11. However, refluxing cluster 11, premade, in acetonitrile does not form the novel cluster 12,
which rules out the formation of cluster 12 from the alkylation of 11 at the iron center. Upon alkylation with Cl(CH2)3Cl, only
the monoalkylated complex 11 was isolated with no observation of cluster 12, and the reaction with Br(CH2)3Br leads to the
(12) Luo, S.; Whitmire, K. H. J. Organomet. Chem. 1989, 376, 297. Figure 1. ORTEP diagram showing the structure and atom labeling
for the anion of 3.
Scheme 1
Figure 2. ORTEP diagram showing the structure and atom labeling for the anion of 12.
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
formation of the sole product 10. The results indicate that the reactivity of dihalides plays an important role in the formation of 12. However, the detailed mechanism is not very clear, and further investigation may be worthwhile.
To compare the stability of the five-membered ring SbC4and
the six-membered ring SbFeC4, we conducted the reaction with
Br(CH2)4Br in refluxing MeCN. The reaction only yields the
monoalkylated antimony product and the dialkylated antimony complex [Et4N][{µ-(CH2)4}Sb{Fe(CO)4}2] (13), suggesting the
better stability of the SbC4ring than of the SbFeC4ring under
this condition.
Structures of Complexes 2, 3, 9 10, 12, and 13. The
antimony atoms in the clusters 2, 3, 9, 10, 12, and 13 are all in distorted tetrahedral environments. For comparison, the average Fe-Sb-Fe angle, the Sb-Fe distance, and the Sb-C length in these tetrahedral clusters and the related cluster 1 are listed in Table 4. The Sb-C distances in 2 (2.16 Å), 3 (2.15 Å, average), 12 (2.12 Å), and 13 (2.16 Å, average) are close to the sum of single-bond covalent radii (2.2 Å),13and the longer
Sb-C(9) length (2.34 Å) for 9 is probably due to the repulsion between the Me and Et groups. As shown, the average Fe -Sb-Fe angle becomes larger and the Sb-Fe distance becomes
shorter as the number of the alkyl groups increases (i.e., the number of the Fe(CO)4groups decreases) attributable to the
steric hindrance of the Fe(CO)4 fragments. The Sb-Fe distances in these clusters fall in the range of other known Sb -Fe(CO)4distances (2.460-2.715 Å)8,14,15and are compared to those found in other structurally characterized antimony com-plexes such as [ClSb{Fe(CO)2Cp}3]22+
(2.539 Å, average)16and
[Cl2Sb{Fe(CO)2Cp}2]+(2.440 Å, average).17
The dianion of complex 10 is isostructural with the previously reported chloroantimony clusters [ClE{Fe(CO)4}3]2-(E
)Sb, Bi).8,18 The bond distance of Sb(a)
-Br(a) is 2.606 (3) Å, which is normal.13 The average Fe
-Sb(a)-Fe angle in 10 (115.07°) is a bit smaller than that in the analogous complex [ClSb-{Fe(CO)4}3]2
-(115.35°)8due to the larger size of Br. Cluster
12 represents an unprecedented bonding mode in the mixed
group 15 element-iron carbonyl clusters. In 12, the Sb and Fe atoms are linked by a -(CH2)3- group to form a five-membered SbFeC3ring. The average Fe-Sb-Fe angle in 12 is close to that of 2, indicative of the little influence of the ring on the Fe-Sb-Fe tetrahedral angles. While the Sb-Fe distance in 2 is about the same, in 12 the Sb-Fe(1) bond distance, the one involving the alkyl bridge, is longer than the other Sb-Fe bonds because of the effect of the ring. The C(13)-Sb-Fe(1) angle is 93.5 (8)°, which deviates greatly from the tetrahedral angle to fit the requirement of formation of the SbFeC3ring.
Acknowledgment. We thank the National Science Council
of the Republic of China for financial support (Grant No. NSC 85-2113-M-003-003).
Supporting Information Available: Listings of complete crystal-lographic data, atomic positional parameters, bond distances and angles, and anisotropic thermal parameters for 2, 3, 9, 10, 12, and 13 (39 pages). Ordering information is given on any current masthead page. IC9513393
(13) Huheey, J. E.; Keiter, E. A.; Keiter, R. L. Inorganic Chemistry: Principles of Structure and ReactiVity; Harper Collins College Publishers: New York, 1993.
(14) (a) Cowley, A. H.; Norman, N. C.; Pakulski, M.; Bricker, D. L.; Russell, D. H. J. Am. Chem. Soc. 1985, 107, 8211. (b) Arif, A. M.; Cowley, A. H.; Pakulski, M. J. Chem. Soc., Chem. Commun. 1987, 622.
(15) (a) Luo, S.; Whitmire, K. H. J. Organomet. Chem. 1989, 376, 297. (b) Whitmire, K. H.; Leigh, J. S.; Luo, S.; Shieh, M.; Fabiano, M. D. New J. Chem. 1988, 12, 397. (c) Rheingold, A. L.; Gieb, S. J.; Shieh, M.; Whitmire, K. H. Inorg. Chem. 1987, 26, 463.
(16) Trinh-Toan; Dahl, L. F. J. Am. Chem. Soc. 1971, 93, 2654. (17) Einstein, F. W. B.; Jones, R. D. G. Inorg. Chem. 1973, 12, 1690. (18) Ferrer, M.; Rossell, O.; Seco, M.; Braunstein, P. J. Organomet. Chem.
1989, 364, C5. Table 4. Comparison of the Average Fe-Sb-Fe Angles and the
Average Sb-Fe and Sb-C Distances in Related Clusters
complex Fe-Sb-Fe, deg Sb-Fe, Å Sb-C, Å [Sb{Fe(CO)4}4]3 -(1)8 109.56 2.666 [Et4N]2[MeSb{Fe(CO)4}3] (2)a 114.43 2.614 2.160(7) [Et4N][Me2Sb{Fe(CO)4}2] (3)a 123.95 2.53 2.146 [Et4N][MeEtSb{Fe(CO)4}2] (9)a 120.8 2.545 2.22 [Et4N][{µ-(CH2)3}Sb{Fe(CO)4}3] (12)a 115.0 2.617 2.12(2) [Et4N][{µ-(CH2)4}Sb{Fe(CO)4}2] (13)a 120.08 2.542 2.160
aThis work; the detailed crystallographic data for 2, 9, and 13 are
provided in the Supporting Information.
Downloaded by NATIONAL TAIWAN UNIV on August 14, 2009
![Table 2. Selected Bond Distances (Å) and Bond Angles (deg) for [Et 4 N][Me 2 Sb { Fe(CO) 4 } 2 ] (3)](https://thumb-ap.123doks.com/thumbv2/9libinfo/8671004.196009/3.918.83.439.88.347/table-selected-bond-distances-å-bond-angles-deg.webp)