Title: Icodextrin Decreases Technique Failure and Improves Patient Survival in Peritoneal Dialysis Patients
Short title: Icodextrin in PD Patients
I-Kuan Wang1,2,3, MD; Yu-Fen Li 4, PhD; Jin-Hua Chen5, PhD; Chih-Chia Liang2,MD; Yao-Lung Liu, MD; Hsin-Hung Lin2, MD; Chiz-Tzung Chang2, PhD; Wen-Chen Tsai6, MD; Tzung-Hai Yen7,8, PhD; Chiu-Ching Huang2, MD.
1 Graduate Institute of Clinical Medical Science, College of Medicine, China Medical University, Taichung, Taiwan
2 Divisions of Nephrology, China Medical University Hospital, Taichung, Taiwan 3 Department of Internal Medicine, College of Medicine, China Medical University, Taichung, Taiwan
4Biostatistics center and School of Public Health, China Medical University, Taichung, Taiwan
5Biostatistics Center and School of Public Health, Taipei Medical University, Taipei, Taiwan
6Department of Health Services Administration, China Medical University, Taichung, Taiwan
7Division of Nephrology, Chang Gung Memorial Hospital, Taipei, Taiwan. 8Chang Gung University College of Medicine, Taoyuan, Taiwan.
Correspondence: Chiu-Ching Huang, MD, Professor
China Medical University Hospital, 2 Yue-Der Road, Taichung 404, Taiwan Tel: 886-4-22052121 ext. 7387; Fax: 886-4-22032798
E-mail: cch@mail.cmuh.org.tw
Word count: 235 words in the abstract, 3654 words in the text, 38 references, 3 tables, 2 figures, 2 supplement tables.
Funding: This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002), The Bureau of Health Promotion (DOH97-HP-1103, and DOH99-HP-1109), and China Medical University (DMR-101-016, DMR-101-017, and DMR-103-013). Financial Disclosure: No financial conflict of interest was declared.
Abstract
Aim: It remains unclear whether long-term daily icodextrin use can decrease
technique failure and improve survival in PD patients. The aim of the present study was to investigate whether icodextrin use, once daily, can decrease technique failure and prolong patient survival in incident PD patients.
Methods: Incident PD patients who survived more than 90 days were recruited from
the China Medical University Hospital, Taiwan, between January 1, 2007 and December 31, 2011. All patients were followed until transfer to hemodialysis (HD), renal transplantation, transfer to another center, death, or December 31, 2011.
Results: A total of 306 incident PD patients (89 icodextrin users, 217 icodextrin
non-users) were recruited during the study period. Icodextrin users were more likely to have hypertension, diabetes and high or high-average peritoneal transport compared with non-users. During the follow-up period, 43 patients were transferred to HD: 7 (7.87%) of the icodextrin group, and 36 (16.59%) of the non-icodextrin group. Thirty-two patients died during the follow-up period: 5 (5.62%) of the icodextrin group, and 27 (12.44%) of the non-icodextrin group. Icodextrin use was significantly associated with a better prognosis, in terms of technique failure (adjusted HR= 0.32; 95% CI = 0.14-0.72). With regard to patient survival, icodextrin use (adjusted HR= 0.33; 95% CI = 0.12-0.87) was associated with a significantly lower risk of death.
Conclusion: The use of icodextrin once daily may decrease technique failure and
improve survival in incident PD patients.
Key words: End-stage renal disease, icodextrin, patient survival, peritoneal dialysis, technique survival.
Introduction
Compared with hemodialysis (HD), peritoneal dialysis (PD) has an early survival advantage. However, PD is also associated with an increased risk of death in older patients, those with cardiovascular disease, and those with comorbid diabetes. In addition, PD has a higher risk of technique failure, compared with HD.3 Glucose load
from PD dialysate has harmful effects on both diabetic and non-diabetic patients. Glucose exposure may contribute to obesity, insulin resistance, dyslipidemia, and atherosclerosis. Moreover, the high glucose content of dialysate is deleterious to the peritoneal membrane, due to the high osmolality of such solutions, and the associated production of glucose degradation products (GDPs). Long-term peritoneal damage leads to increased peritoneal permeability, and loss of peritoneal ultrafiltration capacity. These changes may result in fluid retention, and increased risk of
cardiovascular disease.6 In addition, a substantial portion of dialysate-derived GDPs is
absorbed, which can accelerate the formation of systemic advanced glycation end-products.7 The higher glucose load from PD solutions is associated with increased risk
of death, and technique failure.8
Icodextrin is an iso-osmolar solution of corn starch-derived glucose polymers, with an average molecular weight of 17,000 Da.9 Icodextrin is absorbed from the
the ultrafiltration gradient is maintained over a much longer time period. Icodextrin induces ultrafiltration by a mechanism resembling colloid osmosis.10 Given that the
solution contains no glucose, its use is associated with a low level of GDP.11
Icodextrin has been available for use as a PD dialysate in Taiwan since March 2003, and was reimbursed in certain high-risk PD patients. However, whether long-term daily icodextrin use can improve patient and technique survival in incident PD patients remains controversial. The aim of this study was to investigate whether icodextrin use, once daily, can prolong patient survival and decrease technique failure in incident PD patients.
Methods
Incident PD patients from China Medical University Hospital, a tertiary medical center in central Taiwan, were recruited between January 1, 2007 and December 31, 2011. Eligible patients who survived less than 90 days were excluded from analysis. The records of all remaining eligible patients were retrospectively reviewed.
Demographic data, comorbid conditions, and laboratory data were collected at the start of dialysis. Patients with cardiovascular comorbidity, defined as a history of coronary artery disease, congestive heart failure, or stroke. All eligible patients used conventional solutions (Dianeal; Baxter Healthcare Corporation). Patients were categorized as icodextrin users if they used the solution for at least 1 month. According to the regulation of the Taiwan health insurance, icodextrin (Extraneal;
Baxter Healthcare Corporation) could be considered to be prescribed for once daily in patients who were diabetic with an HbA1c > 7.0%, required 2.5% or 4.25% dextrose solution in more than half of daily exchanges, or were in high or high-average peritoneal membrane transporter status. However, not all patients meeting these criteria received icodextrin. The prescription of icodextrin was up to medical staffs for enhancing ultrafiltration and glycemic control. Technique failure was defined as transfer to HD. All patients were followed until transfer to HD, renal transplantation, transfer to another center, death, or December 31, 2011. The primary outcomes were technique failure and all-cause mortality. Patient survival and technique survival were compared between the icodextrin group and the non-icodextrin group. This
retrospective observational study complied with the guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of China Medical University (CMUH103-REC2-070). Since this study involved retrospective review of existing data, the Institutional Review Board of China Medical University has
specifically waived the need for consent. All data was de-identified and analyzed anonymously.
Statistical analysis
All values are expressed as mean ± standard deviation or the number and
for categorical variables, and an independent t-test for continuous variables. Death, renal transplantation, transfer to another center, and alive at the end of the study period (December 31, 2011) were censored for technique survival analysis. Transfer to HD, renal transplantation, transfer to another center, and alive at the end of the study period were censored for patient survival analysis. Cox proportional hazards model was used to calculate the hazard ratio (HR) and 95% confidence interval (CI). Variables with a P value < 0.25 in univariate Cox model were analyzed further by multivariate Cox proportional hazards model. The survival curve was plotted using Kaplan-Meier method, and the difference was assessed with the log-rank test. All analyses were performed using SAS statistical software (version 9.3 for Windows; SAS Institute, Inc., Cary, NC, USA). All statistical tests were two-tailed, and the level of significance was set at 0.05.
Results
A total of 306 incident PD patients were recruited from 2007 to 2011 (89 icodextrin users, 217 icodextrin non-users). The demographic data indicated that icodextrin users were more likely to have hypertension and diabetes, compared with non-users (Table 1). Icodextrin users were also more likely to be in high or high average transporter status, compared with non-users (Table 1).The mean follow-up duration of all PD patients was 2.23 ± 1.39 years with a range of 0.25-5 years. The mean duration of
follow-up for icodextrin users was longer than that of icodextrin non-users (2.62 ± 1.34 vs. 2.07 ± 1.39 years); this difference was statistically significant (p = 0.0017). The mean icodextrin treatment time was 19.31 ± 14.20 months. During the follow-up period, 43 patients were transferred to HD: 7 (7.87%) of the icodextrin group, and 36 (16.59%) of the non-icodextrin group. Thirty-two patients died during the follow-up period: 5 (5.62%) of the icodextrin group, and 27 (12.44%) of the non-icodextrin group. In addition, 11 patients received renal transplantation: 2 (2.25%) of the icodextrin group, and 9 (4.15%) of the icodextrin group. One patient in the non-icodextrin group was transferred to another center during the follow-up period. The causes of technique failure are shown in Table S1; causes of death are shown in Table S2. Peritonitis and cardiovascular disease were the major causes of technique failure and death, respectively.
Technique survival
Technique survival was significantly lower in the non-icodextrin group, compared with the icodextrin group (p = 0.01) (Figure 1A). The univariate Cox model analysis of technique failure (Table 2) indicated that poor prognosis was significantly
associated with older age and comorbid DM. A better prognosis was associated with icodextrin use and serum albumin. In the multivariate Cox model analysis, icodextrin use was significantly associated with a better prognosis, in terms of technique
survival, while DM was significantly associated with technique failure (Table 2). Patient survival
Patient survival was significantly lower in the non-icodextrin group, compared with the icodextrin group (p = 0.02) (Figure 1B). The univariate Cox model analysis indicated that icodextrin use and serum albumin were positive prognostic factors. Older age and DM were significantly associated with poorer survival (Table 3). In the multivariate Cox model analysis, older age and DM were associated with poorer survival, while icodextrin use and serum albumin were positive prognostic factors (Table 3).
Use of icodextrin was with a positive prognostic factor for both technique survival and patient survival, regardless of diabetic or non-diabetic status (Figure 2A and Figure 2B).
Discussion
Our study revealed that icodextrin use may decrease technique failure and improve patient survival in incident PD patients. Technique failure and all-cause mortality were significantly lower in the icodextrin users.
The first icodextrin study was performed in the early 1990s, and the solution has become widely used in many developed countries.14 However, the evidence for the
positive impact of icodextrin on patient and technique survival remains inconclusive. Several previous studies showed that icodextrin is used as salvage therapy for
ultrafiltration failure, and extends technique survival. Recently, Han et al. conducted a
post-hoc analysis using the Baxter Korea databases.13 Icodextrin users were defined as
those patients that utilized the solution for >50% of their PD duration. The results indicated that long-term utilization of icodextrin was associated with lower risk of all-cause mortality (HR, 0.69) and technique failure (HR, 0.60). However, the study was limited by a lack of clinical data, including membrane transport type, residual renal function, and adequacy data. Takatori et al. performed a randomized controlled trial in diabetic PD patients over a 2-year period.17 They found that icodextrin users had
significantly better technique survival, compared with non-icodextrin users. However, this study was limited by a small sample size (41 patients). A recent meta-analysis showed that icodextrin has no significant effect on technique failure and patient survival.12 However, studies in this analysis were limited by small patient numbers,
short trial durations, and low event rates. These factors may result in an analysis with insufficient power to draw confident conclusions. Our study may provide evidence to support the benefits of icodextrin in both technique survival and patient survival. There are several possible mechanisms by which icodextrin may confer a survival advantage in PD patients. Icodextrin can enhance ultrafiltration in long dwells, compared with glucose-based solutions, especially in patients with high and high-average transport .18-20 Icodextrin use in therefore associated with improved fluid
balance, and consequently better blood pressure control, and left ventricular mass reduction. Higher extracellular water is associated with high odds of frailty.23 In
addition, the European Automated Peritoneal Dialysis Outcome Study (EAPOS) demonstrated that an ultrafiltration volume ≥750 mL/day was associated with significantly better survival in anuric automated PD patients.24 Enhanced convection
by icodextrin also contributes to increased clearance of small solutes, such as urea and sodium.6 Better adequacy could improve platelet function and reduce the risk of
bleeding. Thus, it can be assumed that the improved technique survival and lower mortality in the icodextrin group could be attributed to improved volume control, and better adequacy.
The low glucose exposure and iso-osmolality of icodextrin is more biocompatible, and therefore preserves the peritoneal membrane. A secondary analysis of data from the EAPOS also showed reduced deterioration of peritoneal membrane function in anuric automated PD patients receiving icodextrin for day dwells.25 An ex vivo study
showed that mesothelial cells present in icodextrin effluent had a greater proliferation than those present in glucose effluent.26 In addition, metabolic effects are favorable
for icodextrin. The use of icodextrin could reduce weight gain, reduce HbA1c, and improve dyslipidemia.27-30 Obesity is associated with an increased risk of technique
increases infection risk in diabetic patients.32 Poor glycemic control is associated with
increased risk of death in diabetic PD patients in a recent study.33 The use of
icodextrin has previously been reported to preserve residual renal function, although another study showed a more rapid decline in residual renal function, probably caused by excessive ultrafiltration.21 The above-mentioned local peritoneal or systemic
effects may contribute to the better outcomes associated with the use of icodextrin. Our study found that there were more causes of technique failure due to burn-out (8, 3.69%), medical problems (7, 3.23%), non-compliance, (1, 0.46%), inadequate dialysis, (1, 0.46%), and mechanical problem,(1, 0.46%) in the non-icodextrin group, but there were no these problems in the icodextrin group. Han et al. also reported that dropout related to non-compliance was significantly lower in patients using
icodextrin.13 Because of better ultrafiltration, icodextrin reduces the need for
additional exchanges during daytime or nighttime and may provide better
convenience for patients requiring an increase in fluid removal. In addition, icodextrin has been demonstrated to improve quality of life, and PD-related symptoms in
randomized controlled trials. These beneficial effects may also contribute to better prognosis in the icodextrin group.
Strengths of this study include a larger sample size, and longer follow-up time. There are several limitations in the present study. A limitation was the observational
and retrospective design. The prescription of icodextrin was up to medical staffs for enhancing ultrafiltration and glycemic control, and there was selection bias. In addition, the icodextrin group was more likely to have diabetes and to be in high or high-average peritoneal membrane transporter status compared to the non-icodextrin group. Diabetes and higher peritoneal transport are associated with increased risk of technique failure or death. Therefore, we adjusted several important clinical variables such as comorbidity, membrane transport type, residual renal function, hematocrit, and serum albumin for comparison. Furthermore, we did not observe a difference in the rates of cardiovascular mortality between the two groups [9 (4.15%) in the non-icodextrin group vs. 4 (4.49%) in the non-icodextrin group]. However, 3 of 9 infectious deaths in the non-icodextrin group were caused by diabetic foot infection, which was related to vascular insufficiency. Therefore, the rate of cardiovascular related
mortality was higher in the non-icodextrin group [12 (5.53%)].
In conclusion, the use of icodextrin, once daily, may reduce technique failure and improve patient survival in patients receiving PD. Further randomized controlled studies with greater patient numbers and longer duration are necessary to confirm our observations.
Acknowledgements
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002), The
Bureau of Health Promotion (DOH97-HP-1103, and DOH99-HP-1109), the research laboratory of pediatrics, Children’s Hospital of China Medical University, China Medical University (DMR-101-016, DMR-101-017, and DMR-103-013). Conflict of interest statement
References
1 Wang IK, Kung PT, Kuo WY, Tsai WC, Chang YC, Liang CC, et al. Impact of dialysis modality on the survival of end-stage renal disease patients with or without cardiovascular disease. J Nephrol. 2013; 26: 331-41.
2 Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol. 2010; 21: 499-506.
3 Sinnakirouchenan R, Holley JL. Peritoneal dialysis versus hemodialysis: risks, benefits, and access issues. Adv Chronic Kidney Dis. 2011; 18: 428-32.
4 Johnson DW, Armstrong K, Campbell SB, Mudge DW, Hawley CM, Coombes JS, et al. Metabolic syndrome in severe chronic kidney disease: Prevalence,
predictors, prognostic significance and effects of risk factor modification. Nephrology
(Carlton). 2007; 12: 391-8.
5 Schwedler S, Schinzel R, Vaith P, Wanner C. Inflammation and advanced glycation end products in uremia: simple coexistence, potentiation or causal relationship? Kidney Int Suppl. 2001; 78: S32-6.
6 Garcia-Lopez E, Lindholm B, Davies S. An update on peritoneal dialysis solutions. Nat Rev Nephrol. 2012; 8: 224-33.
Glucose degradation products in PD fluids: do they disappear from the peritoneal cavity and enter the systemic circulation? Kidney Int. 2003; 63: 298-305.
8 Wu HY, Hung KY, Huang TM, Hu FC, Peng YS, Huang JW, et al. Safety issues of long-term glucose load in patients on peritoneal dialysis--a 7-year cohort study.
PLoS One. 2012; 7: e30337.
9 Qi H, Xu C, Yan H, Ma J. Comparison of icodextrin and glucose solutions for long dwell exchange in peritoneal dialysis: a meta-analysis of randomized controlled trials. Perit Dial Int. 2011; 31: 179-88.
10 Frampton JE, Plosker GL. Icodextrin: a review of its use in peritoneal dialysis.
Drugs. 2003; 63: 2079-105.
11 Posthuma N, ter Wee PM, Niessen H, Donker AJ, Verbrugh HA, Schalkwijk CG. Amadori albumin and advanced glycation end-product formation in peritoneal dialysis using icodextrin. Perit Dial Int. 2001; 21: 43-51.
12 Cho Y, Johnson DW, Badve S, Craig JC, Strippoli GF, Wiggins KJ. Impact of icodextrin on clinical outcomes in peritoneal dialysis: a systematic review of randomized controlled trials. Nephrol Dial Transplant. 2013; 28: 1899-907.
13 Han SH, Ahn SV, Yun JY, Tranaeus A, Han DS. Effects of icodextrin on patient survival and technique success in patients undergoing peritoneal dialysis. Nephrol
Dial Transplant. 2012; 27: 2044-50.
2009; 29: 367-9.
15 Johnson DW, Arndt M, O'Shea A, Watt R, Hamilton J, Vincent K. Icodextrin as salvage therapy in peritoneal dialysis patients with refractory fluid overload. BMC
Nephrol. 2001; 2: 2.
16 Wilkie ME, Plant MJ, Edwards L, Brown CB. Icodextrin 7.5% dialysate solution (glucose polymer) in patients with ultrafiltration failure: extension of CAPD
technique survival. Perit Dial Int. 1997; 17: 84-7.
17 Takatori Y, Akagi S, Sugiyama H, Inoue J, Kojo S, Morinaga H, et al. Icodextrin increases technique survival rate in peritoneal dialysis patients with diabetic
nephropathy by improving body fluid management: a randomized controlled trial.
Clin J Am Soc Nephrol. 2011; 6: 1337-44.
18 Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, et al. Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double-blind randomized controlled trial. J Am Soc Nephrol. 2003; 14: 2338-44.
19 Finkelstein F, Healy H, Abu-Alfa A, Ahmad S, Brown F, Gehr T, et al.
Superiority of icodextrin compared with 4.25% dextrose for peritoneal ultrafiltration.
J Am Soc Nephrol. 2005; 16: 546-54.
20 Lin A, Qian J, Li X, Yu X, Liu W, Sun Y, et al. Randomized controlled trial of icodextrin versus glucose containing peritoneal dialysis fluid. Clin J Am Soc Nephrol. 2009; 4: 1799-804.
21 Konings CJ, Kooman JP, Schonck M, Gladziwa U, Wirtz J, van den Wall Bake AW, et al. Effect of icodextrin on volume status, blood pressure and
echocardiographic parameters: a randomized study. Kidney Int. 2003; 63: 1556-63. 22 Woodrow G, Oldroyd B, Stables G, Gibson J, Turney JH, Brownjohn AM. Effects of icodextrin in automated peritoneal dialysis on blood pressure and bioelectrical impedance analysis. Nephrol Dial Transplant. 2000; 15: 862-6.
23 Johansen KL, Dalrymple LS, Delgado C, Kaysen GA, Kornak J, Grimes B, et al. Association between body composition and frailty among prevalent hemodialysis patients: a US Renal Data System special study. J Am Soc Nephrol. 2014; 25: 381-9. 24 Brown EA, Davies SJ, Rutherford P, Meeus F, Borras M, Riegel W, et al. Survival of functionally anuric patients on automated peritoneal dialysis: the European APD Outcome Study. J Am Soc Nephrol. 2003; 14: 2948-57.
25 Davies SJ, Brown EA, Frandsen NE, Rodrigues AS, Rodriguez-Carmona A, Vychytil A, et al. Longitudinal membrane function in functionally anuric patients treated with APD: data from EAPOS on the effects of glucose and icodextrin prescription. Kidney Int. 2005; 67: 1609-15.
26 Bajo MA, Selgas R, Castro MA, del Peso G, Diaz C, Sanchez-Tomero JA, et al. Icodextrin effluent leads to a greater proliferation than glucose effluent of human mesothelial cells studied ex vivo. Perit Dial Int. 2000; 20: 742-7.
peritoneal dialysis with an overnight icodextrin dwell on parameters of glucose and lipid metabolism. Perit Dial Int. 2001; 21: 275-81.
28 Wolfson M, Piraino B, Hamburger RJ, Morton AR. A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J
Kidney Dis. 2002; 40: 1055-65.
29 Marshall J, Jennings P, Scott A, Fluck RJ, McIntyre CW. Glycemic control in diabetic CAPD patients assessed by continuous glucose monitoring system (CGMS).
Kidney Int. 2003; 64: 1480-6.
30 Paniagua R, Ventura MD, Avila-Diaz M, Cisneros A, Vicente-Martinez M, Furlong MD, et al. Icodextrin improves metabolic and fluid management in high and high-average transport diabetic patients. Perit Dial Int. 2009; 29: 422-32.
31 McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J
Am Soc Nephrol. 2003; 14: 2894-901.
32 Gupta S, Koirala J, Khardori R, Khardori N. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am. 2007; 21: 617-38, vii.
33 Duong U, Mehrotra R, Molnar MZ, Noori N, Kovesdy CP, Nissenson AR, et al. Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus.
Clin J Am Soc Nephrol. 2011; 6: 1041-8.
patients treated with automated peritoneal dialysis. Perit Dial Int. 2006; 26: 405-7. 35 Guo A, Wolfson M, Holt R. Early quality of life benefits of icodextrin in peritoneal dialysis. Kidney Int Suppl. 2002: S72-9.
36 Mistry CD, Gokal R, Peers E. A randomized multicenter clinical trial comparing isosmolar icodextrin with hyperosmolar glucose solutions in CAPD. MIDAS Study Group. Multicenter Investigation of Icodextrin in Ambulatory Peritoneal Dialysis.
Kidney Int. 1994; 46: 496-503.
37 Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Page D. Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. The Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1998; 9: 1285-92. 38 Liao CT, Chen YM, Shiao CC, Hu FC, Huang JW, Kao TW, et al. Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol Dial Transplant. 2009; 24: 2909-14.
Figure 1. Cumulative survival curves for (A) technique survival and (B) patient survival.
Figure 2. Cumulative survival curves for (A) technique survival and (B) patient survival, stratified by diabetes status.
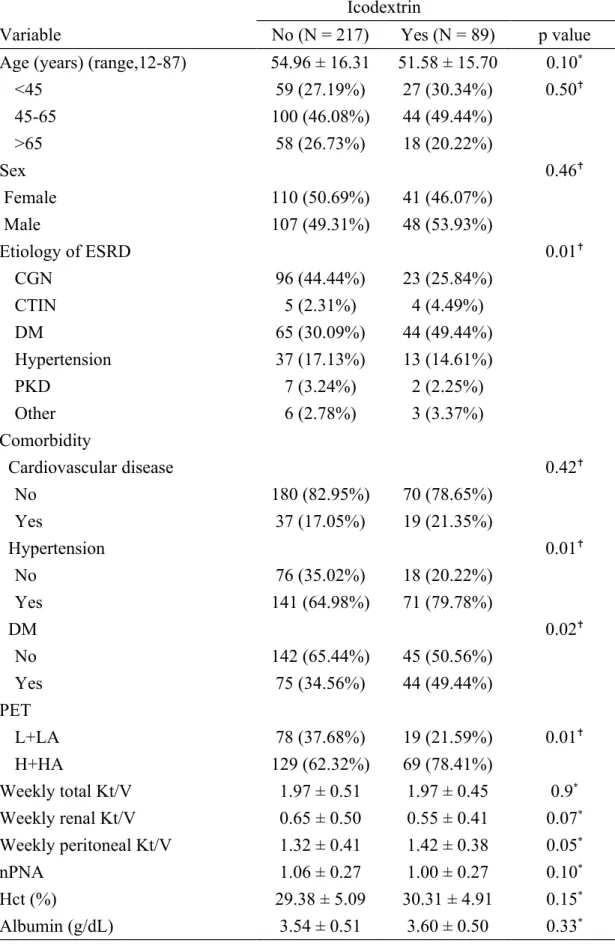
Table 1. Demographic and clinical characteristics of incident peritoneal dialysis patients
Icodextrin
Variable No (N = 217) Yes (N = 89) p value
Age (years) (range,12-87) 54.96 ± 16.31 51.58 ± 15.70 0.10*
<45 59 (27.19%) 27 (30.34%) 0.50 45-65 100 (46.08%) 44 (49.44%) >65 58 (26.73%) 18 (20.22%) Sex 0.46 Female 110 (50.69%) 41 (46.07%) Male 107 (49.31%) 48 (53.93%) Etiology of ESRD 0.01 CGN 96 (44.44%) 23 (25.84%) CTIN 5 (2.31%) 4 (4.49%) DM 65 (30.09%) 44 (49.44%) Hypertension 37 (17.13%) 13 (14.61%) PKD 7 (3.24%) 2 (2.25%) Other 6 (2.78%) 3 (3.37%) Comorbidity Cardiovascular disease 0.42 No 180 (82.95%) 70 (78.65%) Yes 37 (17.05%) 19 (21.35%) Hypertension 0.01 No 76 (35.02%) 18 (20.22%) Yes 141 (64.98%) 71 (79.78%) DM 0.02 No 142 (65.44%) 45 (50.56%) Yes 75 (34.56%) 44 (49.44%) PET L+LA 78 (37.68%) 19 (21.59%) 0.01 H+HA 129 (62.32%) 69 (78.41%) Weekly total Kt/V 1.97 ± 0.51 1.97 ± 0.45 0.9* Weekly renal Kt/V 0.65 ± 0.50 0.55 ± 0.41 0.07* Weekly peritoneal Kt/V 1.32 ± 0.41 1.42 ± 0.38 0.05* nPNA 1.06 ± 0.27 1.00 ± 0.27 0.10* Hct (%) 29.38 ± 5.09 30.31 ± 4.91 0.15* Albumin (g/dL) 3.54 ± 0.51 3.60 ± 0.50 0.33*
Abbreviations: ESRD, end-stage renal disease; CGN, chronic glomerulonephritis; CTIN, chronic tubulointerstitial disease; DM, diabetes mellitus; PKD, polycystic kidney disease; PET, peritoneal equilibrium test; nPNA, normalized protein nitrogen appearance.
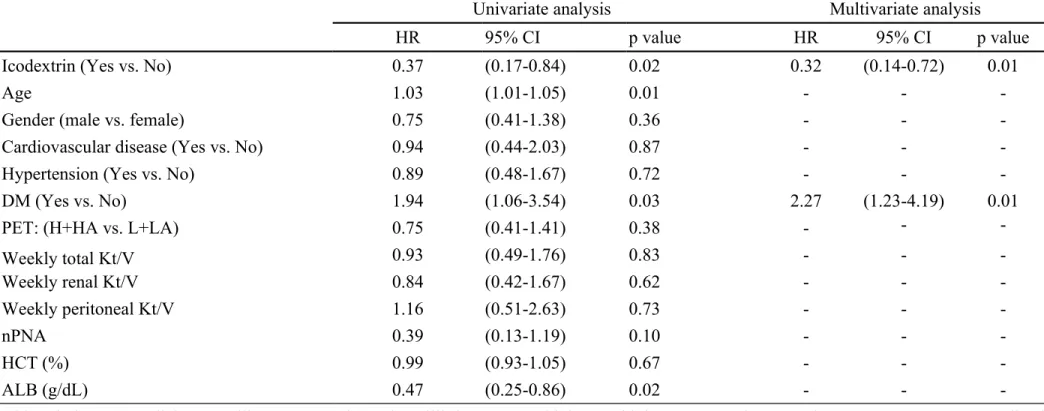
Table 2. Risk factors for technique failure by Cox regression analysis
Univariate analysis Multivariate analysis
HR 95% CI p value HR 95% CI p value
Icodextrin (Yes vs. No) 0.37 (0.17-0.84) 0.02 0.32 (0.14-0.72) 0.01
Age 1.03 (1.01-1.05) 0.01 - -
-Gender (male vs. female) 0.75 (0.41-1.38) 0.36 - -
-Cardiovascular disease (Yes vs. No) 0.94 (0.44-2.03) 0.87 - -
-Hypertension (Yes vs. No) 0.89 (0.48-1.67) 0.72 - -
-DM (Yes vs. No) 1.94 (1.06-3.54) 0.03 2.27 (1.23-4.19) 0.01
PET: (H+HA vs. L+LA) 0.75 (0.41-1.41) 0.38 - -
-Weekly total Kt/V 0.93 (0.49-1.76) 0.83 - - -Weekly renal Kt/V 0.84 (0.42-1.67) 0.62 - - -Weekly peritoneal Kt/V 1.16 (0.51-2.63) 0.73 - - -nPNA 0.39 (0.13-1.19) 0.10 - - -HCT (%) 0.99 (0.93-1.05) 0.67 - - -ALB (g/dL) 0.47 (0.25-0.86) 0.02 - -
-Abbreviations: DM, diabetes mellitus; PET, peritoneal equilibrium test; H, high; HA, high average; L, low; LA, low average; nPNA, normalized protein nitrogen appearance.
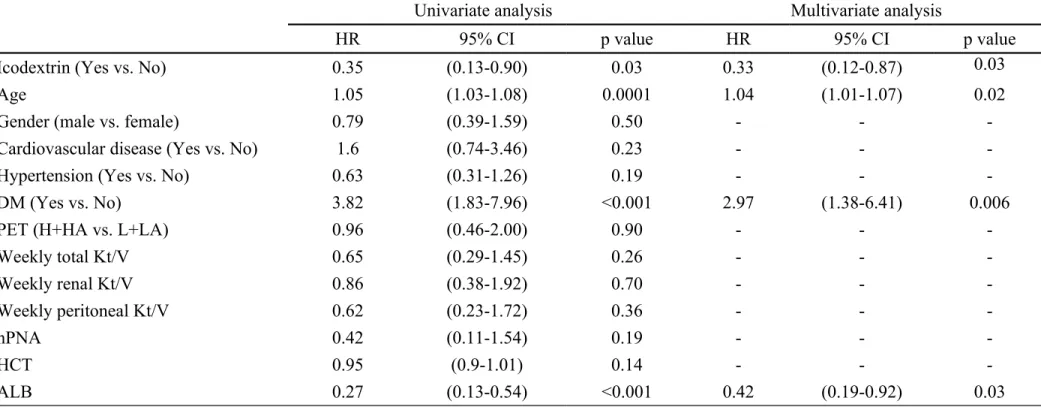
Table 3. Risk factors for death by Cox regression analysis
Univariate analysis Multivariate analysis
HR 95% CI p value HR 95% CI p value
Icodextrin (Yes vs. No) 0.35 (0.13-0.90) 0.03 0.33 (0.12-0.87) 0.03
Age 1.05 (1.03-1.08) 0.0001 1.04 (1.01-1.07) 0.02
Gender (male vs. female) 0.79 (0.39-1.59) 0.50 - -
-Cardiovascular disease (Yes vs. No) 1.6 (0.74-3.46) 0.23 - -
-Hypertension (Yes vs. No) 0.63 (0.31-1.26) 0.19 - -
-DM (Yes vs. No) 3.82 (1.83-7.96) <0.001 2.97 (1.38-6.41) 0.006
PET (H+HA vs. L+LA) 0.96 (0.46-2.00) 0.90 - -
-Weekly total Kt/V 0.65 (0.29-1.45) 0.26 - - -Weekly renal Kt/V 0.86 (0.38-1.92) 0.70 - - -Weekly peritoneal Kt/V 0.62 (0.23-1.72) 0.36 - - -nPNA 0.42 (0.11-1.54) 0.19 - - -HCT 0.95 (0.9-1.01) 0.14 - - -ALB 0.27 (0.13-0.54) <0.001 0.42 (0.19-0.92) 0.03
Abbreviation: DM, diabetes mellitus; PET, peritoneal equilibrium test; H, high; HA, high average; L, low; LA, low average; nPNA, normalized protein nitrogen appearance