~nal of
Biomedical
Science
Original Paper
J Biomed Sci 2002;9:596-606 DOl: 10.1159/000067288 Received: March 14, 2002 Accepted: May 6, 2002Differential Effects of Foods Traditionally
Regarded as "Heating" and "Cooling" on
Prostaglandin E2 Production by a Macrophage
Cell Line
Ching-jang Huang Mei-Chiao Wu
Laboratory of Nutritional Biochemistry, Department of Agricultural Chemistry, National Taiwan University, Taipei, Taiwan, ROC
Key Words
COX-2 • Food • Macrophage • PGE2. Prostaglandin
production. Traditional food belief
Abstract
Some components of natural foods may enhance or
inhibit prostaglandin formation and potentially affect
the inflammation condition. A macrophage cell line,
RAW264.7, was employed to examine the effects of
foods traditionally regarded as 'heating' or 'cooling' on
the production of PGE2, a well-known proinflammatory
mediator. Foods traditionally regarded as 'heating' (lit-
chi, Iongan, and dried Iongan) or 'cooling' (chrysanthe-
mum flower, bitter gourd, and lotus seed plumule) were
extracted sequentially with water and ethyl acetate. The
water extracts (WE) and ethyl acetate extracts (EAE) were
applied to RAW264.7 macrophages in the presence or
absence of LPS (lipopolysaccharide). In the absence of
LPS, the WEs from the 'heating foods', litchi, tongan, or
dried Iongan had a dose-dependent enhancing effect on
PGE2 production, with respective EC50s of 8.4, 16, and
11 mg/ml. This effect was accompanied by significant
induction of COX-2 protein expression, as shown by
Western blot analysis. In contrast, LPS-induced PGE2
production was inhibited in a dose-dependent manner
by the WEs of the 'cooling foods', chrysanthemum flow-
er, bitter gourd, and lotus seed plumule, with respective
tC50s of 0.6, 0.13, and 0.08 mg/ml. At the concentrations
tested, none of the EAEs had any effect on basal PGE2
production, while LPS-induced PGE2 production was in-
hibited or increased by the EAE from bitter gourd and
Iongan, respectively. Water-soluble extracts of foods tra-
ditionally regarded as 'heating' enhanced basal PGE2
production, while those from 'cooling' foods significant-
ly inhibited LPS-induced PGE2 production by the macro-
phage celt line. This subject merits further study to deter-
mine whether appropriate food selection may help pa-
tients suffering from chronic inflammatory conditions.
Copyright © 2002 National Science Council, ROC and S, Karger AG, Basel
Introduction
Prostaglandins (PGs), a family of intercellular and
intracellutar messengers derived from 20-carbon polyun-
saturated fatty acids, are synthesized in a wide range of
tissue types and serve as autocrine or paracrine mediators
signaling changes in the immediate environment. In hu-
mans, PGs are involved in diverse functions, including
blood clotting, ovulation, initiation of labor, bone metab-
olism, nerve growth and development, wound healing,
KAKG E R
Fax+41 61 306 12 34 E-Mail karger@karger.ch www.karger.com
© 2002 National Science Council, ROC S. Karger AG, Basel
1021-7770/02/0096-0596518.50/0 Accessible online at:
ww~'. karger.com/jbs
Ching-jang Huang, PhD
Department of Agricultural Chemistry National Taiwan University
1, Sec. 4, Roosevelt Road, Taipei, 106 Taiwan (ROC) TeL/Fax +886 2 23621301 E-Mail cjjhuang@ccmsmtu.edu.tw
kidney function, blood vessel tone, and the immune
response [42]. The key regulatory enzyme of P G biosyn-
thesis is cyclooxygenase (COX, or prostaglandin H syn-
thase, PGHS), a bifunctional enzyme catalyzing the oxi-
dative cyclisation of the central 5 carbons in 20-carbon
polyunsaturated fatty acids and subsequent peroxidation
[27, 55]. The final PGs produced vary, depending on the
downstream enzymatic machinery present in a particular
cell type.
Nonsteroidal anti-inflammatory drugs (NSAIDs) had
been used in humans for many decades before Vane [53]
proposed that the inflammation suppression produced by
NSAIDs might be due to inhibition of COX, resulting in
less proinflammatory P G production at the injury site.
Molecular cloning studies have identified two forms of
COX [15], COX-l, a constitutive form responsible for
'housekeeping' functions, and COX-2, an inducible form
that mediates many of the inflammatory and inducible
effects. Both forms carry out essentially the same catalytic
reaction and have similar primary sequences [15, 48].
Inhibitors acting only on the inducible COX-2 would
therefore be ideal as NSAIDs free of side effects [11].
PGE2 is the major P G produced by macrophages.
COX-2 expression in macrophages is induced in response
to intrinsic factors, such as cytokines, or extrinsic factors,
such as lipopolysaccharide (LPS), leading to the produc-
tion of PGE2 [45, 18]. PGE2 produced by macrophages is
a well-known pro-inflammatory mediator [ 17].
Some components of natural foods may enhance or
inhibit PG formation and potentially affect the inflamma-
tion condition. For example, phytochemicals and other
components in foods may modulate COX-2 expression
and P G formation [1, 5, 9, 20, 29, 32, 35, 50, 54]. It is
therefore worth screening the effect of foods on COX-2
expression and P G formation which can provide useful
information on food selection for the amelioration of the
pathophysiological conditions of inflammation.
For 2,000 years, people in Chinese and Indian societies
have believed that certain foods are either 'heating' (or
'fire increasing') or 'cooling' (or 'fire reducing') in the
body when eaten [2, 7, 14]. In addition, some physiologi-
cal conditions or disease states are categorized as 'hot' or
'cold'; but this is not related to body temperature. Accord-
ing to traditional Chinese medicine, symptoms for the
diagnosis of a 'heating' disease state include a dry mouth,
thirst, a bitter taste sensation even when not eating or
drinking, flushing, constipation, a small amount of dark-
colored urine, a rough yellow tongue, and a fast pulse [34].
Fever, overstimulation, inflammation, and sore throat
have also been described as conditions with excess heat in
the body [2]. In addition, acne or pimples, hemorrhoids,
and nosebleeds are also thought to be associated with an
excess consumption of heating food [22]. In contrast, dis-
ease states in which the patient is thirsty but is not willing
to drink, and has pallor, diarrhea, a large amount of
diluted urine, a smooth white tongue and a slow pulse are
diagnosed as 'cooling' [34]. Weakness, tiredness, cold
body temperature and shivering are also reported to be
'cooling' conditions [2, 14]. In agreement with the Chi-
nese beliefofyin and yang, health is thought to result from
a proper balance of 'heating' and 'cooling' foods and
activities, and illness is treated with foods or medicines
with properties opposite to those of the disease [2, t 4, 33].
In Taiwan, Hong Kong and among Chinese immigrants in
the USA, in which ahnost all of the population accept
m o d e m medical care, the concept of 'heating' and 'cool-
ing' foods is still prevalent in all socioeconomic classes.
Many individuals select food to avoid suffering from
unfavorable and disturbing conditions, often discounted
by most modern medical doctors [22, 33]. This is because
these traditional rules filled the explanatory and behavior
niches left open in Western medicine [22].
Not all foods are categorized as 'heating' or 'cooling'; a
large variety of foods such as staple foods like plain rice,
noodles and steamed bun are neutral [2, 7, 33]. Moreover,
foods can change categories as a result of different cooking
methods [2, 7, 33]. For example, masted and fried pea-
nuts are typical 'heating' foods, but boiled and steamed
peanuts are not. Fried foods and meat cooked in black
sesame oil with ginger are also good examples of 'heating'
foods produced by a cooking method. Most 'cooling'
foods are of plant origin, especially fruits and vegetables.
Many, but not all, of the 'cooling' fruits and vegetables
have a high water content, e.g. watermelon and radish,
and excretion of a large volume of diluted urine is consid-
ered one of the 'cooling' conditions. However, not all veg-
etables are 'cooling'; many are neutral, and a few fruits,
such as litchi, longan, and durian, are considered to be
'heating'.
In spite that food items listed as 'cooling' or 'heating'
may vary among societies or populations in different geo-
graphical areas because of different climates, environ-
ments and agricultural products available, the central rule
of the hot/cold system prevails. In addition, susceptibility
also varies among individuals which is partially attributed
to an innate 'hot' or 'cold' physical nature. Although there
has been speculation on the significance and rationale of
the 'hot/cold' food belief from a social science aspect [2-4,
14], an empirical rational basis has not been reported. A
chemical feature c o m m o n to 'hot' or 'cold' foods cannot
Heating/Cooling Foods and Macrophage
PGE2 Production
be ruled out simply based on the nutrient composition,
implying that some components with special functions
may be involved. On the other hand, the class of body
regulator that is targeted by 'hot' or 'cold' foods and
mediates the diverse 'hot/cold' syndrome must be one
that has a wide range of functions or can trigger diverse
physiological changes in the body.
Since (1) the extreme case of a 'heating' condition
resembles inflammation in certain ways, and (2) feeding
mice a diet containing frying oil tended to increase PGE2
production by peritoneal macrophages [26] and fried
foods are very common 'heating' foods [2, 33], the effect
of some typical 'heating' and 'cooling' foods on PGE2 pro-
duction was tested using a murine macrophage cell line,
RAW 2 64.7, as an in vitro model [38]. The 'heating'
tbods, litchi, longan, and dried longan [2], and the 'cool-
ing' foods, bitter gourd [14], chrysanthemum flower [2],
and lotus seed plumule, were chosen for testing because
these are natural foods in which the classification of 'heat-
ing' or 'cooling' is most widely accepted. Results of this
study will provide basic information supporting further
welt-controlled in vivo experiments.
Materials and M e t h o d s
Materials
The 'heating' foods chosen for this study were longan
(Nephelium
longana
Camb orEuphoria longana
Lain), litchi (or lychee, leechee or lichi,Litchi sinesis
Sonn), and dried longan (during drying, longan undergoes a marked browning reaction), while the 'cooling' foods chosen were dried chrysanthemum flower(Chrysanthemum morifot-
ium
Hemsl.), fresh bitter gourd(Momordica charantia
L.), and dried plumule of lotus seed(Nelumbo nucifera
Gaertn). Longan, litchi, and bitter gourd, which are common fruit and vegetables in Taiwan, were purchased from a local market. Dried chrysanthemum flower and lotus seed plumule were purchased from a Chinese traditional medi- cine store. Dried longan, the hot-air-dried product of the whole lon- gan fruit including the peel, was purchased from a grocery store.The RAW264.7 macrophage cell line (CCRC60001, originally from the American Type Culture Collection; designation, TIB-71) was obtained from the cell bank of the Food Industry Research and Development Institute, Hsin Chu, Taiwan. Dulbecco's minimal essential medium (DMEM) and fetal bovine serum were purchased from Gibco (Md., USA).
Methods
Extraction of FoodSamptes.
The edible portions oflongan, litchi, dried longan (the aril), and bitter gourd (the flesh) were cut into smalI pieces and homogenized in a Waring blender with a minimal amount of double-distilled water, and the homogenates were filtered through several layers of gauze. Dried chrysanthemum flower was boiled in water (10 ml/g flower) for 5 rain aud filtered. Then the residue was reextracted in the same way and the two filtrates were combined. The dried lotus seed plumule was homogenized with water (10 ml/g plu-mule) and filtered. All filtrates were centrifuged at 10,500 g at 4°C for 30 rain and the clear supernatant, the water extract (WE), was freeze-dried. The ethyl acetate extract (EAE) was prepared by freeze- drying the precipitate and filtered residues from the water extraction and extracting it overnight by shaking at room temperature with ethyl acetate (30 ml/g of residue), followed by filtration and drying of the filtrate in a rotary evaporator (Biichi, Essen, Germany). The yields of dried water extract of longan, litchi, dried longan, bitter gourd, lotus seed plumule and chrysanthemum were 7.15, 14.02, 43.49, 2.76, 28.95 and 34.5 g/100 g of the fresh edible portion (lon- gan, litchi and bitter gourd) or dried material (dried longan, lotus seed plumule and chrysanthemum). The yields of evaporated EAE of longan, litchi, dried longan, bitter gourd and lotus seed plumule were 1.53, 4.01, 2.23, 1.86, and 17.25 g/100 g of the dried residue after water extraction.
For cell culture experiments, the WEs were dissolved in DMEM, while the EAEs were dissolved in a minimal amount of ethanol or dimethyl sulfoxide. Both were diluted to the appropriate concentra- tion with DMEM immediately before use.
Cell Culture
RAW 264.7 cells were grown in 25-cm 2 flasks in DMEM contain- ing 10% fetal bovine serum at 37 °C in a 5% CO2 atmosphere. Near- confluent cells were removed with a cell scraper, seeded on 96-well plates at a concentration of 6 x t0 s cell/ml, incubated for 24 h at 37 °C in 5% CO2, and then washed with phosphate-buffered saline (27 raM, pH 7.4, PBS). To test the effect of food extracts on PGE2 production, the cells were incubated for 18 h at 37 ° C with serum-free DMEM containing various concentrations of sample in the presence or absence of 1 ng/ml of LPS. Then the medium was collected and stored at -70 °C until analysis fbr PGE2. Cell viability was assayed using the MTT method [39]. Experiments were repeated at least three times in triplicate.
To study the effect of the above-mentioned treatment on COX-2 protein expression, confluent cells were seeded on 6-cm 2 dishes and treated for 12 h at 37°C, in the presence or absence of 1 ng/ml of LPS, with WEs or EAEs at the concentration that showed a maximal effect on PGE2 production and did not significantly affect cell viabil- ity. The cells were then washed twice with PBS, suspended in 1 ml of PBS using a cell scraper, and transferred to a microcentrifuge tube. After centrifugation, the supernatants were discarded and the cell pellets stored at -70 °C until analyzed by Western blotting.
Analyses
The collected medium was diluted to an appropriate concentra- tion and analyzed for PGE2 by competitive enzyme-immunoassay using a commercial kit (Cayman, Ann Arbor, Mich., USA). For COX-2 protein analysis, cell pellets were lysed and the cellular pro- teins extracted, separated by polyacrylamide gel electrophoresis, and transferred to a polyvinylidene fluoride membrane, which was then treated sequentially with primary antibody (rabbit anti-COX-2 anti- serum, Cayman), secondary antibody (alkaline-phosphatase-conju- gated anti-rabbit antibody, Tropix, Applied Biosystems, Foster City, Calif., USA), and CDP-star (chemiluminescent substrate of alkaline phosphatase, Tropix). The specific protein band was visualized using X-ray film and quantitated by image analysis.
Statistical AnaIysis
The data for PGE2 production were expressed as the mean + SD for triplicate wells in a representative experiment. The significance of
differences at each sample concentration was analyzed by ANOVA and Duncan's multiple range test using SAS 5.1 software. To calcu- late the percentage enhancement of basal PGE2 production by a sam- ple at a specific concentration, the maximal PGE2 production pro- duced by this sample was taken as 100% and the enhancing effect
calculated using the equation: percent enhancement = [(PGE2 sample -
PGE2 basal)/(PGE2 maximum- PGE2 b~sal)] X 100%, where PGE2 basal is the PGE2 produced by nonstimulated untreated macrophages and PGE2 sample is the PGE2 produced in the presence of a specific concen- tration of the sample. The ECso value, calculated from the curve of
percentage enhancement versus concentration, is the concentration of sample that produces 50% enhancement. To calculate the percent- age inhibition of LPS-stimulated PGE2 production, PGE2 produc- tion in the presence of 1 ng/ml of LPS was taken as 0% inhibition and basal PGE2 production as 100 % inhibition; the percentage inhibition by a sample at a specific concentration was calculated as: percentage
inhibition = [1 - (PGE2 s a m p l e + L P S - PGE2 basaI)/(PGE2 LPs -
P G E 2 basal)] X 1000/0. The ICs0 value, calculated from the curve of
percentage inhibition versus concentration, is the concentration of sample that results in 50% inhibition.
121
6 12==9
g
u73
¢D 13- 12g
~ 7 3 0 A F0 0.01 0.1 0.2 0.5 1 2 Concentration (mg/ml) b ab ab ab FO 0.01 0,05 0.1 Concentration (rng/rnl) i= ECs0 = 11 mg/ml 12 E o "c} O C}. u~ 3 - ( 9 r~ c F0 b c ~ c 1 2 5 10 Concentration (mg/ml) 1 2 ¸- 9 - £g
"o o u~ 3 - (.9 e . B a a a a F0 0.02 0.05 0.1 0,2 Concentration (mg/ml) D ECs0 = 16 mg/ml 0.5 aN
c c a d 0.2 F0 1 5 10 20 50 Concentration (mg/ml) F ECs0 = 8.4 mg/ml 12 9 v o r~~ 3
(.9 IX. 20 F0 1 5 7.5 10 20 Concentration (mg/ml)F i g . 1. Effect of WEs on basal PGE2 production. A Chrysanthemum flower. B Bitter gourd. C Lotus seed plumule. D Longan. E Dried longan. F Litchi. RAW264.7 cells (6 × 104 cells/well) were seeded on 96-well plates and grown for 24 h, then incubated for 18 h with DMEM or DMEM containing various concentrations of WE. PGE2 in the medium was then measured by competitive enzyme-immuno-
assay. The data are the mean + SD for triplicate wells from a repre- sentative experiment. The experiment was repeated at least three times with similar results. F0 indicates basal PGE2 production when cells were incubated in DMEM alone. Means not sharing a common letter (a-d) were significantly different (p < 0.05) when analyzed by ANOVA and Duncan's multiple range test,
Heating/Cooling Foods and Macrophage PGE2 Production
30 l
~25
2o4
E 0 0 ,.,? L9 n A ICso = 0.6 mg/ml 0 0.01 0.1 0.2 0.5 1 2 Concentration (mg/ml) C IC50 = 0.08 mg/ml a a a b 0 5e-3 0 . 0 1 0.05 0.1 0,2 Concentration (mg/mt) 0 0.5 1 5 10 20 Concentration (mg/ml) F0 b F0 F03° t
t
~ 1 0 L~ ~. 53o t
1
15 o K10 u7 5 30-~
25 - - ~ 2 0 - O "~ 1 5 - O ~ . 1 0 - ~9 ~. 5- B IC50 = 0.13 mg/ml 0 0 . 0 1 0.02 0.05 0.1 0.2 Concentration (mg/ml) D 0 0.1 1 5 10 20 Concentration (rng/rnl) F b - r b 0 0.1 0,5 1 Concentration (m /ml) 0.5 F0 50 F0 10 c F0Fig.
2. Effect of WEs on LPS-induced PGE2 production. A Chrysan- themum flower. B Bitter gourd. C Lotus seed plumule. D Longan. E Dried longan. F Litchi. RAW264.7 cells (6 x 104 cells/well) were seeded on 96-well plates and grown tbr 24 h, then incubated for 18 h with DMEM containing 1 ng/ml of LPS with or without various con- centrations of the WEs. The PGE2 concentration of the medium wasmeasured using an enzyme-immunoassay kit. The data shown are the mean + SD for triplicate wells of a representative experiment. The experiment was repeated at least three times with similar results. Means not sharing a common letter (a-t) were significantly different (p < 0.05) when analyzed by ANOVA and Duncan's multiple range test.
R e s u l t s
The basal level of PGE2 production by nonactivated
macrophages (without LPS or any other stimulation) was
low and in the range of 0.5-1 ng/ml. As expected, treat-
ment with 1 ng/ml of LPS significantly increased the
expression of COX-2 protein and PGE2 production.
In this study, a wide range of concentrations of sample
extracts was tested, but the data shown are restricted to
those for experiments in which the concentration of
extract did not significantly affect cell viability, as deter-
mined by the MTT assay. The enhancing and inhibitor)'
effects described below were therefore not secondary to
any change in cell number or viability.
Effects of Food Extracts on PGE2 Production by
RA W264. 7 Cells
Water Extracts. The effects of the different WEs on
basal PGE2 production were first tested. As shown in fig-
ure 1, basal PGE2 production was unaffected by WEs
from chrysanthemum flower (fig. 1A), bitter gourd
(fig. 1B), and lotus seed plumule (fig. 1C), but was in-
creased in a dose-dependent manner by WEs from longan
(fig. 1D), dried longan (fig. 1E), and litchi (fig. 1F). At a
concentration of 50 mg/ml, longan WE increased PGE2
production 8.5-fold compared with the basal level, while,
at 20 mg/ml, the WEs of dried longan and litchi resulted
in 5- and 7.5-fold increases, respectively, over the basal
levels. The EC50s for the WE of longan, dried longan, and
litchi were 16, 11, and 8.4 mg/ml, respectively.
The WEs were then tested for their effect on LPS-
induced PGE2 production. As shown in figure 2, WE from
chrysanthemum flower (fig. 2A), bitter gourd (fig. 2B),
and lotus seed plumule (fig. 2C) inhibited LPS-induced
PGE2 production in a dose-dependent manner. At a con-
centration of 2 mg/ml, the WE from chrysanthemum
flower completely inhibited PGE2 production, while the
WEs from bitter gourd (0.5 mg/ml) and lotus seed plu-
mule (0.2 mg/ml) produced 68 and 63% inhibition,
respectively. At a concentration equal to or greater than
1.0 (bitter gourd) or 0.5 mg/ml (lotus seed plumule), the
WEs significantly reduced cell viability. The ICs0s for the
WEs from chrysanthemum flower, bitter gourd, and lotus
seed plumule were 0.6, 0. t 3, and 0.08 mg/ml, respective-
ly. In contrast, the WEs from longan (fig. 2D), dried lon-
gan (fig. 2E), and litchi (fig. 2F) did not inhibit LPS-
induced PGE2 production, but instead tended to increase
it.
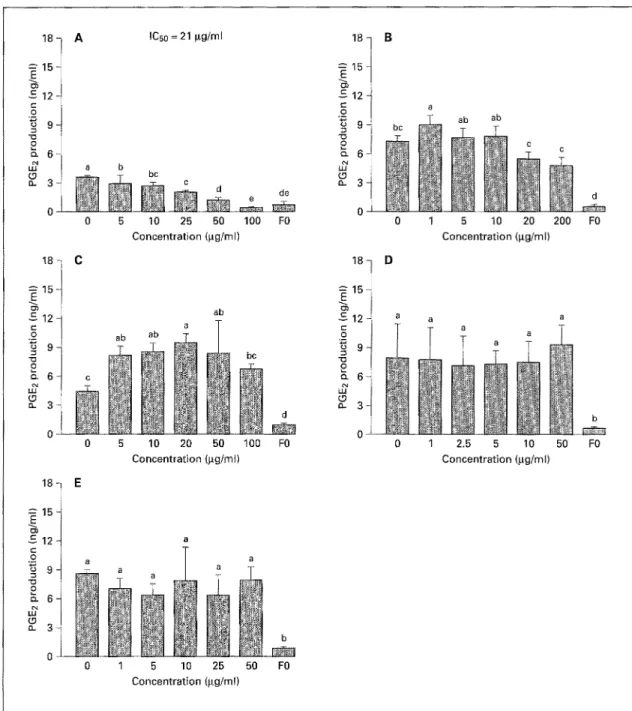
Ethyl Acetate Extracts. Due to their limited solubility,
the EAEs could only be tested in the gg/ml concentration
range. At these levels, none of the EAEs significantly
altered basal PGE2 production (data not shown). How-
ever, when tested for effects on LPS-induced PGE2 pro-
duction, the EAE from bitter gourd showed dose-depen-
dent inhibition (ICs0 = 21 gg/ml, fig. 3A), and, at concen-
trations of 50-100 gg/ml, caused almost complete inhibi-
tion, while the EAE from longan resulted in increased
PGE2 production (fig. 3C).
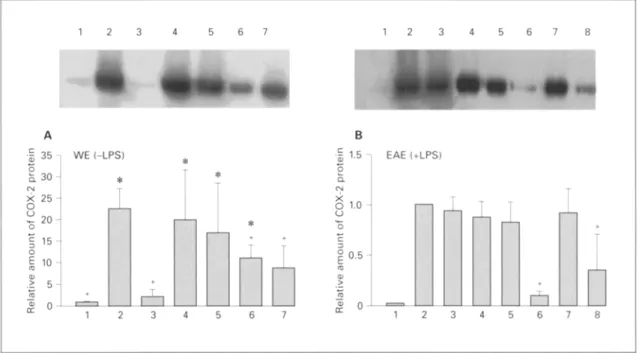
Effects of Food Extracts on COX-2 Protein Expression
by tL4 W264. 7 Cells
Levels of COX-2 protein in RAW 264.7 cells treated
with various samples in the presence or absence of LPS
were determined by Western blot analysis. As shown in
figure 4A, WEs from litchi, longan, dried longan, and
lotus seed plumule induced significant COX-2 protein
expression in nonstimulated macrophages (without LPS).
None of the WEs had any significant effect on the amount
of COX-2 protein induced by 1 ng/mt of LPS (data not
shown); however the EAEs from lotus seed plumule and
longan (100 gg/ml) showed a significant reduction
(fig. 4B). As no significant effect on PGE2 production was
seen when 1L~W264.7 cells were treated with EAE alone,
the effect of EAEs on basal COX-2 protein was not
checked.
Discussion
Among the six foods tested in this study, WEs from the
'heating' foods, longan, litchi, and dried tongan, signifi-
cantly enhanced basal PGE2 production by the macro-
phage cell line RAW264.7 and also tended to increase
LPS-induced PGE2 production. In contrast, WEs from
the 'cooling' foods, bitter gourd, chrysanthemum flower,
and lotus seed plumule, did not stimulate basal PGE2 pro-
duction and inhibited LPS-induced PGE2 production.
These results support our hypothesis that 'heating' or
'cooling' foods may, respectively, stimulate or inhibit
PGE2 production by macrophages. It is therefore worth
testing these effects further in an appropriate in vivo mod-
el and also testing more foods for these effects.
The yields of dried water extract of longan, litchi and
bitter gourd corresponded to a WE concentration of 71.5,
140 and 27.6 mg/g of the fresh edible portion. This is
higher than their respective EC50s of 8.4 (litchi) and 16
mg/ml (longan), and IC50 of 0.13 mg/ml (bitter gourd).
Similarly, the ECs0 of WE from dried longan (11 mg/ml)
and ICs0s of the chrysanthemum flower (0.6 mg/ml) and
lotus seed plumule (0.08 mg/ml) were lower than the WE
concentration in 1% (wt/vol) chrysanthemum or dried
lotus seed plumule beverages or a 30/0 (wt/vol) dried lon-
gan beverage. After eating, the presence of the active com-
ponent at a sufficient concentration, at least in the gas-
trointestinal tract, is therefore possible.
Since the water-soluble component(s) of longan, litchi,
and dried longan stimulated PGE2 production by the
macrophage cell line and also tended to increase the
expression of COX-2 protein (fig. 4 A), it is probable that
Heating/Cooling Foods and Macrophage PGE2 Production
IC50 = 21 gg/ml a b ~ b c
18 l
c 12 ~ c- o 9 " o o o_ 3 B - - - ' d d e o 0 5 10 25 50 100 FO 0 1 5 10 20 200 FOConcentration (lag/ml) Concentration (gg/ml)
1 8 C 18 D 15 15 -£=1 ~ 1 2 - ~ a a a "o "13 O o " ~ 6 ~7 ~7 (.9 0 5 10 20 50 100 FO 0 1 2.5 5 10 50 FO Concentration (gg/ml) Concentration (gg/ml) 0 1 5 10 25 Concentration (gg/m() 50 FO
Fig. 3. Effect of EAEs on LPS-induced PGE2 production. A Bitter gourd. B Lotus seed plumule. C Longan. D Dried longan. E Litchi. The EAEs were dissolved in a minimal amount of dimethylsulfoxide or ethanol, then diluted with DMEM. The experiment was perfbrmed as in figure 2. Means not sharing a common letter (a-d) were significantly different (p < 0.05) when analyzed by ANOVA and Duncan's multiple range test.
the enhancing effect on PGE2 production involved induc-
tion of COX-2 expression.
COX-2 expression is induced in macrophages in re-
sponse to intrinsic factors such as cytokines, or extrinsic
factors such as LPS, leading to the production of PGE2
[ 18, 45]. Most of these factors are molecules that can trig-
ger the signaling pathway by binding to receptor mole-
ctdes in the cell membrane. For example, LPS acts on
macrophages by binding to LPS binding protein and
mCD14, a receptor located on the macrophage surface
membrane [51]. This activates the signal transduction
pathway, which includes protein kinase C [12] and
1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 A 3 5 q WE(-LPS) S, 3 ¢'4
>k2
o ~ L o .> 1 2 3 4 5 B -$ 1.5 EAE (+LPS) Q. x o 1.o 0.5 % -~ 0 6 7 "" 1 2 3 4 5 6 7 8Fig. 4. Western blot analysis of COX-2 protein expression atter treat- ment with extracts. A Treatment with WE or LPS alone: lane 1 = F0 (basal level, no treatment); lane 2 = FOL1, LPS alone (1 ng/ml); lane 3 = bitter gourd WE alone (0.2 mg/ml); lane 4 = dried longan WE alone (5 mg/ml); lane 5 = litchi WE alone (20 mg/ml); lane 6 = lotus seed plumule WE alone (0.2 mg/ml); lane 7 = longan WE alone (20 rag/ ml). B Treatment with LPS (1 ng/ml) with or without EAE: lane 1 = F0 (basal level, no treatment); lane 2 = FOL1 (LPS alone); lane 3 = LPS plus dried longan EAE (t 0 gg/ml); lane 4 = LPS plus litchi EAE (20 gg/ml); lane 5 = LPS plus bitter gourd EAE (100 l,tg/ml); lane 6 = LPS plus lotus seed plumule EAE (200 gg/ml); lane 7 = LPS plus longan EAE (20 btg/ml); lane 8 = LPS plus longan EAE (100 btg/ml).
RAW 264.7 cells (4 x 106/ml) were seeded on a 6-cm 2 dish and grown tbr 24 h. Then the medium was changed to DMEM or LPS alone or DMEM containing EAEs and LPS. The cells were then incu- bated for 12 h and harvested for Western blot analysis of COX-2 protein expression. The upper panels show Western blots fi'om a rep- resentative experiment; the experiment was repeated three times with similar results. The lower panels show image analysis results; the data are the means + SD for three experiments. * p < 0.05 vs. F0 (cells with no treatment) and + p < 0.05 vs. FOL1 (cell treated with LPS only). In the lower panel of A, the p value of the difference between longan (lane 7) and F0 (Lane 1) was 0.059, i.e. nearly signifi- cant.
MAPK [18]. Expression of the COX-2 gene is induced
when these signals activate transcriptional factors, such as
NF-~cB [10, 18, 31]. Theoretically, molecules able to bind
to cell surface receptors, trigger the signaling pathway, or
activate transcription factors could directly or indirectly
induce COX-2 protein expression and result in PGE2 pro-
duction. Molecules that interfere with any point in the
above-mentioned mechanism of action of LPS may lead
to inhibition of PGE2 production [19]. Other potential
factors that could influence PGE2 production include
those affecting phospholipase A2 activity [57] or the fatty
acid composition of the cell membrane [32], both of
which may alter substrate availability, or those that may
directly affect the catalytic activity of COX-2 [6].
Among the dietary and food factors that may affect
PGE2 production, direct inhibition of COX activity has
been demonstrated for resveratrol [20, 35, 50], hydroxy-
stilbenes [47], and curcumine [1]. Enhancement of ex-
pression of COX-2 protein has also been demonstrated by
n-6 fatty acids in the mammary gland [5]. Inhibition of
COX-2 expression in macrophages, epithelial cells, the
RAW 264.7 macrophage cell line, and colorectal cancer
cells has been reported to be caused, respectively, by
genistein [10], resveratrol [50], apigenin [29], and antioxi-
dants [9]; the first three agents are bioflavonoids with
antioxidant activity [44] and it appears that the antioxi-
dant activity is closely related to the inhibitory effect on
COX [35, 37, 52]. Using the same testing system as that
used in this study, we have observed a significant inhibi-
tion on the LPS-induced PGE2 production by a number of
phytochemicals including curcumine, quercetin, apige-
nin, narigenin, resveratrol, and some tea polyphenols
[54].
Heating/Cooling Foods and Macrophage PGE2 Production
For chrysanthemum flower, we only tested the WE,
since it is usually ingested as a drink prepared by brewing
in hot water. When cells were treated with chrysanthe-
m u m flower WE, although inhibition of PGE2 production
was seen in the presence (fig. 2A) of LPS, no significant
effect was seen on COX-2 protein expression (data not
shown). These results suggest that chrysanthemum flower
WE may directly interfere with COX-2 enzyme activity.
Compounds isolated from chrysanthemum flowers, in-
cluding sesquiterpenes and flavones, have been shown to
inhibit LPS-induced nitric oxide production by macro-
phages [56] and to be antioxidative and inhibit cancer cell
growth [4t]. Some flavones may be partially soluble in
water. It is not known if antioxidant flavonoids are
responsible for this inhibition by chrysanthemum flower
WE.
Numerous biologically active components have been
identified in bitter gourd, also known as bitter melon. For
example, seed extracts contain MAP-30, an anti-HIV and
anti-tumor protein [28], momorcharins [43] and momor-
dins [21], which inactivate ribosomes, and momordin Ic
[30] and oleanolic acid glycoside [36], both of which can
alter gastrointestinal transit time and blood glucose. An
extract of bitter gourd fruit has been shown to have hypo-
glycemic activity [46] and to alter the activities of phase I
and phase I! drug metabolizing enzymes in the rat liver
[25]. In this study, the extract was prepared exclusively
from the fleshy part with the seeds removed. Both the WE
and EAE from bitter gourd caused dose-dependent inhibi-
tion ofLPS-induced PGE2 production; again, since COX-
2 protein expression did not change significantly, inhibi-
tion of COX-2 enzyme activity may have been responsi-
ble.
Lotus seed plumule WE showed a marked inhibitory
effect on PGE2 production on a dried weight basis. How-
ever, its effect on PGE2 production and COX-2 protein
expression was complex, since it significantly inhibited
LPS-induced PGE2 production (fig. 2C), but did not af-
fect COX-2 protein expression (data not shown). On the
other hand, a faint COX-2 protein band was seen on the
Western blot of RAW264.7 cells treated with lotus seed
plumule WE in the absence of LPS (fig. 4 A) and this was
accompanied by a very slight increase in PGE2 production
(fig. 1C). The most intriguing result was the effect of the
EAE, which significantly reduced COX-2 protein expres-
sion (fig. 4B), but did not significantly affect PGE2 pro-
duction (fig. 3 B) by RAW264.7 cells treated with LPS.
These results suggest that multiple compounds in lotus
seed plumule may exert diverse, or even opposite, effects
on the RAW264.7 macrophage cell line. Despite this com-
plicated result, the inhibitory effect of lotus seed plumule
WE on LPS-induced PGE2 production is considered most
significant, since brewing in hot water to prepare a drink
is the most common method used for the preparation of
this food material.
Depending on the nature of the inhibitor and the sys-
tem used, COX inhibitors have been shown to reduce [ 12,
24, 29, 50], induce [40], increase [8], or have no effect on
[6] levels of COX-2 mRNA or protein. Dietary bioflavon-
oids, such as genistein [12], apigenin [29], and resveratrol
[50], inhibit COX-2 gene expression, probably by interfer-
ing with the signal transduction pathway that leads to
expression of COX-2 genes. In contrast, COX inhibitors,
such as indomethacin or NS398, can induce COX-2
mRNA expression [40] in a manner similar to peroxisome
proliferators, or increase COX-2 protein levels [8], proba-
bly by stabilization of the enzyme protein. PGE2 can de-
stabilize the COX-2 protein [40]. In the presence of LPS,
the inhibition of PGE2 production without a change in
COX-2 protein levels produced by extracts of bitter
gourd, chrysanthemum flower, and lotus seed plumule are
similar to the results seen with NSAID reported by Bar-
rios-Rodiles et al. [6].
The molecule(s) in litchi, longan, and dried longan
responsible for induction of COX-2 expression in the
macrophage remain to be elucidated. Because of their
water-soluble nature, plant lectins may be candidates.
Lectins from various plant sources have been shown to
bind to macrophage surface glycoproteins [16, 23] and
can result in activation of the macrophages [23]. Further
studies are needed to test this possibility.
Macrophages represent a system of widely dispersed
cells that are able to recognize and destroy invading
microorganisms and altered host components, such as
apoptotic cells. The macrophage plays an important role
in the regulation of the immune response [ 13]. Fixed mac-
rophages, such as alveolar macrophages, Kupffer cells,
histocytes, mesangial cells, and microglial cells, like free
macrophages, are monocyte-derived cells and may serve
additional functions in the tissues in which they reside,
respectively. For example, mesangial cells are involved in
the regulation of the glomerular filtration rate and renal
blood flow [49]. In addition, the final products of COX,
such as PGE2 and other prostaglandins, are involved in
diverse physiological functions and pathophysiological
conditions. The results of this study provide one of several
possible rationales for the effects of 'heating' and 'cooling'
foods. However, given the diverse aspects of physiological
conditions associated with 'heating' and 'cooling', other
possible mechanisms of action await further discovery.
For example, diuretic and antidiuretic actions may be
physiological effects of these so-called 'heating' and 'cool-
ing' foods.
In conclusion, WEs of foods traditionally regarded as
'heating', namely litchi, longan, and dried longan, in-
creased COX-2 protein levels, and basal PGE2 production
by the RAW264.7 macrophage cell line. In contrast, WEs
of foods traditionally regarded as 'cooling', namely chry-
santhemum flower, bitter gourd, and lotus seed plumule,
significantly decreased LPS-induced PGE2 production by
these cells. These results provide preliminary evidence for
a scientific basis for the ancient oriental belief in 'heating'
and 'cooling' foods. As PGE2 produced by macrophages is
an important pro-inflammatory agent, this subject merits
further study, as this could provide useful information on
food selection as a means of treatment for the pathophysi-
ological condition of inflammation.
Acknowledgments
This study was supported by a grant (DOH89-TD-1065) from the D e p a r t m e n t of Health, Taiwan. W e t h a n k Dr. M i n - H s i u n g Lee for advice on food sample extraction and Mr. Kuo-Wey Chen o f the Institute of Food Science a n d Technology, N a t i o n a l Taiwan Univer- sity, for assistance with freeze-dl2eing of samples.
References
1 Ammon HP, Safayhi H, Mack T, Sabieraj J. Mechanism of anti- inflammatory actions of curcumine and boswellic acids. J Ethnophar- maco138:113-119;1993.
2 Anderson EN Jr. 'Heating 'and 'cooling' foods in Hong Kong and Taiwan. Soc Sci Inf 19:237- 268;1980.
3 Anderson EN Jr. 'Heating and cooling' foods re-examined. Soc Sci Meal 23:755-773;1984. 4 Anderson EN Jr, Why is humoral medicine so
popular'? Soc Sci Med 25:331-337;1987. 5 Badawi AF, E1-Sohemy A, Stephen LL, Gho-
shaI AK, Archer MC. The effect of dietary n-3 and i1-6 polyunsaturated fatty acids on the expression ofcyclooxygenase 1 and 2 and levels of p2 lras in rat mammary glands. Carcinogen- esis 19:905-910; 1998.
6 Barrios-Rodiles M, Keller K, Belley A, Chadee K. Nonsteroidal anti-inflammatory drugs in- hibit cyclooxygenase-2 enzyme activity but not mRNA expression in human macrophages. Biochem Biophys Res Commun 225:896-900; 1996.
7 Briggs GM, Calloway DH. Nutrition and Phys- ical Fitness, ed 11. Philadelphia, Saunders, I984, chap 19, pp 481-494.
8 CaUejas NA, Castrillo A, Bosca L, Martin-Sanz P. Inhibition ofprostaglandin synthesis up-reg- ulates cyclooxygenase-2 induced by lipopoly- saccharide and peroxisomal proliferators. J PharmacoI Exp Ther 288:1235-1241;1998. 9 Chinery R, Beauchamp RD, Shyr Y, Kirkland
SC, Coffey, RJ, Morrow JD. Antioxidants re- duce cyclooxygenase-2 expression, prostaglan- din production, and proliferation in colorectal cancer cetts. Cancer Res 58:2323-2327;1998. 10 D'Acquisto FD, Iuvone T, Rombola L, Saute-
bin L, Rosa MD, Carnuccio R. Involvement of NF-KB in the regulation of cyclooxygenase-2 protein expression in LPS-stimulated J774 macrophages. FEBS Lett 418:175-178; 1997. 11 Dewitt DL, Meade EA, Smith WL. PGH syn-
thase isozyme selectivity: The potential for saf- er nonsteroidal anti-inflammatory drugs. Am J Med 95:40S-44S; 1993.
12 Glaser KB, Sung A, Bauer J, Weichman BM. Regulation of eicosanoid biosynthesis in the macrophage. Involvement of protein tyrosine phosphorylation and modulation by selective protein tyrosine kinase inhibitors. Biochem Pharmacol 45:711-721; 1993.
13 Gordon S. The role of the macrophage in im- mune regulation. Res ImmunoI 149:685-688; 1998.
14 Gould-Martin K. Hot cold poison and dirt: Chinese folk medical categories. Soc Sci Med 12:39-46;1978.
15 Herschman HR. Regulation of prostaglandin synthase-1 and prostaglandin synthase-2. Can- cer Metastasis Rev 13:241-256; 1994. 16 Hoenig S, Skutelsky E, Leibovici J, Barot R.
Changes in distribution of Iectin receptors in macrophages activated by Nocardia water-so- luble molecules. Cell Mol Biol 39:843-848; 1993.
17 Humes JL, Bonney R J, Pelus, L, Dahlgren ME., Kuehl FLA, Davies P. Macrophages synthesize and release prostaglandins in response to in- flammatory stimuli. Nature 269:149-I51; 1977.
18 Hwang D, Jang BC, Yu G, Bludreau M. Ex- pression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide. Mediation through both mitogen-activated protein kinase and NF-~B signaling pathways in macro- phages. Biochem Pharmaco154:8%96; 1997. 19 Hwang D, Fischer NH, Jang BC, Tak H, Kim
JK, Lee W. Inhibition of the expression of inducible cyclooxygenase and proinflammato- ry cytokines by sesquiterpine lactones in mac- rophages correlates with the inhibition of MAP kinases. Biochem Biophys Res Commun 226: 810-818;1996.
20 Johnson JL, Maddipati KR. Paradoxical ef- fects of resveratrol on the two prostaglandin H synthases. Prostaglandins Other Lipid Mediat 56:131-143;1998.
21 Kimura Y, Minami Y, Tokuda T, Nakajima S, Takagi S, Funatsu G. Primary structures of N- linked oligosaccharides of momordin-a, a ribo- some-inactivating protein from Momordica
charantia seeds. Agric Biol Chem 55:2031-
2036;1991.
22 Koo LC. The use of food to treat and prevent disease in Chinese culture. Soc Sci Med 18: 757-766;I984.
23 Krugluger W, Lucas T, Koller M, Boltz-Nitu- lescu G, Froster O. Soybean agglutinin binds a
1 6 0 - k D a rat macrophage membrane glycopro- tein and enhances cell differentiation and acti- vation. Immunol Lett 52:53-56;1996. 24 Ku KM, Sansores-Garcia L, Chen XM, Mati-
jevic-Aleksic, N, DuM, Wu KK. Suppression of inducible cyclooxygenase 2 gene transcription by aspirin and sodium salicylate. Proc Natl Acad Sci USA 96:5292-5297; 1999.
25 Kusamran WR, Ratanavila A, Tepsuwan A. Effects of neem flowers, Thai and Chinese bit- ter gourd fruits and sweet basil leaves on hepat- ic monooxygenases and glutathione S-transfer- ase activities, and in vitro metabolic activation of chemical carcinogens in rats. Food Chem Toxico1122:121-126;1998.
26 Lai CC, Lin BF, Lin KW. Effects of high dietary fat and deteriorated frying oil on pros- taglandin E2 production in BALB/c mice. J Chin Agric Chem Soc 35:401-412; 1997. 27 Lands WEM. "Iqae biosynthesis and metabo-
lism of prostaglandins. Annu Rev Physiol 4l: 633-652;1979.
28 Lee-Huang S, Huang PL, Chen HC, Bourin- baiar A, Huang HI, Kuang HF. Anti-HIV and anti-tumor activities of recombinant MAP30
from bitter melon. Gene 161:151 - 156; 1 9 9 5 .
29 Liang YC, Huang YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide syn- thase by apigenin and related flavonoids in mouse macrophage. Carcinogenesis 20:1945- I952;1999.
Heating/Cooling Foods a n d Macrophage PGE2 P r o d u c t i o n
30 Li Y, Matsuda H, Yamahara J, Yoshikawa M. Acceleration of gastrointestinal transit by mo- mordin Ic in mice: Possible involvement of 5- hydroxytryptamine, 5-HT(2) receptors and prostaglandins. Eur J Pharmacoi 392:71-77; 2000.
31 Lo CJ, Cryer HG, Fu M, Lo FR. Regulation of macrophage eicosanoid generation is depen- dent on nuclear factor ~B. J Trauma 45:19-24; 1998.
32 Lokesh BR, Kinsella JE. Modulation of prosta- glandin synthesis in mouse peritoneal macro- phages by enrichment oflipids with. either eico- sapentaenoic or docosahexaenoic acids in vi- tro. Immunobiology 175:406-419;1987. 33 Ludman EK, Newman JM. Yin and yaug in the
health-related food practices of three Chinese groups. J Nutr Educ 19:3-5;1984.
34 Ma CC. Chung I Chert Tuan Hseueh (Diagno- sis in Chinese Traditional Medicine). Taipei, Kuo Lee Bian I Kuan. 139;1980.
35 Martinez J, Moreno JJ. Effect ofresveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem Pharmaco159:865-870;2000. 36 Matsuda H, Li Y, Murakami T, Matsumura N,
Yamahara J, Yoshikawa M. Antidiabetic prin- ciples of natural medicines. IlL Structure-relat- ed inhibitory activity and action mode ofolea- nolic acid glycosides on hypoglycemic activity. Chem Pharm Bull 46:1399- t 403; 1998. 37 Mendez C, Garcia I, Maier, R. Antioxidants
attenuate endotoxin-induced activation of al- veolar macrophages. Surgery 1 I8:412-420; 1995.
38 Miwa M, Kong ZL, Shinohara K, Watanabe M. Macrophage stimulating activity of foods. Ag- ric BioI Chem 54:1863-1866; 1990.
39 Mosmann T. Rapid colorimetric assay for cel- lular growth and survival: Application to pro- liferation and cytotoxicity assay. J Immunol Methods 65:55-63;1983.
40 Pang L, Hoult JR. Induction ofcyclooxygenase and nitric oxide synthase in endotoxin-acti- vated J774 macrophages is differentially regu- lated by indomethacin: Enhanced cyclooxygen- ase-2 protein expression but reduction of indu- cible nitric oxide synthase. Eur J Pharmacol 317:151-155;1996.
41 Peterson J, Dwyer J. Flavonoids: Dietary oc- currence and biochemical activity. Nutr Res
18:1995-2018;1998.
42 Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LBA, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J 12:1063-1073;1998.
43 Pu Z, Lu BY, Liu WY, Jin SW. Characteriza- tion of enzymatic mechanism of gamma-mo- morcharin, a novel ribosome-inactivating pro- tein with lower molecular weight of 11,500
purified from the seeds of bitter gourd (Mo-
mordica charantia). Biochem Biophys Res
Commun 229:287-294; 1996.
44 Rice-Evans CA, Miller NJ, Paganga G. Struc- ture-antioxidant activity relationships of fla- vonoids and phenolic acids. Free Radic Biol Med 20:933-956;1996.
45 Riese J, HoffT, Nordhoff A, Dewitt DL, Resch K, Kaever V. Transient expression of prosta- glandin endoperoxide synthase-2 during mouse macrophage activation. J Leukocyte Biol 55: 476-482; 1994.
46 Sarkar S, Pranava M, Marita R. Demonstra-
tion of the hypoglycemic action of Momordica
charangia in a validated animal model of dia-
betes. Pharmacol Res 33:1-4; 1996.
47 Shin NH, Ryu SY, Lee H, Min KR, Kim Y. Inhibitory effects of hydroxystilbenes on cy- clooxygenase from sheep seminal vesicles. Planta Med 64:283-284; 1998.
48 Smith WL, Garavito RM, DeWitt D L Prosta- gtandin endoperoxide H synthases (cyclooxy- genase)-I and -2. J Biol Chem 271:33157- 33t60;1996.
49 Stock JD, Sansom SC. Regulation of filtration rate by glomerular mesangial cells in health and diabetic renal disease. Am J Kidney Dis 29: 971-981;1997.
50 SubbaramaiaI K, Quing W, Michaluart P, Te- lang N, Tanabe T, Inoue H, Jang M, Pezzuto JM, Dannenberg AJ. Resveratrot inhibits cy- clooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithe- lial cells. J Biol Chem 273:21875-21882;1998. 51 Sweet M J, Flume DA. Endotoxin signal trans-
duction in macrophages. J Leukocyte Biol 60: 8-26;1996.
52 Tetsuka T, Baler LD, Morrison AR. Antioxi- dants inhibit interleukin-1 induced cyclooxy- genase and nitric oxide synthase expression in rat mesangial cells. Evidences for post-tran- scriptional regulation. J Biol Chem 271:11689- 11693;1996.
53 Vane JR. Inhibition ofprostaglandin synthesis as a mechanism of action for aspirin-like dlnags. Nature 231:232-235;1971.
54 Wu MC, Huang, CJ. Inhibition of prostaglan- din E2 production of a macrophage cell line by some phytochemicals. Food Sci Agric Chem 3: 59-71;2001.
55 Yamamoto S. Characterization of enzymes in prostanoid synthesis. In: Curtis-Prior PB, ed. Prostaglandins: Biology and Chemistry of Pros- taglandins and Related Eicosanoids. Edin- burgh, Churchill Livingstone. 37-51; 1988. 56 Yoshinkawa M, Morikawa T, Toguchida I,
Harima S, Matsuda H. Medicinal flowers. 1I. Inhibitors of nitric oxide production and abso- lute stereostructures of five new germacrane- type sesquiterpenes, kikkanols D, D monoace- tate, E, F, and F monoacetate from the flowers of Chrysanthemum indicum L. Chem Pharm
Bull 48:651-656; 2000.
57 Zor U, Reiss N. Regulation of plasma mem- brane phospholipase A2 activity by phosphory- lation/dephosphorylation: Is glucocorticoid ac- tion mediated by induction of protein phospha- tase. In: Bailey JM, ed. Prostaglandins, Leuko- trienes, Lipoxins and PAF. New York, Plenum Press. 135-139;1991.