行政院國家科學委員會專題研究計畫成果報告 Preparation of NSC Project Report
計畫編號:90-2313-B-009-001 執行期限:90 年 8 月 1 日至 91 年 7 月 31 日 主持人:毛仁淡 計畫參與人員:廖純沂、陳文亮 執行機構:交通大學生物科技所 中文摘要:
a-Lactalbumin (a-LA) 及 ß-Lactoglobulin
(ß-LG)是乳清蛋白中之主要蛋白,牛乳的加 工過程中加熱是必要的流程,因此在此難以 估計之加工過程中,檢測牛乳的品質是非常 重要的,因此研究重點在於利用簡單的方式 檢測出乳清蛋白再加工過程中的變化。我們 成 功 的 利 用 native-polyacrylamide gel electrophoresis (PAGE) 發現 ß-LG 在加工過程中會全面性的變性,但 並不會在中 a-LA 發現。此種變性推測與在加 工過程中之熱處理有直接的相關性,我們利 用溫度動力學上的改變研究純化後之 ß-LG 或 a-LA,在 circular dichroic (CD) 分析中 205nm 之 ellipticity 溫度及時間上的改變最 大。ß-LG 在溫度低於 60℃加熱時間 500 秒以 內,結構上並未有顯著上的改變,些許的改 變在溫度 70-80 ℃,但劇烈的改變在 90-95 ℃。在 Westernblot 分析上加工後之牛乳中 ß-LG 會形成大分子結構,再生乳中並未有此 結 構 產 生 , 因 此 可 利 用 native PAGE 及 Westernblot 檢測牛乳中 ß-LG 之變化來評估 牛乳的加工流程。 關 鍵 字 : a-Lactalbumin, ß-Lactoglobulin, Native-PAGE, Westernblot 英文摘要:
α -Lactalbumin ( α -LA) and β -Lactoglobulin ( β -LG) are major protein moieties of bovine whey proteins. As much of the process involves heat treatment during the preparation of milk on an industrial scale, the unpredictable measures of the process are an essential issue in determining the quality of the
milk. The purpose of the present study was to investigate the major change(s) of whey proteins in the processed milk using a simple approach. By a native-polyacrylamide gel electrophoresis (PAGE), β -LG in processed milk was found to be extensively denatured, but
not α -LA. Such denaturation was
presumably associated with the heating procedure used in the process. The kinetics of thermal denaturation of purified β-LG or α -LA at various temperatures was further studied using a circular dichroic (CD) analysis. The maximal changes of ellipticity at 205 nm were correlated to the heating temperature and time. There were no significant changes in CD spectrum of β-LG at the temperature below 60 ℃ within 500 s, mild changes occurred at 70-80 ℃, but the most pronounced changes were at 90-95 ℃ in a time-dependent fashion. Westernblot analysis revealed that the large multiple forms of β -LG were markedly present in processed milk, but not in the raw milk. Therefore, using a simple native PAGE and Westernblot, β-LG may be served as a marker for evaluating the thermal process of manufactured milk.
Keywords: α-Lactalbumin, β-Lactoglobulin, Native-PAGE, Westernblot
緣由與目的
Molten globules are thought to be general intermediates in protein folding and unfolding (1). α-LA or β-LG is one of the major protein moieties of bovine whey proteins, both of them are the most investigated models for understanding the mechanism involved in protein stability, folding and unfolding upon the heating. Recent studies have shown that milk α-LA and β-LG may induce apoptosis in tumor cells (2,3), and possess
immunomodulatory (4-6) and hypocholesterolemic effect (7). As much of the process involves heat treatment during the preparation of milk on an industrial scale, the unpredictable nature of the process has therefore been an essential issue in affecting the physiologic role of the α -LA and β -LG. For this reason, we attempted to investigate some major changes of the whey proteins in the processed milk. In fact, there were no such reports that have been documented concerning the processed milk thus far. In the present study, we demonstrated that there was a substantial loss of β-LG in the whey proteins using a native-PAGE. Since the manufacturers do not disclose the processing procedures of the milk, we established the thermal denaturation curves of the loss of β -LG and α-LA with respective to their heating time and temperatures. Some of the thermal denaturation of each respective α-LA or β -LG has previously been investigated (8,9) ; but the detailed information regarding to their correlation with the kinetic changes in CD spectra at various temperatures has not been simultaneously reported. The present study provided a reference value, in general, for the study of the correlation of conformational changes and electrophoretic properties in both α -LA and β -LG. In addition, the Westernblot analysis on the changes in some molecular form of β -LG in the raw and processed milk, which has not been reported previously, is also investigated.
結果與討論 Result
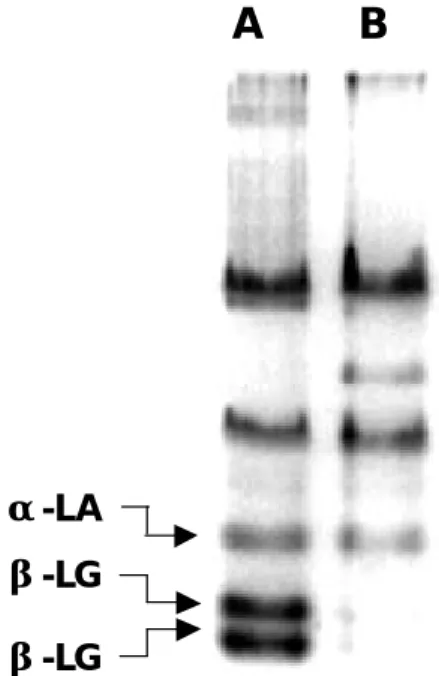
To identify the possible difference in protein profiles between raw and dairy processed milk, freshly prepared whey samples were initially analyzed on a native-PAGE. As shown in Fig. 1, using the same batch of the milk before and after manufactured process, there was a marked decrease in two acidic proteins in processed milk as compared to that of raw milk. N-terminal sequence analysis of these proteins revealed that they were the isoforms of β-LG (Table 1) consisting to the chemical characteristics of β-LG previously
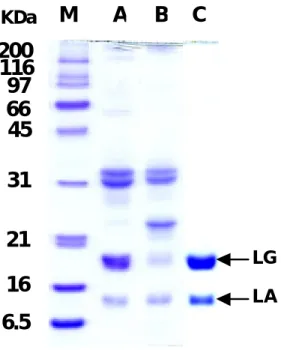
reported (14). In the next experiment, we examined the whey samples on a denatured SDS-PAGE using a PAGE procedure without heat. Similarly, the β-LG (at a molecular weight about 18 kDa) was substantially reduced (Fig. 2). It is worthy to mention that one additional band corresponding to a molecular weight about 25 kDa was observed in the processed milk sample (Fig. 2), but it was not related to β-LG as assessed by a Westernblot (described later). It might be derived from the other protein moiety during the manufacturing process. Thus, our data suggested that the overall negative charges as well as the molecular form of β-LG were altered during the process of dairy milk.
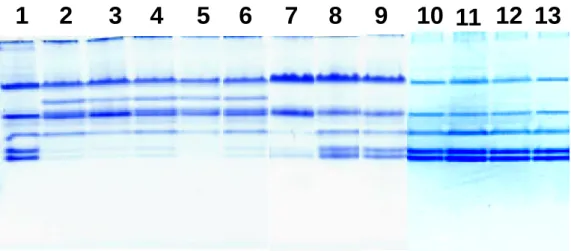
Using a native-PAGE in the next experiment, we determined the feature of whey proteins in other 4 major brands of processed milk purchased from the market in Taiwan (total 5). Fig. 3 shows that the β -LG component, but not α -LA, was extensively denatured in all 5 samples. However, the β -LG remained almost intact in 4 randomly chosen brands from the US market (Fig. 3). In addition, 3 brands of powdered milk imported from Australia, New Zealand, and Denmark were also analyzed (Fig. 3). The Denmark brand exhibited some extent of denaturation in β-LG, but not from that of Australia and New Zealand. Thus, a simple native-PAGE may be used to impart technological difference involved in the process of dairy milk.
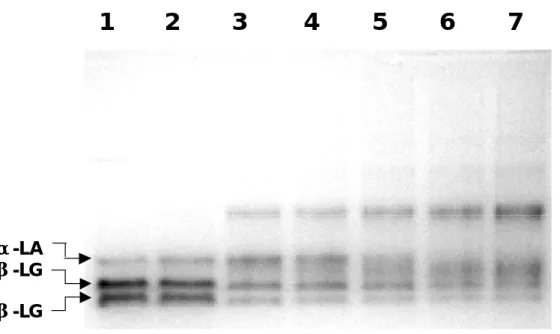
To test the hypothesis that the overall change in negative charges of β-LG was caused by a heat treatment in the processed milk, we heated the isolated β -LG and α -LA at 90 ℃ overtime. On native-PAGE analysis, the acidic property of β-LG was altered in a time dependent manner (Fig. 4). A marked change in heat treatment was observed in β-LG over time up from 30 s. The major extra-band (Fig. 4) from the denaturation was β -LG as confirmed by a Westernblot (described later). Whereas, the α-LA was more resistant (240 s) as compared to β-LG. We further examined the thermal changes in each purified β-LG or α-LA at various temperatures over time using
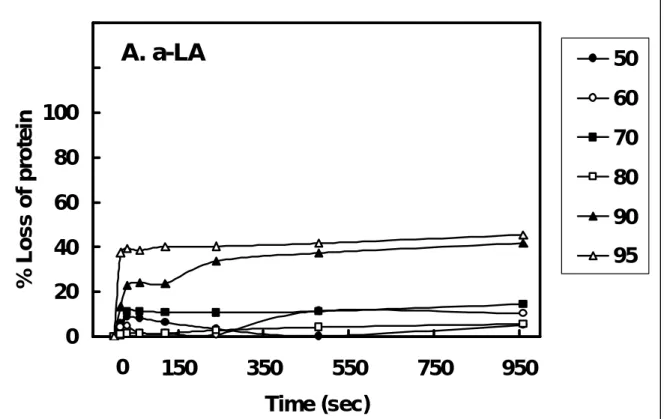
the same native-PAGE. Following the integration using a digital image system from the gel, there was no significant change in β -LG below 60 ℃ over a period of 500 s (Fig. 5A). Some mild changes occurred at 70-80 ℃, but the most pronounced changes occurred between 90 and 95 ℃ and was in a time-dependent fashion. The native form of β-LG was almost abolished when heating was proceeded for longer than 240 s. Similarly, the thermal denaturation curves of α-LA were constructed, but the severity was much less than that of β-LG in both time and temperature responses (Fig. 5B).
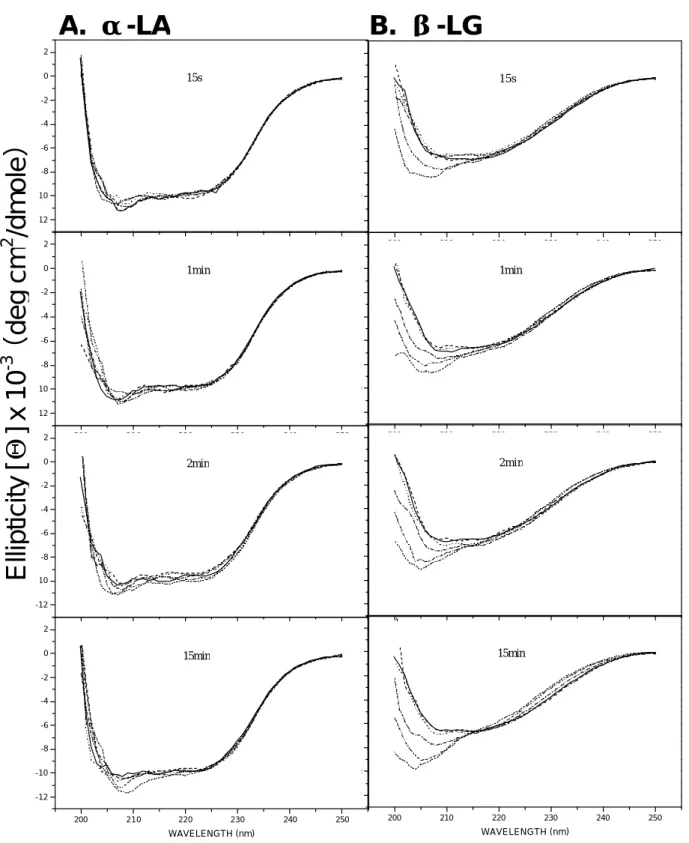
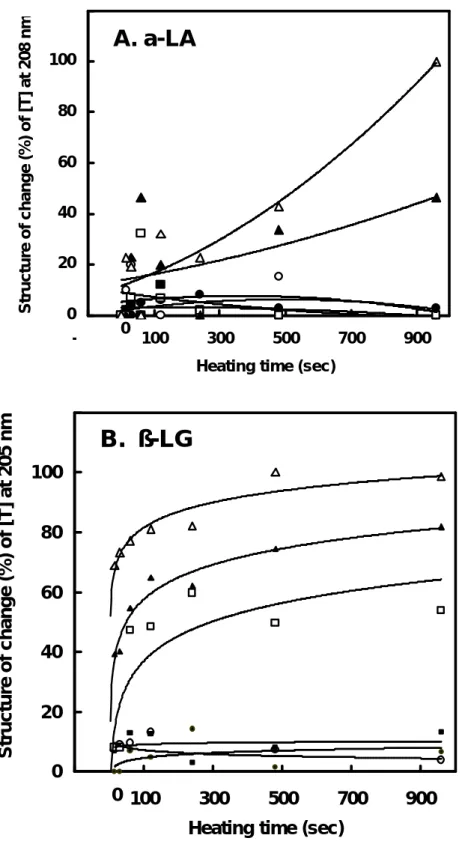
Therefore, our data suggested that β-LG suffered more changes in overall structure than did α -LA upon the heating. To further support this hypothesis, we monitored the structural changes in β -LG and α -LA using a CD spectral measurement. Fig. 6 shows that β-LG exhibited more disordered structure at the temperature up to 80 and 95 ℃ than that of α-LA. The maximal ellipticity changes within our experimental ranges (time vs. temperature) are depicted in Fig. 7. Thus, the conformational changes of β-LG may be responsible for the overall changes of electromobility as that found in native-PAGE shown in Fig. 1.
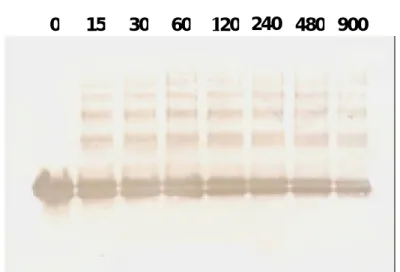
Finally, we employed a polyclonal antibody prepared against native β -LG to further trace the heated β -LG on a Westernblot. This polyclonal antibody reacted equally to the native and heated β-LG on a competitive ELISA indicating that the antibody was capable of recognizing heat denatured antigen (data not shown). Using purified β -LG, the Westernblot demonstrated that both charged property (native-PAGE) (Fig. 8A) and molecular forms (SDS-PAGE) (Fig. 8B) of β -LG were significantly altered upon the heating. Large molecular forms (such as aggregates) of immunoreactive β-LG were demonstrated in Fig. 8B. While, there was no immunoreactive β-LG at 25 kDa (Fig. 8B) further indicating that heat treatment on β-LG did not generate a 25kDa as that displayed in the processed milk
(SDS-PAGE in Fig. 2). Furthermore, Westernblot on commercially processed milk (whey proteins), both domestic and imported powdered milk revealed changes of molecular properties in β -LG as characterized by a native-PAGE (Fig. 9A). One single and no detectable alternation of β-LG was present in raw milk (Fig. 9A). Formation of multiple forms of large molecular weight of β-LG was observed in processed milk as characterized by a SDS-PAGE (Fig. 9B). However, such polymerization was less associated in powdered milk. Again, only raw milk and 4 brands from US revealed undetectable changes.
Discussion
Recent studies (4,5) have indicated that whey protein α -LA appeared to possess immunomodulatory propeties, conferring increased resistance to the growth of tumors. On the other hand, β-LG is associated with the hypocholesterolemic effect (5,7). Because almost all-dairy processes require heat treatments, information on the heat stability of milk with a modified protein component becomes an essential subject. It is conceivable that the dairy industry accounts for a large number of the knowledge and nature of molten globules, since the industry is concerned with improving the process of whole milk and concentrates as well as extending the products used for nutritional source (15). Our original purpose of the present study was to characterize the major changes of α-LA and β-LG, if any, of whey proteins in processed milk. Our study, however, revealed that the heating procedures used in domestic milk was significantly different from those in other countries. Whether or not it may commercially influence the textured dairy products (e.g. yogurt and cheese yield) or nutritional role is presently beyond our objective. β -LG was found severely denatured. If it was due to the excessive heating, it is certainly of worthy to avoid undesirable effects, such as the formation of deposits on walls of heating equipment (16) and the impaired renneting properties (17). Detailed knowledge of the denaturation behavior of β-LG is required to promote the
positive effects and to minimize the deleterious ones (18). In practice, we have found that the deposit of milk remnants did produce the damage on the radiators in some facility of our local dairy manufacturers. Since β-LG can form clot during the heating process (19-26), it may be responsible for such destructible event. The thermal denaturation curves (Fig. 4) and CD spectra (Fig. 5) show that the denaturation ofβ-LG was more rapid and extensive than that of α-LA in both time and temperature response. The result, however, is consistent with the previous reports where the extent of polymerization was limited in α -LA (26). The relatively weak hydrophobic interactions occur between unfolded α-LA that makes it high resistant to thermal changes as compared to β-LG (4).
Although the kinetics of denaturation of whey proteins in milk have been studied by native-PAGE (27), no information is available on the detailed kinetics of heat-induced conformational changes in α-LA and β-LG as judged by both CD and native-PAGE simultaneously (50-95 ℃ for 15s to 15 min). The present study may provide a valuable reference, in general, for the study of the correlation of conformational changes and electrophoretic properties in both α-LA and β-LG.
It is of worth to mention that only the whey proteins, rather than whole milk, were chosen for native-PAGE in the present study. This was due to the lipids (micelles) and casein in whole milk that can considerably affect the performance of gel electrophoresis as described by the others (10,27-29). Furthermore, since some β -LG could associate or polymerize with micelle and casein fractions in overheated whole milk (9,19,30-33), this association is responsible for the partial loss of β-LG in whey protein fraction. Evidently, it may explain the substantial loss of β-LG found in our domestically processed milk (Figs. 1 and 2), in which some loss of β -LG was not completely recovered in gel electrophoresis. Likewise, the immnoreactive β-LG blotted as in multiple large forms (Fig. 9A and 9B) may
sorely represent the partial loss of total β-LG in the processed milk. Nevertheless, the result clearly demonstrated that the Westernblot technique was relatively sensitive in detecting the thermal changes in β-LG, which has not been reported previously. It also suggests that the electrophoretic changes of β-LG found in native-PAGE were structurally irreversible. Thus, using a simple native PAGE as well as a Westernblot, β-LG may be used as a marker for the quality control in thermal process of manufactured milk.
參考文獻
1. Chang, J.-Y., Bulychev, A., and Li, L. (2000) A stabilized molten globule protein. FEBS Lett. 487, 298-300.
2. Svensson, M., Sabharwal, H., Hakansson, A., Mossberg, AK., Lipniunas, P., Leffler, H., Svanborg, C., and Linse, S. (1999) Molecular characterization of alpha-lactalbumin folding variants that induce apoptosis in tumor cells. J. Biol. Chem. 274, 6388-6396.
3. Håkansson, A., Zhivotovsky, B., Orrenius, S., Sabharwal, H., and Svanborg, C. (1995) Apoptosis induced by a human milk protein. Proc. Natl. Acad. Sci. USA 92, 8064-8068. 4. Rattray, W., and Jelen, P. (1998) Thermal
stability of skim milk/whey protein solution blends. Food Res. Int. 30, 327-334.
5. Wong, C. W., and Watson, D. L. (1995) Immunomodulatory effects of dietary whey protein in mice. J. Dairy Res. 62, 359-368. 6. McIntosh, G. H, Regester, G. O., LeLeu, R.
K., Royle, P. J., and Smithers, G. W. (1995) Dairy products protect against dimethlhydrazine-induced intestinal cancers in rats. J. Nutr. 125, 809-816.
7. Nagaoka, S., Yu, F., Keiji, M., Takako, A., Kouhei, Y., Yoshihiro, K., Kojima, T., and Tamotsu, K. (2001) Identification of novel hypocholesterolemic peptides derived from bovine milk β -lactoglobulin. Biochem. Biophys. Res. Commun. 281, 11-17.
8. Schockker, E. P., Singh, H., and Creamer, L. K. (2000) Heat-induced aggregation of β -lactoglobulin A and B with α-lactalbumin. Int. Dairy J. 10, 843-853.
9. Anema, S. G., and Mckenna, A. B. (1996) Reaction kinetics of thermal denaturation of whey protein in heated reconstituted whole milk. J. Agric Food Chem. 44, 422-428. 10. Oldfield, D. J., Singh, H., Taylor, M. W.
and Pearce, K. N. (1998a) Kinetics of denaturation and aggregation of whey protein in skim milk heated in an ultra-high temperature (UHT) plant. Int. Dairy J. 8, 311-318.
11. McCreath, G. E., Owen, R. O., Nash, D. C., and Chase, H. A. (1997) Preparation and use of ion-exchange chromatographic supports based on perfluoropolymers. J. Chromatogr. A 773, 73-83.
12. Yang, S. J., and Mao, S. J. T. (1999) A simple HPLC Purification Procedure for Porcine Plasma Haptoglobin. J. Chromatagr. B 731, 395-402.
13. Huang, G. S., Wang, S. P., Chang, W. T., Sun, T. J., Wang, S. C., Chu, R., Mao, S. J. T. (1999) Intracellular processing generated MDA-lys epitope in foam cells. Life Sci. 65, 285-296.
14. Molloy, M., P., Herbert, B., R., Yan, J., X., Williams, K., L., and Gooley, A., A. (1977) Identification of wallaby milk whey proteins separated by two-dimensional electrophoresis, using amino acid analysis and sequence tagging. Electrophoresis 18, 1073-1078.
15. Sawyer, L., and Kontopidis, G. (2000) The core lipocalin, bovine β -lactoglobulin. Biochim. Biophys. Acta 1482, 136-148. 16. De Jong, P. (1996) Modeling and
optimization of thermal processes in the dairy industry. NIZO Verslag, Ede, V341. 17. Lieske, B. (1997) The influence of
preliminary treatments on structural properties of casein micelles affecting the rennetability. Le Lait 77, 201-209.
18. Lieske, B., Konrad, G.,and Faber, W. (1997) A new spectrophotometric assay for native β -lactoglobulin in raw and processed bovine milk. Int. Dairy J. 7, 805-812.
19. Ruegg, M., Morr, U., and Blanc, B. (1977) A calorimetric study of the thermal denaturation of whey protein in simulated milk ultrafiltrate. J. Dairy Res. 44, 509-520. 20. De Wit, J. N., Klarenbeek, G. and
Hontelez-Backx, E. (1983) Evaluation of functional properties of whey protein concentrates and whey protein isolates. I. Isolation and characterization. Neth. Milk Dairy J. 37, 37-49.
21. Bernal, V. and Jelen, P. (1984) Effect of calcium binding on thermal denaturation of bovine α -lactalbumin. J. Dairy Sci. 67, 2452-2454
22. Paulsson, M., Hegg, P. O. and Castberg, H. B. (1985) Thermal stability of whey proteins studied by differential scanning calorimetry. Thermochim. Acta 95, 435-440.
23. Paulsson, M., and Dejmek, P. (1990) Thermal denaturation of whey protein in mixtures with casein studied by differential scanning calorimetry. J. Dairy Sci. 73, 590-600.
24. Xiong, Y. L., Dawson, K. A., and Wan, L.
(1993) Thermal aggregation of β
-lactoglobulin: effect pH, ionic environment, and thiol reagent. J. Dairy Sci. 76, 70-77. 25. Hollar, C. M., Parris, N., Hsieh, A. and
Cockley, K. D. (1995) Factors affecting the denaturation and aggregation of whey protein in whey protein concentrate mixtures. J. Dairy Sci. 78, 260-267.
26. Chaplin, L. C., and Lyster, R. L. J. (1986) Irreversible heat denaturation of bovineα -lactalbumin. J. Dairy Res. 53, 249-258. 27. Oldfield, D. J., Singh, H., and Taylor, M. W.
(1998b) Association of β -lactoglobulin and α -lactalbumin with the casein micelles in skim milk heated in an ultra-high temperature plant. Int. Dairy J. 8, 765-770.
28. Oldfield, D. J., Singh, H., Taylor, M. W. and Pearce, K. N. (2000) Heat-induced interactions of β -lactoglobulin and α -lactalbumin with the casein micelles in pH-adjusted skim milk. Int. Dairy J. 10, 509-518.
29. Paulsson, M., Hegg, P., and Castberg, H. B. (1986) Heat-induced gelation of individual whey protein. A dynamic rheological study. J. Food Sci. 52, 87-90.
30. Hines, M. E., and Foegeding, E. A. (1993) Interactions of α-lactalbumin and bovine
serum albumin with β -lactoglobulin in thermally induced gelation. J. Agric. Food Chem. 41, 341-346.
31. De wit, J. N. (1981) Structural and functional behaviour of whey proteins. Neth. Milk Dairy J. 35, 47-64.
32. Wang, Q., and Swaisgood, H. E. (1993) Characteristics of β-lactoglobulin binding
to the all-trans retinal moiety covalently immobilized on celite. J. Dairy Sci. 76, 1895-1901.
33. Jang, H. D., and Swaisgood, H. E. (1990) Disulfide bond formation between thermally denatured β -lactoglobulin and κ-casein in casein micelles. J. Dairy Sci. 73, 900-904.
34.
Table 1: Amino sequence of acidic proteins isolated from the
native-PAGE shown in Fig. 1
Amino sequence
Ac1 LIVTQTMKGLDIQKVAGTWY
Ac2 LIVTQTMKGLDIQKVAGTWY
Fig. 1. Native-PAGE analysis on whey proteins obtained from raw (A) and processed (B) milk. Ten ug of the protein sample were loaded onto each lane. There was a marked decrease in two acidic proteins in the processed milk as compared to that in the raw milk. These two acidic-proteins were subsequently eluted from a transfer blot followed by an amino acid sequencing and was identified as two isoforms of β-LG (Table 1).
A
B
β-LG β-LG α-LA
Fig. 2. SDS-PAGE analysis on whey proteins obtained from raw (A) and processed (B) milk. Ten ug of the protein sample were loaded onto each lane without a conventional pre-heating procedure. M: Markers with each molecular weight standard expressed in kDa, while Lane C was a purified standard of β-LG (18.5 kDa) and α-LA (14 kDa), respectively.
6.5
16
21
31
45
66
97
116
200
M
A
B
C
KDa LG LAFig. 3. Native-PAGE analysis on whey proteins obtained from raw, processed, and powdered milk. Lane 1: Freshly prepared raw milk (from Taiwan); Lanes 2 to 6: Processed milk (5 major brands from Taiwan); Lanes 7 to 9: Powdered milk (from Denmark, Australia, and New Zealand, respectively); Lanes 10 to 13: Processed milk (4 brands from US). Whey proteins loaded on lanes 1 and 2 were freshly prepared from the same batch, and were obtained from a university dairy farm before and after the process.
Fig. 4. Native-PAGE analysis on isolated β-LG and α-LA (1mg/mL)heated at 95 ℃ over time. Lane 1: Native β-LG and α-LA without heat; Lanes 2 to 7: Heated β-LG and α-LA at various time from 30, 60, 120, 240, 480, and 960 seconds, respectively. Ten ug of each protein were loaded on the native gel.
1
2
3
4
5
6
7
β-LG β-LG α-LA
Fig. 5. Effect of heating temperature and time on the loss of α-LA (A) and β-LG (B). The loss of each protein upon the heating was extrapolated from the native-PAGE (similar to that conducted in Fig. 4), while using the sample without heat as 0 % loss. The experiment was conducted independently at various temperatures as indicated : 50 (●); 60 (○); 70 (■) ; 80 (□); 90 (▲); and 95 (△) ℃.
0
20
40
60
80
100
120
140
-50
150
350
550
750
950
Time (sec)
% Loss of protein
50
60
70
80
90
95
A. a-LA
0
0
20
40
60
80
100
120
140
-50
150
350
550
750
950
Time (sec)
% Loss of protein
50
60
70
80
90
95
B. ß-LG
0
Fig. 6. Circular dichroic spectra of heated α- LA and β-LG at various temperatures. A: α- LA heated at 50 ( ), 60 ( --- ), 70 ( ), 80 ( ), 90 ( ) and 95 ℃ ( ) for 15 s to 15min; B: β-LG heated at 50 ( ), 60 ( --- ), 70 ( ), 80 ( ), 90 ( ) and 95℃ ( ) for 15 s to 15min. Notably, the spectrum of unheated was identical to that heated at 50 ℃ for 15 sec (data not shown). α-LA appears to be more resistant to heat than that of LG.
95
50
60
70
80
90
100
A. α-LA
B. β-LG
200 210 220 230 240 250 -12 -10 - 8 - 6 - 4 - 2 0 2 15s 50 60 70 80 90 100 ELLIPTICITY[ ? ] x10 -3 (deg cm 2/dmole) WAVELENGTH (nm) 200 210 220 230 240 250 -12 -10 - 8 - 6 - 4 - 2 0 2 1min 50 60 70 80 90 100 ELLIPTICITY[ ? ] x10 -3 (deg cm 2/dmole) WAVELENGTH (nm) 200 210 220 230 240 250 -12 -10 - 8 - 6 - 4 - 2 0 2 16mins 50 60 70 80 90 100 ELLIPTICITY[ ? ] x10 -3 (deg cm 2/dmole) WAVELENGTH (nm) 200 210 220 230 240 250 -12 -10 - 8 - 6 - 4 - 2 0 2 2mins 50 60 70 80 90 100 ELLIPTICITY[ ? ] x10 -3 (deg cm 2/dmole) WAVELENGTH (nm) 200 210 220 230 240 250 -12 -10 -8 -6 -4 -2 0 2 15s 50 60 70 80 90 100 ELLIPTICITY[ ? ] x10 -3 (deg cm 2/dmole) WAVELENGTH (nm) 200 210 220 230 240 250 -12 -10 -8 -6 -4 -2 0 2 1min 50 60 70 80 90 100 ELLIPTICITY[ ? ] x10 -3 (deg cm 2/dmole) WAVELENGTH (nm) 200 210 220 230 240 250 -12 -10 -8 -6 -4 -2 0 2 2mins 50 60 70 80 90 100 ELLIPTICITY[ ? ] x10 -3 (deg cm 2/dmole) WAVELENGTH (nm) 200 210 220 230 240 250 -12 -10 -8 -6 -4 -2 0 2 16mins 50 60 70 80 90 100 ELLIPTICITY[ ? ] x10 -3 (deg cm 2/dmole) WAVELENGTH (nm) 15minEllipticity [
Θ
] x 10
-3(
deg cm
2/dmole
)
15min .. .. .. __.. __.. __.. __.. .. .. ..Fig. 7. Thermal denaturation curves based on the maximal changes of the ellipticity of α- LA (A) and β-LG (B) heated at 50 (●), 60 (○), 70 (■), 80 (□), 90 (▲) and 95 (△) ℃. The data were extrapolated from Fig. 6.
0 20 40 60 80 100 120 -100 100 300 500 700 900
Heating time (sec)
Structure of change (%) of [T] at 205 nm 0
B. ß-LG
0 20 40 60 80 100 120 -100 100 300 500 700 900Heating time (sec)
Structure of change (%) of [T] at 208 nm
0
A. Native - PAGE
B. SDS - PAGE
Fig. 8. Characterization of heated β -LG using a Westernblot analysis on native-PAGE (A) and SDS-PAGE (B). The experiment was carried out by heating purified β-LG (1mg/ml) at 95℃ prior to the reaction with a polyclonal β-LG antibody. Time course was expressed in seconds as indicated. In panel B, there were no breakdown or aggregates of β-LG on the initial sample (time 0) and no aggregate was observed at 25 kDa as that shown in Fig. 2.
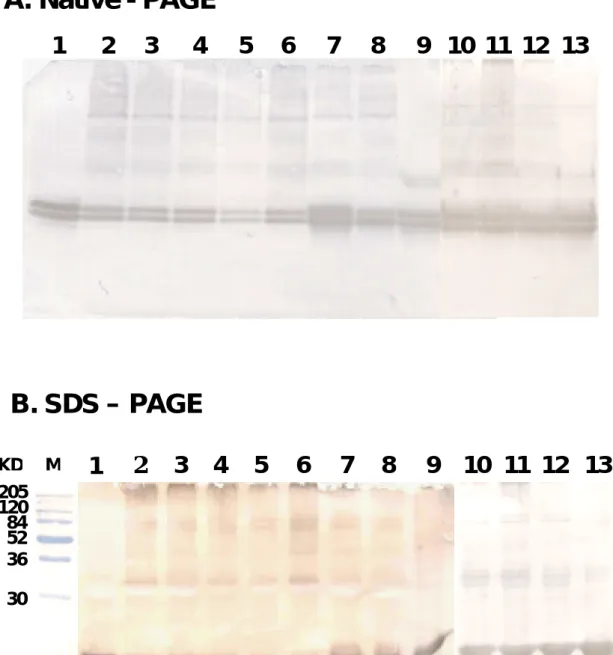
7.4 84 120 205 36 52 30 M 0 15 30 60 120 240 480 900 0 15 30 60 120 240 480 900
A. Native - PAGE
B. SDS – PAGE
Fig. 9. Characterization of the β-LG component following a heat on whey proteins of raw, processed, and powdered milk using a Westernblot analysis on native-PAGE (A) and SDS-PAGE (B). The specificity of the antibody was confirmed by the formation of a single band against the whole whey proteins. Lane 1: Freshly prepared raw milk (from Taiwan); Lanes 2 to 6: Five major brands of processed milk (from Taiwan); Lanes 7 to 9: powdered milk (from Australia, New Zealand, and Denmark, respectively); Lanes 10 to 13: Four brands of processed milk (from US ). Again, there was no immunoreactive β-LG at 25kDa. Notably, the decrease in β-LG was not as sharp as that characterized on Coomassie blue staining due to the extremely high sensitivity of the immunoblot. 7.4 84 120 205 36 52 30 KD M