Static magnetic field attenuates mortality rate of mice by increasing the
production of IL-1 receptor antagonist
SHU-LI LIN
1, WEI-JEN CHANG
2, YUNG-SHENG LIN
3, KENG-LIANG OU
4,5,
CHE-TONG LIN
2, CHIH-PING LIN
2, & HAW-MING HUANG
41
Dental Department, Cathay General Hospital, Taipei,2School of Dentistry, Taipei Medical University, Taipei,3Instrument Technology Research Center, National Applied Research Laboratories, Hsinchu,4Graduate Institute of Biomedical Materials and Engineering, Taipei Medical University, Taipei, and5Research Center for Biomedical Implants and Microsurgery Devices, Taipei Medical University, Taipei, Taiwan
(Received 23 July 2008; Revised 22 April 2009; Accepted 23 April 2009)
Abstract
Purposes: Disseminated intravascular coagulation (DIC) is a complex systemic thrombohemorrhagic disorder involving intravascular coagulation and hemorrhage. The aim of this study is to test whether static magnetic field (SMF) is effective in attenuating lipopolysaccharide (LPS)-induced DIC.
Materials and methods: In vivo experiments were performed in this study using male BALB/cByJ mice. An intraperitoneal injection of 50 mg/kg LPS was shown to lead to approximately 50% mortality and this dose was used in subsequent experiments. To test the effects of SMF on the survival rate of LPS-induced animals, the mice were exposed to 0.25-T SMF for 2 h before LPS injection. In addition, the effect of a 2-h SMF treatment on the production of anti-inflammatory cytokines was evaluated.
Results: In the first set of experiments, we found that the survival rate was higher in the SMF-exposed group than in the sham-exposed group. The circulating platelet (PLT) counts in the SMF-exposed mice were significantly higher than in the unexposed animals. However, no significant changes in inflammatory cytokine, including tumour necrosis factor-a (TNF-a), interleukin-1a (IL-1a), interleukin-6 (IL-6) and monocyte chemotactic protein 1 (MCP-1), in plasma were found after SMF treatment. The results from the second experiment showed that the plasma levels of interleukin-1 receptor antagonist (IL-1ra) were higher in the SMF-exposed group than in the sham group.
Conclusions: Exposure to an SMF increases the plasma levels of IL-1ra. This effect may inhibit the reduction in PLT in plasma, resulting in prevention in LPS induced DIC.
Keywords: Static magnetic field, disseminated intravascular coagulation, lipopolysaccharide, proinflammatory cytokine
Introduction
Septic shock is a severe clinical condition caused by decreased tissue perfusion and oxygen delivery as a result of infection in the circulating blood. Clinical findings indicate that the mortality rate associated with septic shock has been increasing over the past several decades (Hardaway 2000, Sato et al. 2004). Disseminated intravascular coagulation (DIC), an insidious condition that develops frequently in patients with septic shock, is the major cause of the high mortality rate in these patients (Fourrier et al. 1992).
DIC is a pathological syndrome characterised by the imbalance between inflammation, coagulation, and fibrinolysis (Bakhshi and Arya 2003). DIC in patients with septic shock leads to a depletion of platelets, resulting in markedly accelerated systemic coagulopathy and microvascular thrombosis (Sato et al. 2004). Platelet (PLT) activation plays an important role in this pathogenesis of DIC and organ failure associated with sepsis (Weiss and Rashid 1998). It causes platelet accumulation and platelet-platelet aggregation at inflammation sites, which results in a decrease in platelet numbers in the bloodstream (Weiss and Rashid 1998).
Correspondence: Dr Haw-Ming Huang, Graduate Institute of Biomedical Materials and Engineering, Taipei Medical University, 250 Wu-Hsing Street, Taipei, Taiwan. Tel: 886 2 2736 1661, ext. 5128. E-mail: hhm@tmu.edu.tw
ISSN 0955-3002 print/ISSN 1362-3095 online Ó 2009 Informa Healthcare USA, Inc. DOI: 10.1080/09553000902993908
Int J Radiat Biol Downloaded from informahealthcare.com by Taipei Medical University on 05/30/11
Lipopolysaccharide (LPS), the component of the outer membrane of Gram-negative bacteria, plays an important role in the initiation of the DIC (Weiss and Rashid 1998). It triggers the release of thrombin by host cells, which also induces the expression of interleukin-1 (IL-1), interleukin-6 (IL-6) and tu-mour necrosis factor (TNF) in activated monocytes and macrophages. These inflammatory cytokines are thought to play a major role in the pathogenesis and acceleration of LPS-induced DIC (Bakhshi and Arya 2003, Toh and Dennis 2003, Dempfle 2004, Ergonul et al. 2006).
Previous studies have focused on inhibitors of coagulation (Norman et al. 2003, Slofstra et al. 2006). Animal studies have demonstrated that Immunoglobulin (IG) preparations attenuate tissue damage and consumption coagulopathy by suppres-sing the production of cytokines in LPS-induced DIC models (Gullestad et al. 2001, Kishimoto et al. 2003, Asakura et al. 2006). Administration of anti-endotoxin antibodies is another treatment strategy for treating Gram-negative sepsis (Lynn 1998, Rothenburger et al. 2001). However, the benefits of IG administration and antiendotoxin therapy remain controversial (Nahra and Dellinger 2008). Heparin is
also used in treatment of LPS-induced DIC
(Cummins et al. 2001). However, previous clinical studies demonstrate that heparin is not an effective treatment of DIC (Feinstein 1982, Weiss and Rashid 1998). Interleukin-1 receptor antagonist (IL-1ra), a member of the IL-1 family, is produced mainly by monocytes and macrophages after LPS stimulation (Dinarello 1998). It was reported that IL-1ra has an anti-inflammatory effect by binding to IL-1 recep-tors, thereby rendering IL-1 incapable of inducing an inflammatory response (Dinarello 1998). Clinical findings indicate that administration of IL-1ra reduces pathological processes such as septic shock and inflammatory disease (Tilg et al. 1997, Ashdown et al. 2007). Although the subject of years of investigation, clinical applications of IL-1ra for endotoxin-induced DIC remain unavailable. Thus, development of a new method for attenuating the effects of LPS- and sepsis-induced DIC is valuable.
In 1999, Salerno et al. found, for the first time, that exposure to a static magnetic field (SMF) of 0.5 T generated by an magnetic resonance imaging (MRI) unit may induce modifications in the expres-sion of some IL in peripheral blood mononuclear cells (PBMC) (Salerno et al. 1999). Since then, numerous investigations have focused on the effects of continuous SMF on the inflammatory responses in animals and cytokine release by PBMC. Although modifications in release of TNF-a and IL-1 were not detected after short-duration SMF exposure (Salerno et al. 1999, Aldinucci et al. 2003),
long-duration, continuous SMF exposure was found to increase the level of LPS-induced expression of IL-1ra, resulting in suppression of LPS-induced cytotoxicity (Lin et al. 2008). In this study, we investigated whether a static magnetic field is effective in attenuating LPS-induced immune host response in an animal model.
Materials and methods Animal model
Healthy, 5-week-old male BALB/cByJ mice weighing 20–25 g were used as test subjects to qualitatively measure the effects of SMF on DIC. The mice were
obtained from the Laboratory Animal Center,
National Applied Research Laboratories (Hsinchu, Taiwan). All mice were maintained at the animal care facility at the Experimental Animal Center, Taipei Medical University. All of the experimental procedures were approved by the Experimental Animal Ethic Committee, Taipei Medical Univer-sity. The animals were acclimated in the laboratory for at least three days before the experiments were performed. Throughout the experimental period, the mice, kept at a constant room temperature of 208C, were fed a standard diet and had free access to distilled water.
LPS challenge
LPS derived from Escherichia coli (055: B5, Sigma, St Louis, MO, USA) was dissolved in physiologic saline before each experiment. The 50% toxicity concentration (TC50), was defined as the LPS concentration that results in a 50% reduction in survival relative to the control group. To test TC50, mice were challenged with LPS by intraperitoneal injection of 100m1 LPS, at a serial dosage (range
0–200 mg/kg). The survival rate of the
LPS-challenged animals was recorded at 24 h. In the control group, sterile saline was administered to the tested animals via intraperitoneal injection. For a test, six animals were used to calculate the survival rate in each dose group. Triplicate tests were performed for each experimental data. In the further assay, the resulting TC50 was used in all of the following experiments.
SMF exposure
The animals were divided into two groups: the sham-exposed group and SMF-sham-exposed group, and were separately seeded inside two identical plastic animal cages measuring 146868 cm. A pair of neodymium (Nd2Fe14B) magnets measuring 1367 cm with a thickness of 1 cm (Chief-Lion Enterprise Co.,
Int J Radiat Biol Downloaded from informahealthcare.com by Taipei Medical University on 05/30/11
Tainan, Taiwan) was used to produce the SMF (Figure 1). The magnetic poles of the two magnets horizontally faced each other on the outer surfaces of the top and bottom sides of the animal cages. The materials of the rare earth magnets were isolated from the surrounding environment by coating the magnets with a thin layer of nickel. The average magnetic flux density on the surface was monitored by a handheld Gauss meter (Model 5070, FW BELL, Orlando, FL, USA), which showed a flux of 0.4 Tesla (T) at the borders of the magnets. The area where the animals are actually exposed was mea-sured as 0.25 T. In the sham-exposed group, neodymium disks of the same geometric size as the experimental magnets were placed on the second matched animal cages. The neodymium disks were not magnetised, thus the flux density value in the experimental environment was no greater than the level of the natural magnetic field of the earth (0.05 mT).
To test the optimal period for SMF exposure, mice were treated with SMF for 2 h before or after LPS injections, with the TC50 concentration deter-mined in the experiment described above. The experimental mice were divided into three groups of 18 mice, and then exposed to SMF before LPS administration for 0, 1 and 2 h, respectively (defined as pre-exposure). In each pre-exposed group, three sub-groups continuously received SMF treatment after LPS injection for 0, 1 and 2 h, respectively (defined as post-exposure). The survival rate of the LPS-challenged animals was recorded at 48 h after LPS injection. In each sub-group, six animals were used for the calculation of survival rate. Data were obtained from three independent experiments. The optimal SMF exposure protocol, with a maximum survival rate, was determined by screening the various exposure conditions, and used as the SMF treatment method in the following experiments. Subsequently, anti-DIC effects of 0.25 T SMF were observed by comparing the survival rates between
SMF-exposed and sham-exposed (without
pre-exposure and post-pre-exposure) animals.
Platelet counts
To assess the effects of 0.25 T SMF on platelet counts of the experimental mice, separate experi-mental animals were pre-exposed and sham-exposed to 0.25 T SMF. Blood was withdrawn from the heart of the experimental mice using a plastic syringe at 0, 1, 2, 4 h after LPS administration. Mice were scarified under deep anesthesia immediately after blood sampling. Before the assay, the blood samples were mixed with 2 mg/ml ethylenediaminetetraacetic acid (EDTA, Sigma). Platelet counts were per-formed with an automated veterinary hematology analyser (ABC Vet, HORIBA ABX, Montpellier, France). For both SMF-exposed and sham-exposed groups, nine animals were used at each time point.
Cytokines assay
Blood was withdrawn from the heart of the experi-mental DIC mice using a plastic syringe at 0, 2, 4, 8 h after LPS administration. Citrated plasma samples were obtained by centrifugation of whole blood. The supernatant of the samples was collected for cytokine expression assay. Mice were scarified under deep anaesthesia immediately after blood sampling. Detection of cytokine production in plasma was performed using a cytokine antibody array kit (RayBio tech, Norcross, GA, USA). Briefly, 1 ml of 10-fold diluted serum was incubated on a membrane, labeled with 10 different cytokine anti-bodies in duplicate, for 2 h at room temperature. After a series of wash procedures, biotin-conjugated antibodies were added, and the membrane was incubated for an additional 2 h. Horseradish perox-idase-conjugated streptavidin (2.5 pg/ml) was added to the assay for 1 h. Cytokines were detected by exposing the membrane to a film. The images were then developed using a film processor (Kodak M35A Processor, Rochester, New York, USA). Quantifica-tion of the cytokine changes were performed by measuring the related gray level of the positively stained spots using an image analysis software (Image Pro Plus, Media Cybernetics, Inc., Silver Spring, MD, USA).
In order to test the effects of SMF on expression of the anti-inflammatory cytokine IL-1ra, an IL-1ra assay was performed on mice without LPS admin-istration. The experimental animals were pre-exposed and sham-pre-exposed to 0.25 T SMF. Blood was withdrawn from the heart of the experimental mice at 0, 2, 4, 8 h as described above. Plasma levels of IL-1ra in tested mice were determined using a commercialised enzyme-linked immunosorbent as-say (ELISA) kit (R and D System Inc., Minneapolis, MN, USA). During detection, 50m1 serum samples were incubated in microtiter wells precoated with Figure 1. Schematic representation showing the SMF-exposure
system used in this study.
Int J Radiat Biol Downloaded from informahealthcare.com by Taipei Medical University on 05/30/11
anti-IL-1ra antibody. After washing and aspiration of other components, the wells were incubated with a biotinylated polyclonal antibody. Streptavidin horseradish peroxidase activity was determined by the addition of tetramethylbenzidine. The result was read spectrophotometrically at a wavelength of 450 nm (Model 2020; Anthos Labtec Instruments, GmbH, Wals/Salzburg, Austria). In this assay, data were obtained from nine samples.
Statistical analysis
All data measured are presented as mean+ standard deviation (SD). For all assays, one-way analysis of variance (ANOVA) with least significant difference (LSD) multiple comparison was used to test the difference at various time points. Student’s t-test was used to assess the differences between the control and SMF-exposed LPS-induced animals. A p value lower than 0.05 was considered statistically signifi-cant for all tests.
Results
In the TC50 test experiments, LPS-induced DIC assay revealed a dose-dependent decrease in mortal-ity rate in LPS-administered mice (Figure 2). When the LPS concentrations reached 50 mg/kg, the detected survival rate of the LPS-administered mice at 24 h (44.4%) was almost half that of the control counterparts. Thus, in the following experiments, the TC50was defined as 50 mg/kg.
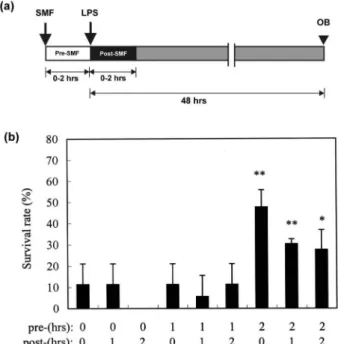
In the experiments for testing the optimal period of SMF exposure, the survival rate of LPS-injected mice that had been pre-exposed to SMF for 0 and 1 h (Figure 3a) was around 10% at 48 h. In LPS-injected mice that had been pre-exposed to SMF for 2 h, the survival rate significantly increased
to 47.7% at the same observational time ( p5 0.01) (Figure 3b). That is, the survival rate was higher in the SMF-exposed group than in the sham-exposed group. However, SMF exposure after LPS injection significantly attenuated the inhibitory effect. The survival rates of mice exposed to SMF for 1 and 2 h after LPS administration were 30.3% and 27.7% at 48 h, respectively. According to these findings, 2-h SMF pre-exposure before LPS injection without additional post-exposure to SMF was chosen as the optimal SMF treatment condition, and was adopted throughout the experiments of platelet counts and inflammatory assays.
Figure 4 demonstrates the SMF effects on
platelet counts of the test animals. In Figure 4, a decreasing trend in circulating platelet counts of the untreated animals was noted at 2 and 4 h after LPS injection. Interestingly, there is no decrease in platelet count with the SMF-treated animals. At 2 and 4 h after LPS treatment (Figure 4a), the circulating platelet counts in the SMF-exposed mice were 1.27 and 1.70-fold higher, respectively, than those in the unexposed animals (Figure 4b) ( p5 0.05).
To determine differences in cytokine induction between SMF-treated and untreated groups, 10 cytokines were detected using a cytokine antibody array composed of ten cytokine antibodies spotted on
Figure 2. Cytotoxic effects of LPS-induced DIC models at different concentrations; LPS concentration is inversely related to survival rate. Error bars indicate the standard deviation of the mean for n¼ 3 independent experiments (**p 5 0.01).
Figure 3. (a) Time-line of the survival rates experiments. White, black and grey bars represented pre-SMF exposed, post-SMF exposed and the observational time, respectively. (b) Comparison of survival rates at 48 h between the nine exposure sub-groups. Error bars indicate the standard deviation of the mean for n¼ 3 independent experiments (Pre: pre-exposure; Post: post-exposure; OB: observational time). (*p5 0.05; **p 5 0.01).
Int J Radiat Biol Downloaded from informahealthcare.com by Taipei Medical University on 05/30/11
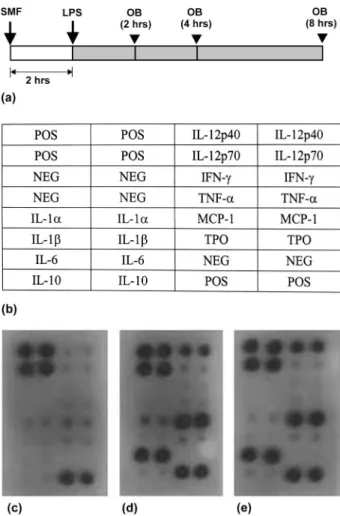
a membrane. Figure 5 illustrates an example of this assay at 4 h after LPS administration (Figure 5a). Positively stained spots of proinflammatory cyto-kines, including IL-1a, IL-6, IL-12 and monocyte chemotactic protein-1 (MCP-1), are clearly identifi-able in the blood sample from an LPS-administered mouse without SMF pre-exposure (Figure 5d); however, there was no obvious change in expression of cytokines after SMF treatment (Figure 5e,
Table I). The staining of anti-inflammatory
cytokine, IL-10, was negative. There was no above-background staining of IL-10 in the blood samples from the SMF-exposed and sham-exposed mice. Similar results were obtained at the other observation time points (data not shown).
The effects of the SMF on anti-inflammatory cytokine production in the experimental mice were tested for 8 h without LPS (Figure 6a). In this study, continuous SMF stimulation over 2 h tended to increase production of IL-1ra in the blood stream. As shown in Figure 6b, IL-1ra production at 2, 4 and 8 h was significantly higher in the SMF-exposed mice than in the control group (2.0-, 1.7- and 1.8-fold for 2, 4 and 8 h, respectively).
Discussion
Lipopolysaccharide is frequently used to induce the coagulation pathway in experimental DIC animal models (Sato et al. 2004, Asakura et al. 2006, Ergonul et al. 2006, Slofstra et al. 2006). We found
that the LPS-induced mortality rate in experimental animals pre-treated with 2-h SMF was significantly reduced ( p5 0.05) at 48 h after LPS infusion (Figure 3). Lin et al. (2008) also found that con-tinuous SMF exposure has an attenuation effect on immune response of LPS-challenged fibroblasts.
Reduced platelet count is a hallmark of consump-tion coagulopathy after LPS infusion (Weiss and Rashid 1998, Norman et al. 2003, Remick 2007),
Figure 4. (a) Time-line of the platelet count experiments. (b) Comparisons of platelet counts between LPS-induced DIC mice with and without SMF exposure. Error bars indicate the standard deviation of the mean for n¼ 3 independent experiments (*p5 0.05; **p 5 0.01).
Figure 5. (a) Time-line of the cytokine experiments. (b) The alignment of the cytokine arrays. (c) stained cytokines at 0 h. (d) and (e) cytokines expression at 4 h after LPS-administration without and with SMF pre-treatment. POS, positive; NEG, negative; IL, interleukin; IFN, interferon; TNF, tumour necrosis factor; MCP, Monocyte chemoattractant protein; TPO, thrombo-poietin.
Table I. Quantification of the positively stained spots of proin-flammatory cytokines demonstrated in Figure 5. Errors indicate the standard deviation of the mean for n¼ 3 independent experiments.
IL-12 p40 IL-1a IL-6 MCP-1
SMF exposure 0.34+ 0.08 0.11 + 0.05 0.49 + 0.06 0.47 + 0.08 Sham exposure 0.25+ 0.06 0.17 + 0.04 0.40 + 0.05 0.38 + 0.04
Int J Radiat Biol Downloaded from informahealthcare.com by Taipei Medical University on 05/30/11
and increased levels of this marker suggest attenua-tion of consumpattenua-tion coagulopathy. In this study, infusion of LPS resulted in a reduction in platelet counts in a dose-dependent manner. However, this reduction was completely inhibited by pre-exposing the experimental animals to a 0.25-T SMF for 2 h (Figure 4b).
Low-dose radiotherapy has been used clinically as an anti-inflammatory treatment. It is believed that the effect of such treatment is through modulation of cytokine production (Rodel et al. 2007). However, long-term treatment with low-dose radiotherapy is risky because of the problem of dose-accumulation. SMF, however, does not involve ionising radiation, which makes it potentially useful in clinical practice (Lin et al. 2008).
Although it is still hard to draw any conclusions regarding the therapeutic effects of SMF, the impact of SMF on human health and its use in the therapeutic treatment is an ancient and contentious subject that remains so to the present (World Health Organisation 2006). The major concern for medical application regards the adverse health effects of such exposure; however, no demonstrable effects of Tesla-scaled SMF exposure on the behaviour or on the growth and development of tumours have been reported (Saunders 2005, World Health Organisa-tion 2006).
It has been reported that anti-inflammatory mediators, such as IL-10, play an important role in disease regression (Short 2004, Kakafika et al. 2007).
However, in the blood samples of SMF-exposed and sham-exposed mice, there was no positive staining of this cytokine after LPS administration (Figure 5). These results suggest that the anti-DIC effect of SMF is independent on the expression of IL-10.
The results of the cytokine array study showed that the amounts of IL-1a, IL-6, IL-12 and MCP-1 in circulation increased significantly after LPS stimula-tion (Figure 5). The net effect of the first three proinflammatory cytokines is the development of systemic microthrombi, and, hence, the develop-ment of DIC (Toh and Dennis 2003, Norman et al. 2003, Ergonul et al. 2006, Asakura et al. 2006). In addition, MCP-1 is an essential chemokine involved in monocyte traffic during inflammation. Therefore, suppression of the production of these cytokines might be an efficient strategy for attenuating the development of DIC (Asakura et al. 2006). Although previous investigations have focused on the effects of continuous SMF on inflammatory responses in animals (Weinberger et al. 1996) and on cytokine release by human peripheral blood mononuclear cells (Aldinucci et al. 2003), our results failed to demonstrate a reduction in such cytokines after SMF treatment (Figure 5).
IL-1ra is mainly produced by monocytes and macrophages after stimulation with LPS. IL-1ra exerts its anti-inflammatory effect by binding to IL-1a and IL-1b (Feinstein 1982). Several recent studies have found that administration of IL-1ra led to remarkable improvement in patients with inflam-matory disease and septic shock (Goldbach-Mansky et al. 2006, Ashdown et al. 2007, Remick 2007). It was, therefore, suggested that elevated levels of IL-1ra in plasma could serve as a marker of non-fatal infection (Ergonul et al. 2006). In this study, we found that pre-treatment of SMF increases IL-1ra production in mice (Figure 6b). These results indicate that the possible mechanism by which SMF prevents the development of DIC is via the upregulation of IL-1ra rather than via the down-regulation of proinflammatory cytokines. That is, the changes in this marker suggest that SMF exerts an anti-inflammatory effect by stimulating the binding of IL-1ra to IL-1 receptors, thereby rendering IL-1 incapable of inducing an inflammatory response (Dinarello 1998).
DIC always starts with a small amount of bacteria infecting a small localised area. The mortal risk of such bacterial infection is due to the following uncontrolled proliferation of the infecting bacteria and either the microbe and/or LPS travelling to the inner organs. It takes time for microbes and/or LPS to travel within the human body. Although pre-exposure with SMF before infection is not an option for treating DIC in humans, if an infection can be identified before it spreads throughout the body, Figure 6. (a) Time-line of the IL-1ra assay. (b) After SMF
pre-exposure, the amounts of IL-1ra in the blood stream differed significantly at the observation time points of 2, 4 and 8 h. Error bars indicate the standard deviation of the mean for n¼ 3 independent experiments (**p5 0.01).
Int J Radiat Biol Downloaded from informahealthcare.com by Taipei Medical University on 05/30/11
SMF pre-exposure for the uninfected organs should be helpful since, in our experiment, IL-1ra increased as a result of SMF.
Based on these observations, it appears reasonable to hypothesise that pre-exposure to 0.25 T SMF for 2 h might improve the outcome of LPS-induced DIC in mice by suppressing IL-1-induced consump-tion coagulopathy. Our evidence demonstrates that this suppressive effect may be achieved by upregulat-ing the amount of IL-1ra in the blood stream and by inhibiting the reduction of platelets in plasma.
Acknowledgements
This study was sponsored by a grant (96CGH-TMU-17) from the Cathay General Hospital, Taipei, Taiwan.
Declaration of interest: The authors report no conflicts of interest. The authors alone are respon-sible for the content and writing of the paper.
References
Aldinucci C, Garcia JB, Oalmi M, Sgaragli G, Benocci A, Meini A, Pessina F, Rossi C, Bonechi C, Pessina GP. 2003. The effect of strong static magnetic field on lymphocytes. Bioelectromagnetics 24:109–117.
Asakura H, Takahashi Y, Kubo A, Ontachi T, Hayashi T, Omote M, Arahata M, Kadohira Y, Maekawa M, Yamazaki M, Morishita E, Takami A, Yoshida T, Miyamoto K, Nakao S. 2006. Immunoglobulin preparations attenuate organ dysfunction and hemostatic abnormality by suppressing the production of cytokines in lipopolysaccharide-induced disseminated intravascular coagulation in rats. Critical Care Medicine 34:2421–2425.
Ashdown H, Poole S, Boksa P, Luheshi GN. 2007. Interleukin-1 receptor antagonist as a modulator of gender differences in the febrile response to lipopolysaccharide in rats. The American Journal of Physiology – Regulatory, Integrative and Compara-tive Physiology 292:R1667–1674.
Bakhshi S, Arya LS. 2003. Etiopathophysiology of disseminated intravascular coagulation. Journal of the Association of Physicians of India 51:796–800.
Cummins D, Segal H, Hunt BJ, Awad R, Maddox A. 2001. Chronic disseminated intravascular coagulation after surgery for abdominal aortic aneurysm: Clinical and haemostatic response to dalteparin. British Journal of Haematology 113:658–660. Dempfle CE. 2004. Coagulopathy of sepsis. Thrombosis and
Haemostasis 91:213–224.
Dinarello CA. 1998. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. International Reviews of Immunology 16:457–499.
Ergonul O, Tuncbilek S, Baykam N, Celikbas A, Dokuzoguz1 B. 2006. Evaluation of serum levels of interleukin (IL)- 6, IL-10, and tumor necrosis factor-A in patients with Crimean-Congo hemorrhagic fever. The Journal of Infectious Diseases 193:941–944.
Feinstein DI. 1982. Diagnosis and management of disseminated intravascular coagulation: The role of heparin therapy. Blood 60:284–287.
Fourrier F, Chopin C, Goudemand J, Hendryscx S, Caron C, Rime A, Marey A, Lestavel P. 1992. Septic shock, multiple
organ failure, and disseminated intravascular coagulation. Compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest 101:816–823.
Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, et al. 2006. Neonatal-onset multisystem inflamma-tory disease responsive to interleukin-1b inhibition. The New England Journal of Medicine 355:581–592.
Gullestad L, Aass H, Fjeld JG, Wikeby L, Andreassen AK, Ihlen H, Simonsen S, Kjekshus J, Nitter-Hauge S, Ueland T, Lien E, Frøland SS, Aukrust P. 2001. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation 103:220–225.
Hardaway RM. 2000. A review of septic shock. The American Surgeon 66:22–29.
Kakafika A, Papadopoulos V, Mimidis K, Mikhailidis DP. 2007. Coagulation, platelets, and acute pancreatitis. Pancreas 34: 15–20.
Kishimoto C, Shioji K, Kinoshita M, Iwase T, Tamaki S, Fujii M, Murashige A, Maruhashi H, Takeda S, Nonogi H, Hashimoto T. 2003. Treatment of acute inflammatory cardiomyopathy with intravenous immunoglobulin ameliorates left ventricular function associated with suppression of inflammatory cytokines and decreased oxidative stress. Inter-national Journal of Cardiology 91:173–178.
Lin CT, Lee SY, Chen CY, Chen CA, Lin CP, Huang HM. 2008. Long-term continuous exposure to static magnetic field reduces lipopolysaccharide-induced cytotoxicity of fibroblasts. International Journal of Radiation Biology 84:219–226. Lynn WA. 1998. Anti-endotoxin therapeutic options for the
treatment of sepsis. Journal of Antimicrobial Chemotherapy 41:A71–80.
Nahra R, Dellinger RP. 2008. Targeting the lipopolysaccharides: Still a matter of debate? Current Opinion in Anaesthesiology 21:98–104.
Norman KE, Cotter MJ, Stewart JB, Abbitt KB, Ali M, Wagner BE, Wallace WA, Forlow SB, Hellewell PG. 2003. Combined anticoagulant and antiselectin treatments prevent lethal intravascular coagulation. Blood 101:921–928. Remick DG. 2007. Pathophysiology of sepsis. The American
Journal of Pathology 170:1435–1444.
Rodel F, Keilholz L, Herrmann M, Sauer R, Hildebrandt G. 2007. Radiobiological mechanisms in inflammatory diseases of low-dose radiation therapy. International Journal of Radiation Biology 6:357–366.
Rothenburger M, Soeparwata R, Deng MC, Berendes E, Schmid C, Tjan TDT, Wilhelm MJ, Erren M, Bo¨ cker D, Scheld HH. 2001. The impact of anti-endotoxin core antibodies on endotoxin and cytokine release and ventilation time after cardiac surgery. Journal of the American College of Cardiology 38:124–130.
Salerno S, Lo Casto A, Caccamo N, D’Anna C, De Maria M, Lagalla R, Scola L, Cardinale AE. 1999. Static magnetic fields generated by a 0.5 T MRI unit affects in vitro expression of activation markers and interleukin release in human peripheral blood mononuclear cells (PBMC). International Journal of Radiation Biology 75:457–463.
Sato H, Konishi Y, Tanaka H, Takahashi O, Tanaka T. 2004. Annexin V inhibits lipopolysaccharide-induced procoagulant activity on human monocytes. Thrombosis Research 114:45–49. Saunders R. 2005. Static magnetic fields: Animal studies. Progress
in Biophysics and Molecular Biology 87:225–239.
Short MA. 2004. Linking the sepsis triad of inflammation, coagulation, and suppressed fibrinolysis toinfants. Advances in Neonatal Care 4:258–273.
Slofstra SH, Cate HT, Spek CA. 2006. Low dose endotoxin priming is accountable for coagulation abnormalities and organ damage observed in the Schwartzman reaction. A comparison between a single-dose endotoxemia model and a double-hit
Int J Radiat Biol Downloaded from informahealthcare.com by Taipei Medical University on 05/30/11
endotoxin-induced Shwartzman reaction. Thrombosis Journal 4:13–19.
Tilg H, Dinarello CA, Mier JW. 1997. IL-6 and APPs: Anti-inflammatory and immunosuppressive mediators. Immunol-ogy Today 18:428–432.
Toh CH, Dennis M. 2003. Disseminated intravascularcoagula-tion: Old disease, new hope. British Medical Journal 327: 974–977.
Weinberger A, Nyska A, Giler S. 1996. Treatment of experimental inflammatory synovitis with continuous magnetic field. Israel Journal of Medical Sciences 32:1197–1201.
Weiss DJ, Rashid J. 1998. The sepsis-coagulant axis: A review. Journal of Veterinary Internal Medicine 12:317–324. World Health Organisation. 2006. Environmental health criteria
232 – Static fields. Geneva: WHO Press, World Health Organisation.
Int J Radiat Biol Downloaded from informahealthcare.com by Taipei Medical University on 05/30/11