行政院國家科學委員會專題研究計畫 期中進度報告
奈米金屬及金屬化合物之合成(2/3)
計畫類別: 整合型計畫
計畫編號: NSC92-2113-M-009-022-
執行期間: 92 年 08 月 01 日至 93 年 07 月 31 日
執行單位: 國立交通大學應用化學系
計畫主持人: 裘性天
計畫參與人員: 裘性天 博士後張裕煦 研究生
報告類型: 精簡報告
報告附件: 出席國際會議研究心得報告及發表論文
處理方式: 本計畫可公開查詢
中 華 民 國 93 年 5 月 25 日
Synthesis of Silicon Carbide Nanostructures via Simplified Yajima Process - Reaction
at the Vapor-Liquid Interphase
Jia-Hsing Wang,1 Yu-Hsu Chang,1 Ming-Yu Yen,2 Chih-Wei Peng,1,3 Chi-Young Lee,4 and Hsin-Tien
Chiu1,*
1 Department of Applied Chemistry, National Chiao Tung University, Hsinchu, Taiwan 30050, R.O.C. 2 Materials Research Laboratories, Industrial Technology Research Institute, Hsinchu, Taiwan 31040, R.O.C. 3 Institut des Matériaux Jean Rouxel, Laboratoire de Physique Cristalline, 2 rue de la Houssinière, BP 32229, 44322
Nantes Cedex 3, France
4 Materials Science Center, National Tsing Hua University, Hsinchu, Taiwan, 30043, R.O.C. RECEIVED DATE (automatically inserted by publisher); E-mail: htchiu@cc.nctu.edu.tw Silicon carbide is a wide bandgap semiconductor material with
many potential high technology applications.1 There are many
ways to prepare silicon carbide powders and thin films. These include cabothermic reduction of silica,sol-gel synthesis, and various chemical vapor deposition techniques.2-5 In 1975, Yajima
reported a preceramic polymer route to prepare β-SiC fibers industrially.6 In the process, Na was employed to couple SiMe
2Cl2
monomers in hydrocarbon solvents into polydimethylsilane, -(Me2Si)n-. The preceramic polymer -(Me2Si)n- was further
decomposed thermally into the β-SiC fibers. Now, we wish to demonstrate that the original Yajima process can be simplified to generate β-SiC nanostructures efficiently. Cubic cages, cubic shells and nanoparticles of β-SiC are formed via the reactions involving vapors of methylchlorosilanes, SiMe2Cl2 and SiMeCl3,
and liquid Na.
At 623 K and under 1 atm of Ar, NaH powders were decomposed to form small droplets of liquid Na. Then, the liquid Na was reacted with the vapors of SiMe2Cl2 and SiMeCl3 to
generate particle precursors Pre-I and Pre-II, respectively. After the precursors were decomposed at 1273 K under vacuum, fine powders were collected as the final products. Black powders I and yellow powders II were obtained from Pre-I and Pre-II, respectively.
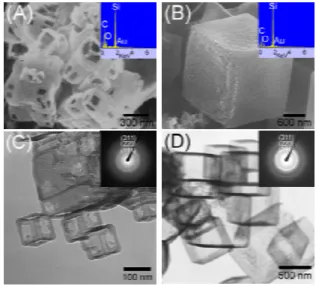
As shown by the scanning electron microscopic (SEM) images in Fig. 1, the particles of I display two types of morphology. The major type, Ia, appears as hollow cubic cages with an edge length of 60 - 400 nm (Fig. 1a). Many of the cages show an edge thickness of ca. 40 nm. An energy dispersive spectrum (EDS) suggested that Ia contains Si, C, and some O atoms. The minor type, Ib, is cubes with an edge length of 1 - 2 µm as shown in Fig. 1B. The EDS of Ib suggests that the cages are composed of Si, C and a small amount of O also. Transmission electron microscopic (TEM) images of Ia and Ib are shown in Figs. 1C and 1D, respectively. The TEM image of Ia confirms that the inner space defined by the cage’s edges is empty. The electron diffraction (ED) study of Ia shows a diffused ring pattern indicating that the structure is polycrystalline. The ED rings can be indexed to the (111), (220) and (311) planes of a cubic structure. The lattice parameter a is estimated to be 0.251 nm, close to the value of β-SiC.7 The TEM image in Fig. 1D shows that Ib is composed of
hollow cubes with a wall thickness of ca. 20 nm. The ED pattern and the derived lattice parameter, a = 0.251 nm, suggest that Ib is polycrystalline β-SiC too.7 Furthermore, X-ray diffraction (XRD)
and other spectroscopic data, such as Fourier transform infrared (FT-IR), 29Si solid-state nuclear magnetic resonance (29Si SSNMR)
and X-ray photoelectron spectroscopy (XPS), support that I is β-SiC.
Figure 1. Images of I, prepared from SiMe2Cl2. SEM and EDS (inset) of (A)
Ia and (B) 1b. TEM and ED (inset) of (C) Ia and (D) 1b.
Employing SiMeCl3 into the reaction, Pre-II was generated
first at 623 K. After the precursor was decomposed at 1273 K, II was isolated. An SEM image in Fig. 2A shows the morphology of
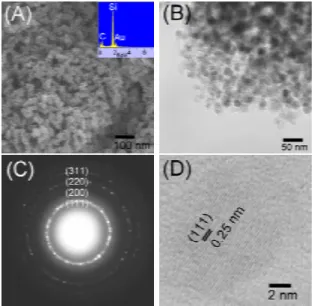
II. It consists of spherical nanoparticles with a diameter of ca. 10
nm. The EDS result shows the presence of Si and C atoms. A TEM image in Fig. 2B confirms that II has a nearly nonagglomerated spherical shape with an average diameter of 10 nm. An ED study in Fig. 2C shows a ring pattern which can be assigned to the diffractions from the (111), (200), (220), and (311) planes of a cubic phase polycrystals. From the ED, the lattice parameter a is estimated to be 0.251 nm. This is consistent with the literature value of β-SiC.7 A high resolution TEM (HRTEM)
image of a nanoparticle of II is shown in Fig. 2D. The particle has an averaged diameter of 10 nm. The fringes, spaced 0.25 nm apart, can be observed clearly. These are assigned to the (111) planes of β-SiC. The value is close to the literature data for β-SiC, 0.252 nm.7 In addition, II is confirmed to be β-SiC based on the results
Na(l) 623 K Me2SiCl2(g) Ar 623 K 623 K Ar 1273 K Vacuum - NaCl Vacuum - NaCl NaH(s) Ar, - H2 1273 K β-SiC I MeSiCl3(g) β-SiC II Pre-II NaCl Na(l) Na(l) Pre-I NaCl Si Si Si Si H H H H H H H H m Si Me n Si Me H CH2 n Si Me n Me
Figure 2. (A) SEM and EDS (inset), (B) TEM, (C) ED, and (D) HRTEM images of II, prepared from SiMeCl3.
Scheme 1. Reaction steps to form I and II.
We studied how the physical shapes of I and II were influenced by the precursors Pre-I and Pre-II. Formed at 623 K, Pre-I showed cubic structures under SEM. EDS and XRD studies indicated the presence of NaCl, a byproduct in the reaction. FT-IR and 29Si SSNMR data supported that Pre-I was a mixture of
polydimethylsilane and polycarbosilane.8 According to the
original Yajima studies, the polydimethylsilane, which was the initial product generated from the coupling of SiMe2Cl2 by Na,
was transformed into polycarbosilane near the reaction temperature.6 Based on the above information, we suggest that
Pre-I had cubic cores of NaCl crystals, which served as
self-generated templates, to support flexible shells composed of the linear polymers. At 1273 K under vacuum, the inner cores were vaporized while the outer shells decomposed further into I, showing the cubic cage and shell structures of β-SiC. The overall steps to the products are summarized in Scheme 1. For Pre-II, the presence of NaCl and polycarbosilane was also supported by XRD, FT-IR and 29Si SSNMR data. However, since II were
nanoparticles, displaying a physical shape distinctively different from I, we speculate that Pre-II did not have a tightly attached NaCl/polycarbosilane core/shell structure similar to that observed for Pre-I. Instead, the polymer and the salt might grow into phases separated widely and with little physical and structural
interactions. Based on a previous report,9 the initial coupling
product between SiMeCl3 and Na at 623 K is proposed to be
polymethylsilyne, (MeSi)n, a highly cross-linked network polymer.
In the 29Si SSNMR spectrum, a broad signal near –60 ppm was
observed and assigned to this type of bonding environment for Si. This material probably reacted further into a rigid cross-linked polycarbosilane based nanoparticles of Pre-II. Then, at 1273 K, these nanoparticles were converted into β-SiC of II.
In conclusion, we have synthesized β-SiC cages, cubes and nanoparticles via vapor-liquid reactions employing methylchlorosilanes and Na. The route is a simplified solvent-free Yajima process. This interesting example shows how nonvolatile products produced at the vapor-liquid interphase may cooperatively influence the physical appearances. Both the precursors Pre-I and Pre-II were composed of mixtures of organosilicon polymers and NaCl. In Pre-I, the more flexible polymers probably wrapped the NaCl crystals, which served as cubic templates. Consequently, the cubic shape was maintained in the final product I. In contract, in Pre-II, the more rigid cross-linked polymers probably separated from the NaCl crystals completely. The result is that little structural influence and correlation was observed between the two. As a result, the final product II stayed as featureless nanoparticles. The differences at the precursor srage generated pronounced shape variations in the final products of β-SiC. Clearly, the appearance difference between I and II is the consequence of the interaction difference among the phase-separated solid products, generated at the interphase of vapor-liquid/solid reactions. This type of behavior has been observed in other systems.10,11 Search for potential
applications of these new materials is in progress.
ACKNOWLEDGMENT This work was supported by NSC92-2113-M-009-022 of the National Science Council of Taiwan, the Republic of China.
Supporting Information Available:. Experimental section. SEM,
XRD, FT-IR, Raman, SSNMR and XPS data of the particles isolated from this study. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
(1) Fantoni, R.; Borsella, E.; Piccirillo, S.; Ceccato, R.; Enzo, S. J.Mater. Res. 1990, 5, 143.
(2) (a) Kilinger, N.; Strauss, E.; Komareh, K. L. J. Am. Ceram. Soc. 1996, 49, 369. (b) Biernacki, J. J.; Wotzak, G. P.; J. Am. Ceram. Soc. 1989, 72, 122.
(3) (a) Hatakeyama, F.; Kanzaki, S. J. Am. Ceram. Soc. 1990, 73, 2107. (b) Seog, I. S.; Kim, C. H. J. Mater. Sci. 1993, 28, 3227.
(4) Kamlag, Y.; Goossens, A.; Colbeck, I.; Schoonman, J. Chem. Vapor. Deposition 2003, 9, 125.
(5) Chiu, H.-T.; Huang, S.-C. J. Mater. Sci. Lett. 1993, 12, 537.
(6) (a) Yajima, S.; Hayasht, J.; Omori, M. Chem. Lett. 1975, 931. (b) Yajima, S.; Okamura, K.; Hayasht, J. Chem. Lett. 1975, 1209. (c) Yajima, S.; Omori, M.; Hayasht, J.; Okamura, K. Chem. Lett. 1976, 551. (d) Yajima, S.; Hayasht, J.; Hasegawa, Y.; Iimura, M. J. Mater. Sci. 1978, 13, 2569. (e) Yajima, S.; Hasegawa, Y.; Omori, M.; Okamura, K. Nature, 1976, 261, 683.
(7) Joint Committee for Powder Diffraction (JCPDS) File No. 29-1129. International Center for Diffraction Data, 1982.
(8) Iseki, T.; Narisawa, M.; Katase, Y.; Oka, K.; Dohmaru, T. Okamura, K. Chem. Mater. 2001, 13, 4163.
(9) Bianconi, P. A.; Weidman, T. W. J. Am. Chem. Soc. 1988, 110, 2342. (10) Yen, M.-Y.; Chiu, C.-W.; Shia, C.-H.; Chen, F.-R.; Kai, J.-J.; Lee, C.-Y.;
Chiu, H.-T. Adv. Mater. 2003, 15, 235.
(11) Hsia, C.-H.; Yen, M.-Y.; Lin, C.-C.; Chiu, H.-T.; Lee, C.-Y. J. Am. Chem. Soc. 2003, 125, 9940.
Synopsis
By reacting vapors of SiMe2Cl2 and SiMeCl3 with liquid Na at 623 K, precurosrs containing organosilicon polymers
and NaCl were obtained. Decomposition of the precursor from SiMe2Cl2 at 1273 K under vacuum generated β-SiC
cubic cages (edge length 60 - 400 nm, edge thickness 40 nm) and shells (edge length 300 nm, shell thickness 20 nm). Decomposition of the precursor from SiMeCl3 under a similar condition, β-SiC nanoparticles (diameter 5 - 10
nm) were obtained. Na(l) 623 K Me2SiCl2(g) 623 K Ar Vacuum - NaCl NaH(s) Ar, - H2 1273 K β-SiC I MeSiCl3(g) β-SiC II Na(l) Na(l) 623 K Ar Vacuum - NaCl 1273 K