行政院國家科學委員會補助專題研究計畫成果報告
※※※※※※※※※※※※※※※※※※※※※※※※※
※ ※
※ 蛋白質酪胺酸磷酸酯水解酵素標示之研究 ※
※
(Mechanism-Based Labeling of Protein Tyrosine Phosphatases)※
※ ※
※※※※※※※※※※※※※※※※※※※※※※※※※
計畫類別:個別型計畫
計畫編號:NSC 90-2113-M-002-028-
執行期間:90 年 08 月 01 日至 91 年 07 月 31 日
計畫主持人:羅禮強
執行單位:臺灣大學化學系
中 華 民 國 九十一 年 十一 月 一 日
行政院國家科學委員會專題研究計畫成果報告
NSC Pr oject Repor ts
蛋白質酪胺酸磷酸酯水解酵素標示之研究
(Mechanism-Based Labeling of Protein Tyrosine Phosphatases)
計畫編號:NSC 90-2113-M-002-028-
執行期限:90 年 08 月 01 日至 91 年 07 月 31 日
主持人:羅禮強 臺灣大學化學系 (
lclo@ccms.ntu.edu.tw)計畫參與人員:周子超、蔡長昇、謝忠憬
中文摘要: 本研究乃是開發化學/酵素方法,利用 alcalase 所具有的極佳立體選擇性的特點來製 備 具 有 高 光 學 活 性 的 (4aS,5S)-4,4a,5,6,7,8-hexahydro-5-hydroxy-4a-methylnaphthalen-2(3H)-one,我們在比較四種不同碳鏈長度的酯類衍生物之後,發現其中丁酸酯為最佳的 酵素受質,而其立體選擇性則是與文獻報導有關 alcalase 的活性中心環境相符合。經過 酵素水解後的羥基烯酮產物,可以經由一簡單的萃取步驟與殘留之酯類受質達到良好分 離之效果,本研究成果可以應用於大量製備上述重要具高光學活性的羥基烯酮衍生物。 關鍵詞:蛋白質水解酵素、立體選擇性、光學分割、羥基烯酮、光學活性 Abstr act:We have developed a convenient chemoenzymatic method for the preparation of (4aS,5S)-4,4a,5,6,7,8-hexahydro-5-hydroxy-4a-methylnaphthalen-2(3H)-one by taking advantage of the excellent enantioselectivity of alcalase. Four different esters were compared and the butanoate ester was found to be the best substrate. The stereochemistry of the product is the same as the one predicted from the binding model of alcalase. A simple extraction/partition procedure was used to separate the hydroxyenone product from the remaining ester. This practical procedure would be very useful in a gram-scale operation for securing the title compound in high optical purity.
KEY WORDS: alcalase, lipase, Wieland-Miescher ketone, enantiopreference, resolution,
hydroxyenone, optical activity
Intr oduction:
Optically pure Wieland-Miescher ketone (1)1 (Figure 1) and its enantiomer are important starting materials for the syntheses of a variety of natural products, including terpenoids and steroids.2 A number of recent publications have focused on how to secure large quantities of optically active 1. Three different approaches, including asymmetric synthesis,3 enantioselective microbial reduction4 and classical resolution through a hemiphthalate derivative,5 are currently utilized for this purpose. In the first approach, asymmetric cyclization of achiral 2-methyl-2-(3-oxobutyl)-1,3-cyclohexanedione (7) in the presence of (S)-(-)-proline would offer optically enriched Wieland-Miescher ketone (1) (Scheme 1). However, the process is time-consuming (3-5 days) and the optical purity of the product is frequently variable (40-70%).3,6 A more recent example of this cyclization using catalytic
antibodies,7 which gave Wieland-Miescher ketone in high optical purity, is yet to be applied to large-scale processes. The other two approaches also have room for improvement, as they would require either careful manipulations of the incubation medium and microbes or tedious crystallization procedures. The first step in many synthetic applications of (S)-1 often involved its regio- and diastereoselective reduction with NaBH4 to give
(4aS,5S)-(+)-4,4a,5,6,7,8-hexahydro-5-hydroxy-4a-methylnaphthalen-2(3H)-one (2).8 Therefore, this hydroxyenone intermediate (4aS,5S)-2 becomes an interesting target and could also serve as a starting point for future applications. Since compound 2 carries a secondary hydroxyl group, we envision that it would be well suited for enzyme-catalyzed kinetic resolution. We hereby report a convenient chemoenzymatic method using alcalase as an alternative approach for the preparation of (4aS,5S)-2 (Scheme 2).
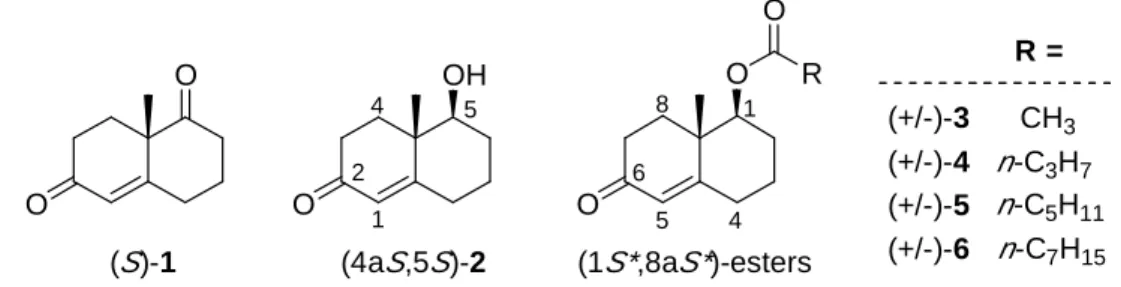
CH3 n-C3H7 n-C5H11 n-C7H15 R = (+/-)-3 (+/-)-4 (+/-)-5 (+/-)-6 O OH O O (4aS,5S)-2 (S)-1 O O R O 1 2 4 5 6 8 1 4 5 (1S*,8aS*)-esters
Figur e 1. Structures of Wieland-Miescher ketone 1, hydroxyenone 2 and racemic ester
derivatives 3-6. O O (S)-1(40-70%e.e.) O O O 7 (S)-(-)-proline
Scheme 1. Asymmetric cyclization of trione 7 in the presence of (S)-proline to form (S)-1.
Results and Discussion:
Alcalase®,9 prepared from submerged formation of a selected strain of Bacillus licheniformis, is an inexpensive additive widely used in detergent formulations and food industry. Its major enzyme component is a serine protease, subtilisin Carlsberg. Alcalase also possesses esterase activity with a high turnover rate and shows good stability in various organic solvents.10 In the meantime, the mechanism of subtilisin action in water and in various anhydrous solvents has been thoroughly studied and found to be the same.11 The enantiopreference of subtilisin for the resolution of secondary alcohols was reported to be largely determined by the relative size of the two substituents.12 The preferred binding of S-substrate in the active site is depicted in Figure 2, where L stands for the large substituent and M for the medium-sized. The extensive information about the enantiopreference of alcalase together with its readily availability have made it a useful reagent in organic synthesis.13 Based on this binding model, we predict that the secondary alcohol (4aS,5S)-2 would meet the stereochemical requirement in the binding pocket of alcalase. We therefore prepared a series of four racemic esters 3-6 and carried out a preliminary screening using these substrates for alcalase-catalyzed hydrolytic reactions (Scheme 2). Esters 3-6, including acetate-3, butanoate-4, hexanoate-5 and octanoate-6, are mainly different in the length of their carboxylate moiety (Figure 1). Their syntheses were achieved by acylation of the racemic alcohol 2 with suitable acylating agents in high yields.
O O R O phosphate buffer, pH 7.0 37oC alcalase O OH
+
O O R ORacemic esters (4aS,5S)-2
15% DMF
(1R,8aR)-enriched esters
Scheme 2. Alcalase-catalyzed hydrolysis of racemic esters 3-6.
O
L
M
R O Ser-OH (S)-substrateFigur e 2. Preferred binding of (S)-substrate in the active site of subtilisin; L stands for the large substituent, and M for the medium-sized.
Table 1. Results of alcalase-catalyzed hydrolysis of racemic esters 3-6.
Esters R Time (h) Conversion (%)a Enantiomeric excess (%)b
(1R,8aR)-ester (4aS,5S)-2 E valuec 3 CH3 48 NR - - -4 n-C3H7 6 17 20 > 99 -4 n-C3H7 12 27 36 > 99 -4 n-C3H7 24 45 73 90 43 4 n-C3H7 48 52 90 84 41 5 n-C5H11 48 NR - - -6 n-C7H15 48 NR - -
-aNR indicates no reaction when checked with TLC. The conversions for ester 4 were
calculated from the following equation, c = ees/(ees + eep),14 where ees and eep represent the
enantiomeric excess of ester starting material and alcohol product, respectively.
bThe enantiomeric excess was determined by HPLC with a Chiralcel® OJ column. The
retention times for the two pairs of enantiomers 4 and 2 are shown in parentheses (min); (1S,8aS)-4 (7.36), (1R,8aR)-4 (8.20), (4aR,5R)-2 (18.47), and (4aS,5S)-2 (23.76).
c
E (Enantioselectivity) = ln[1-c(1+eep)]/ln[1-c(1-eep)]
The hydrolytic reactions were performed by incubating individual racemic ester substrate (50 mM) with alcalase (2.4 AU/g)9 in 0.3 M phosphate buffer (pH 7.0) containing 15% DMF
at 37oC. Reactions were stopped at intervals to check the conversions as well as the enantiomeric excess of the remaining ester and the hydroxyenone product 2. Under this screening condition, those four esters displayed a dramatic difference in their rates toward hydrolysis (Table 1). Only butanoate-4 gave a reasonable conversion after 12 h (~26%), whereas the other three substrates (3, 5 and 6) showed no appreciable reactions even after 48 h when checked with TLC. This interesting result indicates that the carboxylate moiety also plays a critical role in the rate of hydrolysis in addition to the steric requirement for the substituents of the alcohol. This observation is not uncommon in enzyme-catalyzed reactions, as in some cases the rate and specificity could be manipulated by partially altering the structure of the substrates.15 Since the reaction only occurred with butanoate-4, the results were further examined.
Butanoate-4 is not only a good substrate for alcalase-catalyzed hydrolysis, it also exhibits excellent enantioselectivity14 as inferred from the optical purity of both remaining ester and hydroxyenone product-2 (Table 1). Determination of the enantiomeric excess was achieved by using HPLC with a Chiralcel® OJ column. The absolute configuration of the major hydroxyenone product was established by comparing its optical rotation with that of authentic sample. The optical purity of (4aS,5S)-2 remained high (e.e. > 99%) at the conversion of 27%. It slightly dropped to 90% when the conversion reached 45%, and further dropped to 84% when the conversion was over 52%. The calculated E values14 were larger than 40, favoring the formation of (4aS,5S)-(+)-2. The influence of DMF content on the rate and enantioselectivity was also studied, and the results showed that the enzyme still performed well in the presence of 15-35% DMF.
Since alcalase has great enantioselectivity on butanoate-4 (E > 40), we then combine this feature with the availability of (1S,8aS)-enriched ester 4 to develop a practical method for the large-scale preparation of enantiomerically pure (4aS,5S)-2. For a gram-scale operation, the condition for the resolution was the same as the one depicted in Scheme 2, except (1S,8aS)-enriched butanoate-4 (58% e.e.) instead of the racemate was used as the substrate. Optically enriched (S)-13,6 (Scheme 1) was first reduced to hydroxyenone 2, which was subjected to acylation with butanoyl chloride to offer (1S,8aS)-enriched butanoate-4 (58% e.e.). After enzymatic hydrolysis, both (4aS,5S)-2 and the remaining ester were extracted from the mixture with EtOAc. These two compounds could easily be separated by partitioning in n-hexane/H2O. The remaining ester goes to the hexane layer, while
(4aS,5S)-2 stays in aqueous phase. This simple extraction/partition procedure is especially convenient and suitable for a large-scale operation. It avoids the need of extensive chromatography for the separation of (4aS,5S)-2 from the remaining ester. Hydroxyenone (4aS,5S)-2 of high optical purity (> 97% e.e.) could thus be obtained in 57% yield.
Conclusion:
In conclusion, we have developed a convenient chemoenzymatic method for the preparation of (4aS,5S)-4,4a,5,6,7,8-hexahydro-5-hydroxy-4a-methylnaphthalen-2(3H)-one (2) by taking advantage of the excellent enantioselectivity of alcalase. Butanoate-4 was found to be the best substrate, and it gave a product with the predicted absolute stereochemistry. The carboxylate moiety of butanoate-4 not only plays an important role in the rate of alcalase-catalyzed hydrolysis, it also offers the advantage of simplifying the purification procedure after enzymatic resolution. Although only alcalase was studied in this report, its low cost and readily availability would make this procedure very useful in a gram-scale operation for securing (4aS,5S)-(+)-2 in high optical purity. We are currently surveying other hydrolytic enzymes, including esterases and lipases, for the hydrolysis of the butanoate-4. It is interesting to note that lipases might display a reverse enantiopreference for this substrate, based on the knowledge of the binding pocket of lipases.12b A recent example of
lipase-catalyzed transesterification on a structurally similar secondary alcohol also supports this prediction.16 We will report the results in due course.
Exper imental Section:
Gener al Methods. Melting points are uncorrected. 1H and 13C NMR spectra were recorded at 400 and 100 MHz in CDCl3, respectively. Analytical TLC (silica gel, 60F-54,
Merck) and spots were visualized under UV light and/or phosphomolybdic acid-ethanol. Flash column chromatography was performed with silica gel 60 (70-230 mesh, Merck). HPLC was performed on a Chiralcel® OJ column (250 x 4.6 mm, n-hexane/i-PrOH = 94/6, 1 mL/min) monitored at 235 nm. Both racemic and optically enriched 2 were prepared according to literature procedures.3,8
Acetic acid (1S*,8aS*)-(±)-8a-methyl-6-oxo-1,2,3,4,6,7,8,8a-octahydr o-naphthalen-1-yl ester (3):17 To an ice-cooled solution of the racemic hydroxyenone 2 (760 mg, 4.22 mmol) in 6 mL of pyridine was slowly added 0.5 mL of Ac2O (5.29 mmol). The reaction
was kept at rt and stirred overnight. The progress of the reaction was monitored by TLC (hexane/EtOAc = 4/6). After the reaction was complete (~12 h), a few drops of H2O were
added to quench the reaction. The mixture was stirred for another 30 min and pyridine was removed under reduced pressure. The residual oil was dissolved in EtOAc and washed consecutively with NaHCO3 (x3), 5% citric acid (x3), H2O (x2) and brine (x1). After drying
over anhydrous Na2SO4 and filtration, the desired ester 3 (890 mg, 95%) was purified by silica
gel column chromatography eluted with hexane/EtOAc (80/20). Rf = 0.35 (hexane/EtOAc =
65/35). 1H NMR (400 MHz, CDCl3): δ 5.80 (d, J = 1.9 Hz, 1 H), 4.64 (dd, J = 11.1, 4.1 Hz,
1 H), 2.48-2.28 (m, 3 H), 2.22 (m, 1 H), 2.07 (s, 3 H), 1.98-1.62 (m, 5 H), 1.49 (m, 1 H), 1.26 (s, 3 H). 13C NMR (100 MHz, CDCl3): δ 198.9, 170.3, 166.7, 125.8, 79.2, 40.4, 34.0, 33.5,
31.8, 26.9, 22.9, 21.1, 16.6. IR (neat): 2959, 2873, 1732, 1679, 1626, 1461, 1169, 1090,1003, 864 cm-1. HRMS calcd for C13H19O3 (M+1)+ 223.1334, found 223.1332.
Gener al pr ocedur e for the pr epar ation of r acemic ester s 4-6: The procedure is
similar to that for acetate 3, except suitable acyl chlorides (butanoyl, hexanoyl and octanoyl) instead of anhydrides were used as the acylating agents. The desired esters 4-6 were purified by silica gel column chromatography eluted with hexane/EtOAc (8/2 → 6/4).
Butanoic acid (1S*,8aS*)-(±)-8a-methyl-6-oxo-1,2,3,4,6,7,8,8a-octahydr o-naphtha-len-1-yl ester (4): Yield 98%, m.p. 43-44oC. Rf = 0.47 (hexane/EtOAc = 65/35). 1H NMR
(400 MHz, CDCl3): δ 5.77 (d, J = 1.9 Hz, 1 H), 4.62 (dd, J = 12.0, 4.1 Hz, 1 H), 2.45-2.14 (m,
6 H), 1.98-1.58 (m, 7 H), 1.46 (m, 1 H), 1.24 (s, 3 H), 0.93 (t, J = 7.3 Hz, 3 H). 13C NMR (100 MHz, CDCl3): δ 198.9, 172.9, 166.8, 125.8, 78.9, 40.4, 36.5, 34.0, 33.5, 31.8, 26.9, 22.9,
18.6, 16.7, 13.7. IR (neat): 2952, 1737, 1673, 1241, 1038 cm-1. HRMS calcd for C15H23O3
(M+1)+ 251.1647, found 223.1644. Optically enriched (1S,8aS)-4 was similarly prepared from (4S,5aS)-enriched 2.
Hexanoic acid (1S*,8aS*)-(±)-8a-methyl-6-oxo-1,2,3,4,6,7,8,8a-octahydr o-naphtha-len-1-yl ester (5): Yield 90%. Rf = 0.52 (hexane/EtOAc = 65/35). 1H NMR (400 MHz, CDCl3): δ 5.76 (d, J = 1.9 Hz, 1 H), 4.61 (dd, J = 11.7, 4.1 Hz, 1 H), 2.43-2.18 (m, 6 H),
1.98-1.55 (m, 7 H), 1.46 (m, 1 H), 1.32-1.27 (m, 4 H), 1.23 (s, 3 H), 0.86 (t, J = 7 Hz, 3 H).
13
C NMR (100 MHz, CDCl3): δ 198.9, 173.1, 166.8, 125.8, 78.9, 40.4, 34.5, 34.0, 33.5, 31.8,
31.2, 26.9, 24.7, 22.9, 22.3, 16.7, 13.9. IR (neat): 2957, 2864, 1738, 1682, 1240, 1170, 1006, 774 cm-1. HRMS calcd for C17H27O3 (M+1)+ 279.1960, found 279.1964.
Octanoic acid (1S*,8aS*)-(±)-8a-methyl-6-oxo-1,2,3,4,6,7,8,8a-octahydr o-naphtha-len-1-yl ester (6): Yield 90%. Rf = 0.60 (hexane/EtOAc = 65/35). 1H NMR (400 MHz, CDCl3): δ 5.76 (d, J = 1.8 Hz, 1 H), 4.61 (dd, J = 12.7, 4.1 Hz, 1 H), 2.37-2.22 (m, 6 H),
1.98-1.58 (m, 7 H), 1.57-1.36 (m, 1 H), 1.32-1.27 (m, 11 H), 0.85-0.82 (m, 3 H). 13C NMR (100 MHz, CDCl3): δ 198.8, 172.9, 166.7, 125.6, 78.7, 40.3, 34.4, 33.8, 33.3, 31.6, 31.5, 28.9,
28.7, 26.7, 24.9, 22.8, 22.4, 16.5, 13.9. IR (neat): 2934, 2863, 1738, 1684, 1469, 1452, 1166, 1006 cm-1. HRMS calcd for C19H31O3 (M+1)+ 307.2273, found 307.2271.
Alcalase-catalyzed hydr olysis of r acemic ester s 3-6 (analytical scale): Two stock
solutions were first prepared; solution A contains 0.333 M of individual substrate in DMF and solution B contains 25% Alcalase® 2.4L9 (v/v) in 0.3 M phosphate buffer (pH 7.0). For each reaction, 60 µL of solution A and 300 µL of phosphate buffer (0.3 M, pH 7.0) were first mixed. It was then added 40 µL of solution B (total volume = 400 µL) and placed in a shaker at 37oC. The reactions were terminated at 6h, 12h, 24h and 48h, respectively, by addition of EtOAc (20 mL) and H2O (10 mL). The organic layer was collected, washed with
H2O (10 mL x2), brine (10 mL x1). It was dried over anhydrous Na2SO4, filtered and
concentrated to dryness. The residue was dissolved in n-hexance/i-PrOH for HPLC analysis.
Alcalase-catalyzed hydr olysis of (1S,8aS)-enr iched butanoic acid 8a-methyl-6-oxo-1,2,3,4,6,7,8,8a-octahydr o-naphthalen-1-yl ester (4) (gr am-scale): To a solution of
(1S,8aS)-enriched butanoate-4 (1.00 g, 58% e.e.) in 12 mL of DMF, was added 66 mL of phosphate buffer (0.3 M, pH 7.0) and 2 mL of alcalase. The mixture was placed in a shaker at 37oC for 48 h. After enzymatic hydrolysis, both the hydroxyenone product 2 and the remaining ester 4 were extracted from the mixture with EtOAc (200 mL x3). The EtOAc extracts were combined and concentrated. The residual oil was partitioned between hexane/H2O. The remaining ester 4 goes to the hexane layer, while the hydroxyenone
product 2 stays in aqueous phase. The aqueous phase was extracted with EtOAc (x3) and the desired product was purified by silica gel column chromatography eluted with hexane/EtOAc (6/4) to give (4aS,5S)-(+)-2 (0.41 g, 57% yield). Its e.e. was found to be 98% by HPLC analysis, [α]25
D = +174 (c 1.0, benzene). 1H NMR (400 MHz, CDCl3): δ 5.76 (d, J = 1.9 Hz,
1 H), 3.40 (dd, J = 11.7, 4.4 Hz, 1 H), 2.50-2.22 (m, 3 H), 2.21-2.10 (m, 2 H), 1.90-1.58 (m, 5 H), 1.39 (m, 1 H), 1.17 (s, 3 H). 13C NMR (100 MHz, CDCl3): δ 199.8, 168.8, 125.4, 78.2,
41.6, 34.2, 33.6, 32.0, 30.2, 23.1, 15.2. HRMS calcd for C11H17O2 (M+1)+ 181.1228, found
181.1228. The spectroscopic data were identical to those reported in the literature.4,8
Refer ences:
1. Wieland, P.; Miescher, K. Helv. Chim. Acta 1950, 33, 2215-2228.
2. Examples from (S)-1: (a) Smith, A. B., III; Kingery-Wood, J.; Leenay, T. L.; Nolen, E. G.; Sunazuka, T. J. Am. Chem. Soc. 1992, 114, 1438-1449. (b) Danishefsky, S. J.; Masters, J. J.; Young, W. B.; Link, J. T.; Snyder, L. B.; Magee, T. V.; Jung, D. K.; Isaacs, R. C. A.; Bornmann, W. G.; Alaimo, C. A.; Coburn, C. A.; Di Grandi, M. J. J. Am. Chem. Soc. 1996, 118, 2843-2859. (c) An, J.; Wiemer, D. F. J. Org. Chem. 1996, 61, 8775-8779. Examples from (R)-1: (d) Harada, N.; Sugioka, T.; Ando, Y.; Uda, H.; Kuriki, T. J. Am. Chem. Soc. 1988, 110, 8483-8387. (e) Paquette, L. A.; Wang, H.-L. J. Org. Chem. 1996, 61, 5352-5357. (f) Paquette, L. A.; Backhaus, D.; Braun, R.; Underiner, T. L.; Fuchs, K. J. Am. Chem. Soc. 1997, 119, 9662-9671.
3. (a) Eder, U.; Sauer, G.; Wiechert, R. Angew. Chem., Int. Ed. Engl. 1971, 10, 496-497. (b) Hajos, Z. G.; Parrish, D. R. J. Org. Chem. 1974, 39, 1615-1621. (c) Gutzwiller, J.; Buchschacher, P.; Fürst, A. Synthesis 1977, 167-168. (d) Buchschacher, P.; Fürst, A. Org. Synth. 1985, 63, 37-43.
4. (a) Prelog, V.; Acklin, W. Helv. Chim. Acta 1956, 39, 748-757. (b) Fuhshuku, K.; Funa, N.; Akeboshi, T.; Ohta, H.; Hosomi, H.; Ohba, S.; Sugai, T. J. Org. Chem. 2000, 65, 129-135. (c) Hioki, H.; Hashimoto, T.; Kodama, M. Tetrahedron: Asymmetry 2000, 11, 829-834.
5. Newkome, G. R.; Roach, L. C.; Montelaro, R. C.; Hill, R. K. J. Org. Chem. 1972, 37, 2098-2101.
2189-2190.
7. (a) Zhong, G.; Hoffmann, T.; Lerner, R. A.; Danishefsky, S.; Barbas, C. F., III. J. Am. Chem. Soc. 1997, 119, 8131-8132. (b) Hoffmann, T.; Zhong, G.; List, B.; Shabat, D.; Anderson, J.; Gramatikova, S.; Lerner, R. A.; Barbas, C. F., III. J. Am. Chem. Soc. 1998, 120, 2768-2779.
8. (a) Yeo, S.-K.; Hatae, N.; Kanematsu, K. Tetrahedron 1995, 51, 3499-3506. (b) Cheung, W. S.; Wong, H. N. C. Tetrahedron 1999, 55, 11001-11016.
9. Alcalase®, available from Novo Nordisk Biochem North America, Inc., Franklinton, NC, was a gift from Trump Chemical Corp., Taipei, Taiwan. The enzyme activity is expressed in Anson Units per gram (AU/g). http://www.novozymes.com/eventure/demo/b1145a-gb.pdf.
10. (a) Chen, S.-T.; Wang, K.-T.; Wong, C.-H. Chem. Commun. 1986, 1514-1516. (b) Zaks, A.; Klibanov, A. M. J. Biol. Chem. 1988, 263, 3194-3201. (c) Chen, S.-T.; Chen, S.-Y.; Wang, K.-T. J. Org. Chem. 1992, 57, 6960-6965.
11. (a) Kanerva, L. T.; Klibanov, A. M. J. Am. Chem. Soc. 1989, 111, 6864-6865. (b) Adams, K. A. H.; Chung, S.-H. Klibanov, A. M. J. Am. Chem. Soc. 1990, 112, 9418-9419.
12. (a) Fitzpatrick, P. A.; Klibanov, A. M. J. Am. Chem. Soc. 1991, 113, 3166-3171. (b) Kazlauskas, R. J.; Weissfloch, A. N. E. J. Mol. Catal. B: Enzym. 1997, 3, 65-72.
13. (a) Chen, S.-T.; Wang, K.-T. Synthesis 1987, 581-582. (b) Chen, S.-T.; Chen, S.-Y.; Hsiao, S.-C.; Wang, K.-T. Biotch. Lett. 1991, 13, 773-778. (c) Wong, C.-H.; Whitesides, G. M. In Enzymes in Synthetic Organic Chemistry; Baldwin, J. E.; Magnus, P. D., Eds.; Elsevier: Oxford, 1994; pp. 41-131. (d) Chen, S.-T.; Wang, K.-T. J. Chin. Chem. Soc. 1999, 46, 301-311.
14. (a) Chen, C.-S.; Fujimoto, Y.; Girdaukas, G.; Sih, C. J. J. Am. Chem. Soc. 1982, 104, 7294-7299. (b) Sih, C. J.; Wu, S.-H. Top. Stereochem. 1989, 19, 63-125.
15. (a) Oberhauser, T.; Bodenteich, M.; Faber, K.; Penn, G.; Griengl, H. Tetrahedron 1987, 43, 3931-3944. (b) Scilimati, A.; Ngooi, T. K.; Sih, C. J. Tetrahedron Lett. 1988, 29, 4927-4930. (c) Wu, S.-H.; Lo, L.-C.; Chen, S.-T.; Wang, K.-T. J. Org. Chem. 1989, 54, 4220-4222.
16. Franssen, M. C. R.; Jongejan, H.; Kooijman, H.; Spek, A. L.; Bell, R. P. L.; Wijnberg, J. B. P. A.; de Groot, A. Tetrahedron: Asymmetry 1999, 10, 2729-2738.
17. Harada, N.; Kohori, J.; Uda, H.; Nakanishi, K.; Takeda, R. J. Am. Chem. Soc. 1985, 107, 423-428.
Acknowledgement:
This work was supported by the National Science Council (NSC 90-2113-M-002-028 to L.-C.L.). We thank Professors Shih-Hsiung Wu and Jim-Min Fang for helpful discussions.