Vol. 17: 247-254, 1999 AQUATIC MICROBIAL ECOLOGY
Aquat Microb Ecol Published June 18
Temperature versus substrate limitation of
heterotrophic bacterioplankton production
across trophic and temperature gradients
in the East China Sea
Fuh-Kwo S h i a h l . * , Kon-Kee ~ i u ~ ,
Gwo-Ching
Gong3
'Center of Oceanographic Research, National Taiwan University, Taipei, Taiwan, ROC 'PO Box 23-13, Institute of Oceanography. National Taiwan University, Taipei, Taiwan, ROC3Department of Oceanography, National Taiwan Ocean University, Keelong, Taiwan, ROC
ABSTRACT: A survey of heterotrophic bacterioplankton biolnass (BB; 4 to 64 mg C m-3), production (BP; 0.3 to 14.7 mg C m-3 d-l) and specific growth rates (p; 0.07 to 0 38 d.') as well as concentrations of nitrate (NO3-, <O 1 to 15.6 PM), partlculate organic carbon (POC; 1 to 60 PM) and chlorophyll a (chl; 0.1 to 4.0 mg chl m-3) in the euphotic zone was conducted over the continental shelf of the East China Sea during the s p r ~ n g seasons of 1996 and 1997 Concentrations of N O 3 were high in the coastal areas and decreased offshore. In contrast, temperature (12.2 to 24.5'C) showed the opposlte trend, with cool (<2O0C) and warm (>2O0C) waters distributed Inside and outside the shelf edge, respectively Inside the shelf edge, bacterial rate parameters (BP and p) were positively correlated with temperature but showed no correlation with POC, values of Q,, for BP and p were 2.7 and 3 3 respectively. From the shelf edge to the adjacent open ocean, bacterial rate parameters were positively correlated with POC only These results lmply that during the spring season, nlechanisms controlling the spatial patterns of bacterial rate parameters over the shelf area are system-dependent. It is concluded here that tempera- ture plays a more dominant role than substrate supply inside the shelf edge; however, from the shelf edge to the open ocean, the situation is reversed
KEY WORDS: Bacterial production . Bacterial growth rates . Bottom-up control . Continental shelf . Kuroshlo . Q j o
INTRODUCTION
Heterotrophic bacterioplankton a r e the major organ- isms respon$ihle for fhp deconzposition of dissolved organic compounds in the sea (Fuhrman 1992 a n d cita- tions therein). The study of the mechanisms controlling bacterial growth has been a n important issue within the perspectives of organic carbon cycling a n d plank- tonic trophodynamics. Bottom-up control (the variation of bacterial variables is controlled by the supply rates of dissolved organic substrate) a n d top-down control (the variation of bacterial variables is controlled by bacterivory and viral lysis) have all been proposed as
'E-mail. frank@odb03 qcc.ntu edu tw
explanations for bacterial biomass a n d production variations in the field (see Ducklow & Carlson 1992, Fuhrman 1992 for review). Recent studies have further demnnstraterl that the seasonal variations of bacterial rate parameters (production a n d specific growth rates) in eutrophic ecosystems (coastal, estuarine a n d brack- ish-water systems) might primarily b e regulated by temperature, with substrate supply playing a lesser role (White e t al. 1991, Ducklow & Shiah 1993, Hoch & Kirchman 1993, Berman e t al. 1994, Griffith e t al. 1994, Shiah & Ducklow 1994a,b, 1995). However, the applic- ability of such hypotheses to the continental shelf ecosystem (mesotrophic to oligotrophic) is a significant question that has never been fully answered (Kemp 1994 a n d citations therein).
248 Aquat Microb Ecol17: 247-254, 1999
MATERIALS AND METHODS
The continental shelf is affected by processes Physical mixing processes among these 4 water derived from land and the open ocean simultaneously masses make the continental shelf of the ECS a system
(Biscaye et al. 1994). The shelf receives a large amount characterized by strong gradients of inorganic nutri- of inorganic nutrients from river discharge and coastal ents and organic substrate, either in terms of concen- erosion processes, which results in high standing stock trations or supply rates. In addition, the mixing of the and production rates of organic matter (Mantoura et al. cold (CCW & KSW) and the warm water masses (KW & 1991) fueling bacterial growth. The heterogeneous TSW) may result in a broad range (ca 10 to 25OC) of patterns of physical, chemical and biological variables temperature change over the entire shelf area during across the shelf system (see below) may create a con- spring. These variations provide an ideal opportunity
tinuous trophic gradient for bacterial growth. A to examine temperature and substrate supply effects detailed examination of the spatial patterns of bacter- on the bacterial rate parameters.
ial biomass and rate parameters in the shelf system
may provide some insights into bacterial growth con- trolling mechanisms.
During spring, water properties in the shelf of the
East China Sea (ECS) may be affected by 4 end mem- Study area and sampling. Data were collected from bers. Two of these serve as important sources of inor- 2 cruises conducted on the continental shelf of the ganic nutrients into the shelf area (Liu et al. 1992 and southern ECS north of Taiwan (Fig. 1) during the citations therein). They are the cold (<15OC), low salin- spring seasons of 1996 (9 sampling stations) and 1997 ity (<32.000 psu) China Coastal Waters (CCW) and the (29 sampling stations). Seawater was collected from a cool (<20°C), high salinity Kuroshio Subsurface Waters SeaBird CTD-General Oceanic Rosette assembly with (KSW) which outcrops at the shelf edge all year round. 20 1 Go-Flo bottles. Light intensity was measured with The other 2 oligotrophic end members are the a light meter (QSP200L; Biospherical), while the depth Kuroshio Waters (W) and the Taiwan Strait Waters of the euphotic zone was defined as 1 % of the surface (TSW). Both the KW and TSW are characterized by light level.
high temperature (22 to 25°C) and high salinity Bacterial biomass, production and growth rates. (>34.000 psu), with their main streams flowing north- Bacterial abundance was determined by using the
ward on the eastern and western sides of Taiwan, Acridine Orange Direct Count method (Hobbie et al.
respectively. Primary production and chlorophyll con- 1977). Samples fixed with glutaraldehyde (final conc.,
centrations in areas more affected by the KSW and 1 %) were stained with acridine orange (final conc.,
CCW are about 2- to 4-fold higher than those observed 0.01 %) for 2 min before being filtered through 0.2 pm in the KW and the TSW (Gong et al. 1996, Shiah et al. polycarbonate filters prestained with an Irgalan black
1996). solution. Slides were enumerated by epifluorescence
microscopy (Zeiss, Axioplan). Biomass
120'E 122'E 124'E was calculated using a carbon conversion
28'N 2 8 ' ~ factor of 2 X 10-14 g cell-' (Lancelot &
Billen 1984). Bacterial production was estimated by the method of 3H-thyrnidine (Fuhrman & Azam 1982) incorporation with a conversion factor of 1.18 X 1 0 ' ~ cells m01 thymidhe-' (Cho & Azam 1988). Triplicate 30 to 40 m1 aliquots of water samples were incubated with 3H-
26'N 26'N [methyl]-thymidine (S, A . , 6.7 Ci mmol-l;
f i n d conc., 20 EM) ir. c!esn pe!;7c.-rhn~ste tubes at in situ temperature. Reactions were stopped by adding formaldehyde
(final conc., 1 %). The killed samples
including time zero controls were filtered through 0.2 pm cellulose nitrate filters.
24'N 24'N These filters were then rinsed 3 times
120'E 122.E 124'E each with ice cold 5 % trichloroacetic acid
Fig. 1. Map of the southern East China Sea showing sampling stations for and ice cold 80% ethyl alcohol sequen-
cruises 1996 (A) and 1997 ( 0 ) . Dashed lines indicate the bottom depths in t i a l l ~ . Scintillation cocktail (6 ml; Ultima
Shiah et al.. Shelf bacterial production 249
filters were dissolved completely in 0 . 5 m1 of ethyl acetate. Radioactivity was determined by liquid scintil- lation counting (Packard 1600). Bacterial (specific) growth rates were calculated by dividing the bacterial production by the bacterial biomass.
Particulate organic carbon and nitrogen concentra- tions. Water samples (0.5 to 1.0 1) for particulate organic carbon (POC) and nitrogen (PN) measure- ments were filtered through a 200 mm mesh to remove zooplankton. After filtration (25 mm GF/F filters; pumping pressure < l 0 0 mm H g ) , the samples were wrapped in aluminum foil and stored at -4'C. Both the filters and aluminum foils had been pre-combusted at 550°C for 1 h before these filtration processes. POC and PN concentrations were measured by a CHN ana- lyzer (Fisons; NA1500) after samples had been dried and acid-fumed.
Chlorophyll a and nitrate concentrations. Chl a a n d nitrate concentrations were measured following the methods of Parsons et al. (1984). Water samples for nutrient analyses were subsampled with clean 100 m1 polypropylene bottles and frozen immediately with liquid nitrogen. Nitrate was analyzed with a self- designed flow injection analyzer (Gong e t al. 1995) and was reduced to nitrite with a cadmium wire which was activated with a copper sulfate solution. For chl a , 1 to 2 1 of seawater were filtered through 25 mm Whatman GF/F filters which were then immediately stored at -20°C. Back at the laboratory, the filters were ground in 10 m1 90% acetone followed by extraction in a 4°C shaking incubator for 2 h. After centrifugation at 1000 rpm (200 X g) for 5 min, the concentrations of chlorophyll in the supernatant were measured on a Turner fluorometer (model 10-AU-005).
Data analyses. The euphotic zone integrated data set was generated by integrating measured variables over the euphotic zone by trapezoid method. Parametric methods including correlation a n d regression analyses were performed with the Macintosh software Sta- ViewTM I1 (Abacus Concepts, Inc.). The non-parametric (Spearman rank correlation) method in that software was used to double check the results derived from parametric analyses, especially for the data set with small sampling sixe (Le the ~ 2 0 ° C : data set; see below). Both methods yielded the same conclusion.
RESULTS
Data collected from the 1997 cruise (29 sampling sta- tions) were used to describe the spatial patterns of the measured variables, while the 1996 data (9 sampling stations) were presented in the statistical analyses together with the 1997 data. The depth of the euphotic zone was about 30 to 40 m in the inner shelf but
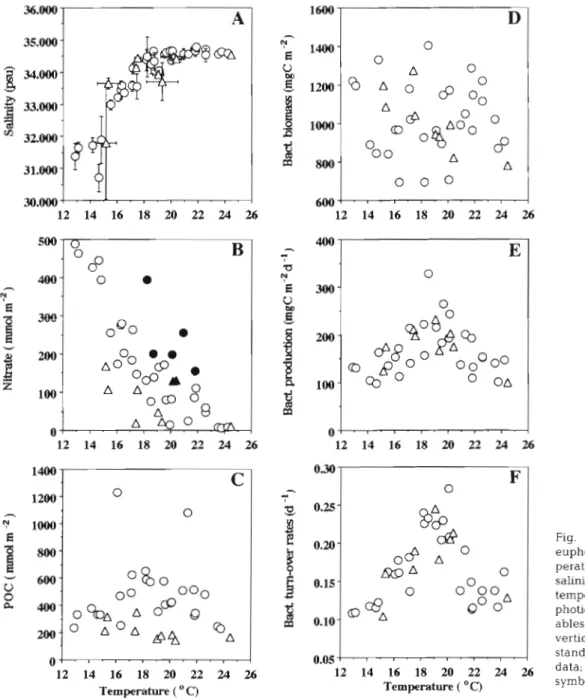
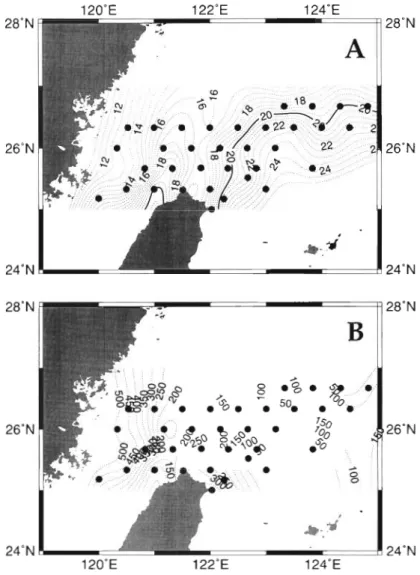
increased to about 65 to 70 m in the outer shelf. With only a few exceptions, the euphotic zone of each sta- tion was well mixed, as judged from the homoge- neously distributed temperature and salinity profiles (Fig. 2A). The average temperature of the euphotic zone changed by more than 1O0C, with the cool (ca 12 to 22°C) and warm (ca 20 to 24°C) waters located at the inner shelf and outside the shelf edge, respectively (Fig. 3A). A warm water mass (TSW) with tempera- tures > 20°C was found west of Taiwan. This northward flowing TSW mixed with the CCW and the KW thereby creating a strong temperature gradient in the middle shelf area. Salinity was positively correlated with tem- perature in areas of <20°C (Fig. 2A). More than half (21 out of the 38) of the sampling stations had similar salinity (-34.500 psu), but temperature varied from 17.0 to 24.5"C. This indicates that temperature is prob- ably a more suitable index than salinity in exploring the magnitude of the mixing among water masses over the shelf. This is particularly true in distinguishing the upwelled KSW and the oligotrophic KW since both water masses were characterized by similar salinity but different temperature and distinct nitrate concen- trations (see below).
Surface nitrate concentrations (NO3-; 0.2 to 15.1 PM) and euphotic zone integrated nitrate concentrations (INO; 6 to 488 mm01 m-2; Fig. 3B) were high in the inner shelf region, yet decreased offshore. Two I N 0 anomalies separated by low I N 0 ( < l 0 0 mm01 m-') waters were observed around the shelf e d g e . One was located at the upwelling area off northeastern Taiwan with I N 0 > 250 mm01 the other ( I N 0 > 150 mm01 m-2) was situated at 26.3'N, 124.0°E. Values of I N 0 were negatively correlated with temperature (Fig. 2B) and salinity when anomalies (7 data) derived from the upwelling areas were excluded ( p < 0.01, n = 31).
Concentrations of particulate organic carbon (POC; 1.2 to 60.4 pM) and the integrated POC (IPOC; 230 to 1220 mm01 m-') varied respectively about 50- a n d 5- fold over the study a r e a (Table 1 ) . In 1997, most of the high IPOC values (>400 mm01 m-') were recorded in the middle shelf with intermediate temperatures (17 to 22°C; Fig. 2C). Values of IPOC in the inner and middle shelf seemed to be higher (>?On mm01 m-2) than those in the outer shelf on the 1996 cruise. Chl a concentra- tions varied 40-fold with values ranging from 0.1 to 4.0 mg chl m-3. On both cruises, the spatial pattern of the integrated chl (Ichl; 6 to 122 mg chl m-') was almost identical to those of the IPOC. The correlation coeffi- cient of Ichl versus IPOC was >+0.90 ( n = 38, p < 0.01). IPOC was positively correlated with euphotic zone integrated particulate nitrogen (IPN; 627 to 135 mm01 N m-2; n = 25, p < 0.01); their relationship can b e expressed as IPOC = 23
+
7.1 (rt 0.5) X IPN with a R2 (coefficient of determination) value of 0.95.250 Aquat Microb Ecol l?: 247-254, 1999
Fig. 2. Scatter plots of the euphotic zone averaged tem- perature versus averaged salinity (A) and averaged temperature versus other eu- photic-zone integrated vari- ables (B-F). Horizontal or vertical bars in (A) indicate standard deviation. (A) 1996 data; (0) 1997 data. Solid symbols in ( B ) indicate up-
welling stations 0 . 0 5 - I . ~ . ~ ~ ~ . ~ . ~ . ~ ' 12 14 16 18 20 22 24 Temperature ( 'C) O ~ . ~ . . . - . ~ . ~ . ~ . I 12 14 16 18 20 22 24 26 Temperature ( O C)
Bacterial biomass (BB; 4 to 64 mg C m-3) varied 16-fold over the study area. Bacterial production (BP) and indi-
vidual growth rates (p; BP/BB; Table l ) val-led >40-fold (0.3 t.n Zd 7 mr; C m-3 d-') and >fi-fo!c! (n 07 to 0.33 c!-'). respectively. The integrated values of BB and BP (IBB and IBP) as well as the averaged growth rates (pze; IBP/IBB), were in the ranges of 694 to 1406 mg C m-', 98
to 328 mg C m-' d-' and 0.11 to 0.27 d-l, respectively. In 1997, no clear spatial pattern for IBB existed in areas in-
side the shelf edge. However, in warm water areas
(>20°C), IBB tended to decrease from the shelf edge to the open ocean (Fig. 2D). On the 1996 cruise, IBB de- creased from the inner to outer shelf, as indicated by the negative trend between IBB and temperature (Fig. 2D).
On both cruises, in terms of their relationships with temperature, bacterial rate parameters (IBP and pZe) showed similar patterns over the shelf (Fig. 2E-F). In areas with temper;ti~res c20°C. IBP and !l,, increased with rising temperature; above 20°C these parameters showed no significant trend relative to temperature (see below). The use of the averaged value (p,,) to rep- resent bacterial individual growth rates at different depths (i.e. 1.1) seems reasonable since the values of p
did not vary much within the euphotic zone at any given station. For almost all of the sampling stations, the standard deviations of p were quite small, while the coefficients of variation (CV = standard deviation X 100/average) seldom exceeded 20%. The correlation
Shidh et a1 Shelf bacterial production 25 1 --
coefficient (i.e. r) of p versus pze was signifi- 120"E 122"E 124'E
cant (r = 0.92, n = 38, p < 0.01). 28"N 28'N
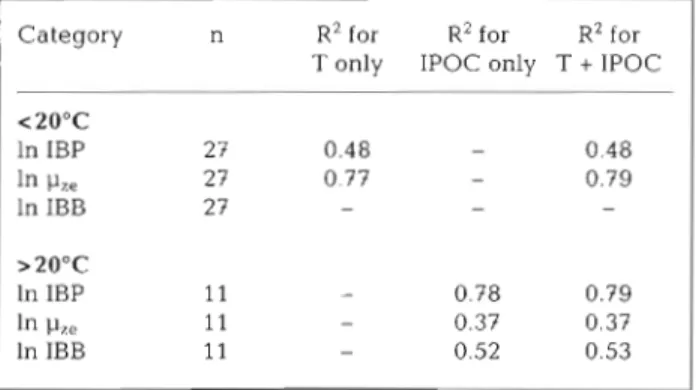
The relationship of temperature versus IBP (and pze) was examined with a polynomial curve fitting method, which justified that the apex of that best-fitted curve was located at 20°C (n = 38, R2 = 0.67, p < 0.01). After this, the multiple regression of natural log trans-
formed IBP (1nIBP) and pze (1npre) on tem- 26-N 26'N
perature and IPOC (independent variables) in the ranges of <20°C and >20°C was per- formed. Inside the shelf edge (<20°C), more than 50 % of the variations (R2 = 0.48 to 0.77; p < 0.01) of IBP and p,, could be explained by temperature alone (Table 2); in fact, the
slopes of In IBP on temperature for the 1996 24'N 24'N
(0.10 k 0.04) and 1997 (0.10 + 0.02) cruises
were almost identical (ANCOVA; p > 0.05). 28'N Analysis of In p,, versus temperature
showed the same trend with slope values of 0.12 * 0.05 and 0.12
+
0.01, respectively. The estimated Qlo (the increase in the growth rate for each 10°C increase in temperature) values of IBP and p,, for the pooled data setwere 2.7 and 3.3, respectively. Neither tem- 26'N perature nor IPOC correlated with IBB.
Conversely, IBP, p,, and IBB values recorded outside the shelf edge were posi- tively correlated with IPOC, with R2 values of 0.37 to 0.78 (n = 11, p < 0.01). For the <20°C data, the addition of IPOC as an extra
independent variable only slightly increased 24'N
the R2 values of IBP and pze (Table 2). On the 120"E 122"E 124"E
other hand, IPOC was the predominant fac- Fig 3 Contours of the euphotic-zone averaged temperature ( A . 'C) and tor in explaining the variance of IBP, pze and the euphotic-zone integrated nitrate concentrations (B mm01 m-2) IBB in the warm water areas.
DISCUSSION
Variables Symbol Unit 1996 1997
Temperature T "C 13.6/24.5 12.2/24.3 Salinity S psu 30.043/34.497 30.012/35.700 Nitrate NO3- pM <0.2*/9.6 <0.2/15.6 Particulate organic POC p M 1.2/52.2 3.2/60.4
carbon Chlorophyll Chl mg chl m-3 0.2/2.4 0.2/4.0 Bacterial biomass BB mg C m-3 4/64 14/35 Bacterial production BP rng C m-3 d-' 0.3/14.7 1.3/11.5 Bacterial turn-overb p d-' 0.07/0.31 0.03/0.38 aDetection limit bCalculated a s BP/BB
Table 1. Ranges of measured variables collected from the euphotic zone
The spatial patterns of temperature over in the continental shelf of the southern East China Sea d u n n g the spring
seasons of 1996 and 1997 the shelf give rise to 2 important implications in this study. Physiologically, temperature may directly affect bacterial growth rates and thus production, which peaked at 20°C (Fig. 2E-F). This confirms many studies (Shiah & Ducklow 1995 and citations therein) whlch have indicated that in temperate a n d subtropical areas, the optimal temperature for bacterial growth was in the range of 20 to 25°C. From a hydrographic point of view, the 20°C isotherm (Fig. 3A) located a t the shelf edge served as a mark separating the study area Into 2 systems with distinct temperature (cold vs warm) and trophic (mesotrophlc vs oligotrophic) characteristics. Temperature
252 Aquat Microb Ecol 17: 247-254, 1999
Table 2. Coefficient of determination ( R ~ ) (all significant at p < 0.01 level) for multiple regression analyses of temperature (T)
and the euphotic-zone integrated particulate organlc carbon concentrations (IPOC) versus bacterial measurements (the euphotic-zone integrated bacterial production [IBP], bacterial
biomass [IBB] and averaged growth rates [p,,; IBP/IBB] after
natural log transformation). T, IPOC independent variables.
n: sampling size
Category n R2for R2for for
T only IPOC only 7
+
IPOC<2O0C In IBP 27 0.48 - 0.48 In 27 0.77 0.79 In IBB 27 - - >2O0C In IBP 11 0.78 0.79 ln p,, 11 - 0.37 0.37 In IBB 1 1 - 0.52 0.53
was an indication of the inorganic nutrient supply (Figs. 2B & 3B) which, in turn, affected algal biomass, activity (i.e. primary production) and, hence, the
potential supply rates of substrate (IPOC; see below) required for bacterial growth. This might be particu- larly true for bacteria outside the shelf edge in warm
and oligotrophic waters (Fig. 2C). Since bacterial rate
parameters peaked at 20°C and the 20°C isotherm roughly separated the study area into 2 different
trophic systems, we chose the temperature of 20°C as
the cut-off point in statistical analyses.
Primary production and dissolved organic carbon concentration data were not available in this study. IPOC was used as the index of substrate supply since they were positively correlated with Ichl. In fact, IPOC might be more indicative than Ichl since all biological components (except most bacteria and viruses), dead or alive, are included.
The results here suggest that the controlling mecha- nisms of bacterial growth might be quite different inside and outside the shelf edge, as judged from their distinct relationships with temperature and IPOC in different temperature ranges (<20°C and >20°C;
Fig. 2F, Table 2). More specifically, it is hypothesized hero !ha! di~rinrj the cprir?g seasnn, bacteria! grnw?h inside and outside the shelf edge are primarily con- trolled by temperature and substrate supply, respec- tively.
Substrate supply has long been recognized as a dominant factor in regulating bacterial growth rates in aquatic ecosystems (Cole et al. 1988, see also Ducklow & Carlson 1992, Fuhrman 1992 for review). However,
several recent studies have suggested that the strength
of bottom-up control might be system dependent and might a.lso change temporally within a system. Duck-
low & Shiah (1993) first proposed that bacterial growth in estuaries might be temperature limited, with the substrate supply playing a less important role. This hypothesis has been confirmed by experiments per- formed in Delaware Bay (Hoch & Kirchman 1993), Chesapeake Bay (Shiah & Ducklow 199413, 1995) and Kiel Fjord (Berman et al. 1994). These findings are of little surprise because many estuaries are eutrophic, showing large amounts of external inputs, either in terms of inorganic nutrients or organic substrate. Under such conditions, bacterial growth might actually be saturated by high substrate supply rates during most seasons of the year.
The data in this study lend support to the fact that the temperature dominance hypotheses could also be applied in explaining the spatial pattern of bacterial growth rates in a mesotrophic environment. As argued by Ducklow & Shiah (1993) and others, if bacterial growth is primarily controlled by factors other than temperature, then a strong relationship between bac- terial growth rates and temperature is less likely to occur. That is, bacteria simply cannot grow without the proper amount of substrate supply, even when growth temperature is optimal (see also below). More impor- tantly, the Qlo value derived from this study (3.3) was very similar 'to the ones reported by Shiah & Ducklow (1994a, 1995) in their seasonal studies in Chesapeake Bay (averaged Qlo = 3.2) and in a salt marsh tidal creek (overall Q l o = 3.2). White et al. (1991) reported an aver- age Qlovalue of 3.3 for bacterial growth rates based on data collected from 57 studies conducted in fresh, marine and estuarine/coastal waters. Interestingly, those 3 studies addressed the temperature effects on bacterial temporal variability within eutrophic sys- tems, whereas the present one emphasized spatial variability in mesotrophic environment (i.e. areas inside the shelf break). This indicates that the temper- ature dependence of bacterial growth rates (Qlo) might be comparable in both systems and over both temporal and spatial scales.
In, warm water areas, a strong correlation between
bacterial growth rates and IPOC (Table 2) was observed. This indicates that substrate supply rates rather than temperature might be the major factor in
r ~ g i l l a t i n r ~ bacterial growth rates in areas outside the shelf edge. The following scenario is proposed as an explanation. In areas next to the oligotrophic environ- ment (the KbV), temperature (20.0 to 24.5"C) itself was probably optimal for bacterial growth (Shiah & Ducklow 1994a,b, 1995). However, as temperature increased, the system became more oligotrophic (Fig. 2B) which resulted in the reduction of substrate supply and thus bacterial growth rates. This implies that as the system became more of an oligotrophic con- dition, the more significant substrate control of bacter-
Shiah et al.: Shelf bacterial production 253
ial growth might become. Outside the shelf edge, IBB was positively correlated with IPOC with an R2 value of 0.52. This observation supported the conclusion of Sanders et al. (1992) who suggested that bottom-up (substrate supply) control dominated in regulating bactellal bion~ass in oligotrophic environments.
In addition to temperature and substrate supply rate, other factors, such as bacterivory and viral lysis (top- down control; see Ducklow & Carlson 1992, Fuhrman 1992 for review) also play significant roles in regulating bacterial biomass and production. Bacterial biomass in- side the shelf edge showed no distinct relationships with any other environmental variables. It is very possi- ble that the spatial pattern of bacterial biomass inside the inner shelf was more affected by these 2 processes. However, no tangible conclusion regarding this can be made due to the lack of bacterivory and viral lysis data. The outcrop of the nutrient-enriched Kuroshio Sub- surface Waters (KSW) caused by the change of bottom topography definitely has a strong influence on the hydrography and thus, the spatial patterns of biologi- cal phenomena. The most recent study (Liu et al. 1999) showed that for the continental shelf of the ECS, nutri- ent (nitrate as example) flux derived from the KSW (5.5 to 7.1 km01 N S-') at the shelf edge adjacent northern Taiwan (i.e. the first I N 0 anomaly in Fig. 3B) was at least 2-fold higher than that from coastal input (i.e. river runoffs; 2.1 km01 N S-'; Zhang 1996). Our obser- vation suggests that there might be other upwellings (i.e. the second I N 0 anomaly in Fig. 3B) along the shelf edge. Their occurrence frequency (persistent or episodic) and possible contribution in importing nutri- ents into the continental shelf of the ECS and the olig- otrophic Kuroshio waters merit further investigation.
Another interesting phenomenon which is worthy of further study is that the values of IPOC were also more or less peaked at the boundary of the inner and outer shelf regions (ca 16 to 20°C; Fig. 2C). The high correla- tion observed between Ichl and IPOC (see above) indi- cated that the biomass (and perhaps activities) of algae and other planktoners within this 'transition zone' were high. In comparison to this area, nutrient-rich waters in the inner shelf (see also below) were colder and more
turbid, which were not favorahle for the devel.opment of high Ichl and IPOC (Shiah et al. 1996). More interest- ingly, the nitrogen to phosphate ratios (N/P ratios) from the river runoffs were in the range of 46 to 84 (Edmond et al. 1985, Zhang 1996), while the N/P ratios of the KSW (14.0 to 15.4; Liu et al. 1999) were slightly lower than the Redfield ratio of 16. Wong et al. (1998) de- duced that such serious N/P imbalance probably was the major factor in limiting algal biomass (and produc- tivity) in the inner shelf areas. Finally, differential graz- ing pressure from zooplankton might be an important reason but needs to be verified in the future.
During spring, the spatial variation of bacterial growth rates and production in the continental shelf of the East China Sea were interactively regulated by both temperature and substrate supply. However, the relative strength of these 2 controlling factors varied, with temperature and substrate supply dominating inside and outside the shelf edge, respectively. The strong relationship observed between bacterial growth rates and temperature implies that during spring, bac- terial growth in the shelf system might not be limited by substrate supply. In view of other published results derived from the eutrophic environment, it seems fea- sible that in both mesotrophic and eutrophic ecosys- tems, temperature plays a more dominant role than substrate supply in regulating bacterial growth rates, at least during certain seasons (e.g. spring) of the year. In addition, similar Q,,, values derived from this and other studies further suggest that temperature depen- dence of bacterial growth and production might be con~parable in these ecosystems.
Acknowledgements. CORE-NSC contribution paper number 7. Support for this research was provided by the National Sci- ence Council (NSC), Taiwan, ROC. Valuable comments from Dr J. Christian and 2 anonymous reviewers on this manuscript are deeply appreciated. We thank the officers and crew mem- bers of the RV 'Ocean Researcher I' for cruise assistance.
LITERATURE CITED
Berman T, Hoppe H, Gocke K (1994) Response of aquatic bac- terial populations to substrate enrichment. Mar Ecol Prog Ser 104:173-184
Biscaye PE, Flagg CN, Falkowski PG (1994) The shelf-edge exchanges processes experiment, SEEP-11: a n introduction to hypotheses, results and conclusions. Deep-Sea Res Part 11 41 (2/3):231-252
Cho BC, Azam F (1988) Major role of bacteria in biogeochem- ical fluxes in the ocean's interior. Nature 332:441-443 Cole JJ, Findlay S, Pace ML (1988) Bacterial production in
fresh and saltwater ecosystems: a cross-system overview. Mar Ecol Prog Ser 43:l-10
Ducklow HW, Carlson CA (1992) Oceanic bacterial produc- tion. In: Marshall KC (ed) Advance in microbial ecology. Plenum, New York, p 113-181
Ducklow HW, Shiah FK (1993) Bacterial production in estuar- ies. in: Ford T (ed) Aquatic microbiology: an ecological approach. Blackwell Sci Publ, Boston, p 261-287
Edmond JM, Spivack A, Grant BC, Hu MH, Chen Z, Chen S, Zeng X (1985) Chemical dynamics of the Changjiang estu- ary. Cont Shelf Res 4:17-36
Fuhrman J A (1992) Bacterioplankton roles in cycling of organic matter: the microbial food web. In: Falkowski PG, Woodhead AD (eds) Primary productivity and bio- geochemical cycles in the sea. Plenum, New York, p 361-383
Fuhrman J A , Azarn F (1982) Thymidine incorporation as a measurement of heterotrophic bacterioplankton produc- tion in marine surface waters: evaluation and field results. Mar Biol66:109-120
254 Aquat Microb Ecol17: 247-254, 1999
Gong GC, Liu KK, Pai SC (1995) Prediction of nitrate concen- tration from two end member mixing in the southern East China sea. Cont Shelf Res 15:827-842
Gong G, Shiah F, Liu K. Chuang W, Chang J (1996) Effect of Kuroshio intrusion on the chlorophyll distribution in the southern East China sea north of Taiwan during spring, 1993. Cont Shelf Res 17(1):79-94
Griffith P, Shiah F, Gloersen K, Ducklow HW, Fletcher M (1994) Activity and distribution of attached bacteria in Chesapeake Bay. Mar Ecol Prog Ser 108:l-10
Hobbie JE, Daley RJ, Jasper S (1977) Use of nuclepore filters for counting bacteria by fluorescence rnicroscopy. Appl Environ Microbiol 33 (5):1225-1228
Hoch MP, Kirchman DL (1993) Seasonal and inter-annual variability in bacterial production and biomass in a tem- perate estuary. Mar Ecol Prog Ser 98:283-295
Kemp PF (1994) ~MicrobiaI carbon utilization on the continen- tal shelf and slope during the SEEP-I1 experiment. Deep- Sea Res Part I1 4 1 (2/3):563-582
Lancelot C, Billen G (1984) Activity of heterotrophic bacteria and its coupling to primary production dunng the spring phytoplankton hloorn in the South Bight of t h ~ North Sea. Limnol Oceanogr 29(4):721-730
Levitus S (1982) Climatological atlas of the world ocean. NOAA, Seattle
Liu K, Gong G, Shyu C, Pai S, Wei C, Chao S (1992) Response of Kuroshio upwelling to the onset of northeast monsoon in the sea north of Taiwan: observations and a numerical simulation. J Geophys Res 97:12511-12526
Liu KK, Tang V, Gong GC, Chen LY, Shiah FK (1999) Cross- shelf and along-shelf nutrient fluxes from flow fields and chemical hygrography observed in the southern East Chlna Sea off northern Taiwan. Cont Shelf Res (in press) Editorial responsibility: Farooq Azam,
La Jolla, California, USA
Mantoura RFC, Martin JM, Wollast R (1991) Ocean margin processes in global change Wiley h Sons, New York Parsons TR, Maita Y, Lalh C M (1984) A manual of chemical
and biological methods for seawater analysis. Pergamon, New York
Sanders RW, Caron DA, Berninger U (1992) Relationships between bacteria and heterotrophic nanoplankton in marine and fresh waters: an inter-ecosystem comparison. Mar Ecol Prog Ser 86:l-14
Shiah F, Ducklow HW (1994a) Temperature and substrate regulation of bacterial abundance, production and speclf~c growth rate in Chesapeake Bay, USA. Mar Ecol Prog Ser 103:297-308
Shiah F, Ducklow HW (1994b) Temperature regulation of het- erotrophic bactenoplankton abundance, production, and specific growth rate in Chesapeake Bay. Lirnnol Oceanogr 39(6).1243-1258
Shiah F, Ducklow HW (1995) Multi-scale variability in bacte- rioplankton abundance, production and specific growth rate in a temperate salt marsh tidal creek. Limnol Oceanogr 40(1):55-66
Shiah F; Gong G, Liu K (1996) Light effects on vhytoplankton photosynthetic performance in the southern East Chlna Sea north of Taiwan. Bot Bull Acad Sin 37:133-140 Wong GTF, Gong GC, Liu KK, Pai SC (1998) 'Excess
nitrate' in the East China Sea. Estuar Coast Shelf Sci (in press)
White PA, Kalff J , Rasmussen JB, Gas01 JM (1991) The effects of temperature and algal biomass on bacterial production and specific growth rate in freshwater and marine habi- tats. Microb Ecol21:99-118
Zhang J (1996) Nutrient elements in large Chinese estuaries. Cont Shelf Res 16:1023-1045
Submitted: February 9, 1998; Accepted: August 31, 1998 Proofs received from author(s1: May 26, 1999