行政院國家科學委員會專題研究計畫 成果報告
預測罹患嚴重急性呼吸道症候群發生急性呼吸衰竭之高危
險群
計畫類別: 個別型計畫 計畫編號: NSC92-2751-B-002-016-Y 執行期間: 92 年 07 月 01 日至 93 年 06 月 30 日 執行單位: 國立臺灣大學醫學院內科 計畫主持人: 余忠仁 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 12 月 14 日
行政院國家科學委員會補助專題研究計畫
■ 成 果 報 告
□期中進度報告
預測罹患嚴重急性呼吸道症候群發生急性呼吸衰竭之高危險群
計畫類別:■ 個別型計畫 □ 整合型計畫
計畫編號:NSC 92-2751-B-002-016-Y
執行期間:
92 年 7 月 7 日至 93 年 6 月 30 日
計畫主持人:余忠仁
共同主持人:
計畫參與人員:
成果報告類型(依經費核定清單規定繳交):□精簡報告 ■完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、
列管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開查詢
執行單位:國立台灣大學醫學院內科
中 華 民 國 93 年 12 月 14 日
中文摘要 關鍵詞:嚴重急性呼吸道症候群、急性呼吸衰竭 至今年7 月 31 日止,全世界共有 8,098 確定 SARS 病例,774(9.6%)例死亡。大約 10-20%之 SARS 患者發生呼吸衰竭需要呼吸器與重症醫療。死亡主要與年齡有關。 對於持續惡化的病人,及早在瀕臨呼吸衰竭前插管並給予合乎標準的呼吸照護被認 為是目前最有效的治療。由先前的觀察大致可以歸納出會發生疾病惡化之危險因 子,包括高齡、中性白血球偏高、高 LDH、嚴重的淋巴球缺乏、延遲給予類固醇與 Ribavirin 合併治療及慢性 B 型肝炎感染。然而,除了年齡之外,並沒有一個簡易的 疾病標誌可供辨認哪位病患會發生呼吸衰竭以及死亡。每天重複監測各項實驗室檢 查數據是唯一辨識這些高危險群的方法。胸部影像檢查也可以提供重要的預後資 訊,但是,也必須每天重複檢查胸部影像。有時候,病情發展太快,胸部影像檢查 一日數變,增加照護上的困難。雖然 SARS 治療指引上也明確規範在危險病患早期 插管,但有時確然難以辨識誰為危險病患。而一但為緊急插管,常會導致不佳的後 果。台大醫院在上一波 SARS 盛行期間共收治 76 位確認 SARS 病患,其中 27 位發 生急性呼吸衰竭,15 位死亡,多數死亡均因併發急性呼吸窘迫症候群或多器官衰竭。 本計畫中,我們希望利用這76 位確認 SARS 病患之臨床資訊,包括症狀、理學檢查
與一系列之實驗室檢查之資料,利用Oracle Clinic 與 Classification and Regression Tree analysis (CART)分析方式,建立一預測系統,以早期辨識會發生呼吸衰竭之高危險群 病患,可幫助醫護人員對於這些病患提供更密切的監測,以降低死亡率。
英文摘要
Keywords:Severe acute respiratory syndrome, acute respiratory failure
Severe Acute Respiratory Syndrome (SARS) is an acute respiratory illness caused by infection with a new coronavirus, SARS-CoV, identified in March, 2003. The disease is characterized by high contagious, may progress rapidly to respiratory failure and is potentially lethal in severe cases. As of June 5, 8,402 cases of SARS had been reported in the world, and 772 (9.2%) died of the disease. About 10-20% of SARS patients developed respiratory failure requiring intensive care and mechanical ventilation. Mortality majorly depends on the age group affected, with an overall estimate of 14% to 15%. For patients with progressive deterioration, early intubation for impending respiratory failure and providing best supportive and respiratory care are considered to be of primary importance. Risk factors that have been reported to be associated with a progressive disease are: older age, high neutrophil count, high LDH peak; severe lymphopenia, impaired ALT, delayed starting of ribavirin and steroids; chronic Hepatitis B infection. However, besides age, no single early markers or clinical parameters have been identified to predict the progression to respiratory failure or fatal outcome. Tedious daily laboratory monitoring is necessary to figure out who will develop progression of the disease. Chest radiographs offer important prognostic clues for disease progression. However, serial chest films are necessary, and sometimes the clinical deterioration is too rapid that a regular chest film may not be useful, dramatic change may appear within one single day. Although a guideline had been proposed for caring SARS patients, which advised elective and early intubation for SARS patients with impending respiratory failure, clinical prediction of high risk patient is sometimes not possible. Emergent intubation or the need of resuscitation seems related to the fatal outcome. In this study, we plan to establish a prediction system by using the clinical parameters early in the disease course to identify patients with high risk for acute respiratory failure, which will help doctors to provide a more intensive monitoring of high-risk patients.
Background information
Severe Acute Respiratory Syndrome (SARS) is an acute respiratory illness caused by infection with a new coronavirus, now named SARS-CoV, which was identified in March, 2003. The virus is predominantly spread by droplets or by direct and indirect contact. Medical personnel, physicians, nurses, and hospital workers are among those commonly infected. The disease is characterized by high contagious, may progress rapidly to respiratory failure and is potentially lethal in severe cases. As of June 5, 8,402 cases of SARS had been reported in the world, and 772 (9.2%) died of the disease. The clinical presentation of SARS is not specific, resembling those of other forms of "atypical pneumonia" caused by legionella, mycoplasma and chlamydia species. After an incubation period of 2 to 10 days, patients develop a fever (> 38.0° C) associated with other symptoms including chills, rigors, headache, dizziness, malaise, and myalgia (1-5). Sputum production, sore throat, coryza, nausea and vomiting, and diarrhea are less common (2). The most common laboratory abnormalities include lymphopenia, leukopenia, thrombocytopenia, elevated lactate dehydrogenase (LDH) levels, elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, and elevated creatine kinase (CK) levels (2-6). The severity of the illness is highly variable, ranging from mild symptoms to a severe disease process with respiratory failure (> 20 %) and death. (2,4). Body temperature normally remains above baseline during the progression of the disease. In the first published follow-up study, patients developed recurrent fever (85.3%) on day 8.9 ± 3.1 (range 4 to 18), watery diarrhea (73.3%) on day 7.5 ± 2.3 (range 3 to 15), radiological deterioration (80%) on day 7.4 ± 2.2 (range 3 to 13) and respiratory deterioration (45.3%) on day 8.6 ± 3 days (range 5 to 19) (7).
At present, the most efficacious treatment regimen for SARS, if any, is unknown. The combination of ribavirin and steroids is generally thought to be responsible for some clinical improvement in SARS patients (2,3,6). However, not every patient with severe SARS responds to the above treatment. About 10-20% of SARS patients developed respiratory failure requiring intensive care and mechanical ventilation. Mortality majorly depends on the age group affected, with an overall estimate of 14% to 15% (8,9). For patients with progressive deterioration, early intubation for impending respiratory failure and providing best supportive and respiratory care are considered to be of primary importance.
Risk factors that have been reported to be associated with a progressive disease are: older age, high neutrophil count, high LDH peak; severe lymphopenia, impaired ALT, delayed starting of ribavirin and steroids; chronic Hepatitis B infection. However, besides age, no single early markers or clinical parameters have been identified to predict the progression to respiratory failure or fatal outcome. Tedious daily laboratory monitoring is necessary to figure out who will develop progression of the disease.
Chest radiographs offer important prognostic clues for disease progression. In particular, when, after approximately one week, unilateral, predominantly peripheral areas of consolidation progress to bilateral patchy consolidation. The extent of the lung opacities is correlated with the deterioration in respiratory function (2). However, serial chest films are necessary, and sometimes the clinical deterioration is too rapid that a regular chest film may not be useful, dramatic change may appear within one single day.
In Taiwan, after the identification of first SARS patient in March, 2003 and the outbreak from HoPing Hospital in late April, 2003, a total of 678 probable SARS patients have been identified, 81 died of the disease, with a mortality rate of 11.9%. In NTUH, 79 probable SARS patients had been admitted in our hospital, 14 died. All of the deceased had acute respiratory failure, and most of them received mechanical ventilation and intensive care due to morbid condition. A guideline had been proposed for medical personnel to care SARS patients, which advised elective and early intubation for SARS patients with impending respiratory failure. However, prediction
of high-risk patient beforehand the deterioration of disease is sometimes not possible, and emergent intubation or the need of resuscitation related to the fatal outcome. In this study, we plan to establish a prediction system by using the clinical and laboratory parameters early in the disease course to identify patients with high risk for acute respiratory failure, which will help doctors to provide a more intensive monitoring of high-risk patients. A prediction system will be built based on the clinical and laboratory parameters of SARS patient hospitalized to the hospital between March 7, 2003 and June 7, 2003. The validity of the prediction model will be tested in patient population admitted to the hospital after August 1, 2003. The clinical parameters include: age, sex, comorbidity, treatment modality; laboratory parameters include: complete blood cell counts, lymphocyte count, LDH, CPK, AST, ALT, CRP, and certain experimental plasma/serum surrogate markers which had been reported to be useful in predicting acute lung injury.
Subjects and methods
Study population
We performedthe study at National Taiwan University Hospital, which isa medical center with approximately 2000-bed. The study includes all SARS patientsidentified from March 2003 through April2004.
Study Design
A retrospectivecohort analysis will be performed to establish such predicting model. All the data existed in the medical charts and individual collecting of spreadsheet will be uniformly assembled and collected. Model to build as a predicting solution is the primary goal. We will use the state-of-art data base system together with clinical trial program, Oracle Clinics, as the data collecting solutions. This will not only cover the data have been generated since the outbreak of SARS, but also the potential patients to be seen in the future. Thus, a model is to be established using the retrospective manner as the learning sample, data to be collected in the future from the clinics and emergency will be used as the testing sample of the model.
Data Collection
Clinical data. Data regarding the following characteristics were obtained for each patient from medical records: age,sex, comorbidities present, initial and serial chest X-ray findings and scores, initial and follow-up laboratory (electrolyte, LDH, CPK, AST, ALT, CRP) and ABG data.
The presence of thefollowing comorbid conditions wasdocumented: diabetes mellitus, heartfailure, chronic obstructive pulmonarydisease (COPD), chronic hepatitis,renal failure (as indicatedby the necessity fordialysis), history of cerebrovascularaccident, malignancy, alcoholism, administrationof immunosuppressive drugs (receiptof >20 mg ofprednisolone per day orof an equivalent corticosteroidfor 14 days beforethe onset of SARS,or receipt of anyantineoplastic chemotherapy in the3 months before theonset of SARS).
Clinical database and data entry program
Though in a retrospective manner of this study, all the data collected from the clinics either through the regular outpatients units or from the emergency will be carefully collected and recorded. Instead of the common spread sheet type data collecting and recording tools such as MS Excel; or the simple database such as MS Access to be used. Oracle Clinics (OC) software widely used in the clinical trials area will be utilized and used for 100% GCP compliance principles. Reasons for this OC to be used are that it
can perform the online data entry, real time data checking, online query, monitoring and real time data reporting.
Features of the Oracle Clinics
With the Study Design and Management subsystem, we can design protocols and amendments as well as specify how patient data is tracked especially in the timing data tracking of the any recategorization of the SARS. Oracle Clinical features sophisticated site, patient, and visit tracking to:
• Assign and maintain information on investigators and sites
• Visualize the planned, projected, and actual patient enrollment and study timelines • Develop detailed visit schedule specification and tracking, including the identification of missing and late Case Report Forms (CRFs)
• Track patient availability and progressing information
Study Data Definition
The study data definition subsystems enable a single study to be defined and conducted at several worldwide locations concurrently with minimal additional effort. The essential subsystems include: global library management, study data definitions, a data validation facility, and lab reference range management. Using the specific features in defining the SARS protocols, one can easily derive the necessary information for the identification of SARS.
Study Conduct and Validation
We will use OC to capture and edit data and edit the screen layout to parallel the CRF layout. This data validation can reduce the time spent identifying and finding data problems. This is accomplished through a library of procedures that can be used and reused continually. During this process, each data problem identified creates a discrepancy record that can be tracked and summarized. Data, validation checks, and discrepancies are all synchronized so that when a change is made to any unique component, the system automatically reflects the change in all areas.
Data Access and Reporting
Oracle Clinical stores all data results in a universal format. This universal format means that study set-up, data collection, and data extract do not require specialist database design skills. This includes::
• Automatically create views corresponding to each CRF and automatically extract data into SAS for analysis
• Create custom views combining data from multiple CRFs
• Create any number of data snapshots for interim analysis during normal data processing
• Query the data through an online query facility
• Include locked or frozen data, as well as discrepancy status information, in extracted data
Using these features described above, one can setup the SARS database in the state-of-art data collecting architecture which to ensure the data collected from the sites in the best quality.
Data Coordinating Center
The Bioinformatics and Biostatistics Core Facility of the National Taiwan University Hospital headed by Dr. Chee Jen Chang, have the access and ability to perform such tasks in designing the database of OC for the SARS data collection. Through the NTUH internal research grant, this center is able to provide such ability in the process described
above.
Statistical Methods
Variables in the data entry programs stored, then retrieved, and exported for analysis will be through the SAS system. For those variables interested for analysis will be examined using QA/QC procedures to ensure the best quality of the data. For those continuous variables, mean, median, standard deviation of the descriptive statistics will be derived and tabulated. Ordinal and nominal categorical variables, frequencies and percentage of the level of each of the discrete variables will be derived and tabulated. Any necessary comparisons of both continuous and categorical variables will be made. Both parametric and non-parametric statistical methods will be utilized.
CART
Classification and Regression Tree analysis (CART) a statistical method to handle multiple variables on one measure, either binary (classification) or continuous (regression) outcome, are feasible in survey and epidemiological field (14). This has been widely used in exploring the homogeneous subgroups in the genetic studies (15,16). In the field of early prediction of the risk factors to a certain disease such as SARS, CART is considered as important tool for managing heterogeneity problem. Together with common predicting statistical tools such as the logistic regression and multiple regression, one can easily demonstrate the possible risk factors to the SARS with acute respiratory failure in this protocol.
Results and conclusions
63 patients with SARS were enrolled into analysis, 16 of them were intubated due to refractory hypoxemia under oxygen therapy. The difference of demographic data and
outcome between those who were or were not intubated were calculated. (Table 1-3) Using CART analysis , we had established a diagnostic tree for identifying patients with higher risk of developing acute respiratory failure due to SARS.(Fig. 1). However, since no outbreak of SARS has occurrred after July 31, 2003, the diagnostic tree can not be validated.
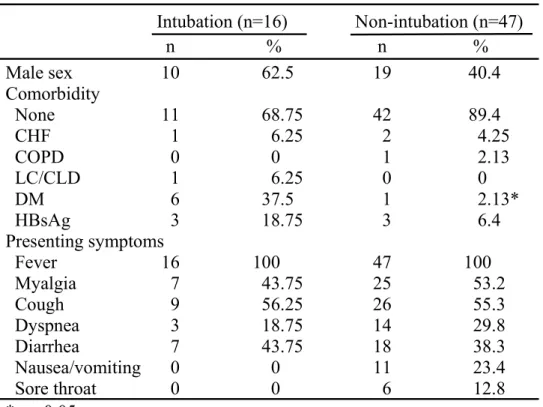
Table 1. Demographic data of SARS patients
Intubation (n=16) Non-intubation (n=47) n % n % Male sex 10 62.5 19 40.4 Comorbidity None 11 68.75 42 89.4 CHF 1 6.25 2 4.25 COPD 0 0 1 2.13 LC/CLD 1 6.25 0 0 DM 6 37.5 1 2.13* HBsAg 3 18.75 3 6.4 Presenting symptoms Fever 16 100 47 100 Myalgia 7 43.75 25 53.2 Cough 9 56.25 26 55.3 Dyspnea 3 18.75 14 29.8 Diarrhea 7 43.75 18 38.3 Nausea/vomiting 0 0 11 23.4 Sore throat 0 0 6 12.8 * p <0.05
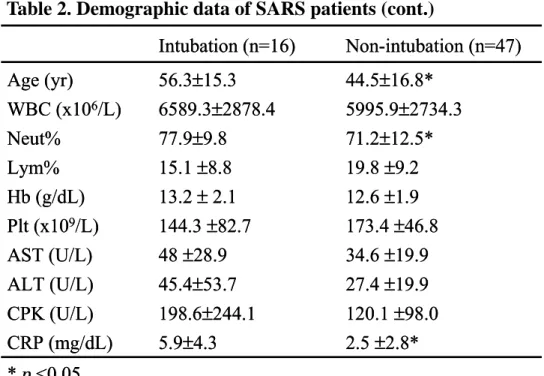
Table 2. Demographic data of SARS patients (cont.)
Table 3. Outcome of SARS patients
Intubation (n=16) Non-intubation (n=47) Age (yr) 56.3±15.3 44.5±16.8* WBC (x106/L) 6589.3±2878.4 5995.9±2734.3 Neut% 77.9±9.8 71.2±12.5* Lym% 15.1 ±8.8 19.8 ±9.2 Hb (g/dL) 13.2 ±2.1 12.6 ±1.9 Plt (x109/L) 144.3 ±82.7 173.4 ±46.8 AST (U/L) 48 ±28.9 34.6 ±19.9 ALT (U/L) 45.4±53.7 27.4 ±19.9 CPK (U/L) 198.6±244.1 120.1 ±98.0 CRP (mg/dL) 5.9±4.3 2.5 ±2.8* * p <0.05 Intubation (n=16) Non-intubation (n=47) Age (yr) 56.3±15.3 44.5±16.8* WBC (x106/L) 6589.3±2878.4 5995.9±2734.3 Neut% 77.9±9.8 71.2±12.5* Lym% 15.1 ±8.8 19.8 ±9.2 Hb (g/dL) 13.2 ±2.1 12.6 ±1.9 Plt (x109/L) 144.3 ±82.7 173.4 ±46.8 AST (U/L) 48 ±28.9 34.6 ±19.9 ALT (U/L) 45.4±53.7 27.4 ±19.9 CPK (U/L) 198.6±244.1 120.1 ±98.0 CRP (mg/dL) 5.9±4.3 2.5 ±2.8* * p <0.05 Intubation (n=16) Non-intubation (n=47) n % n % Death 9 56.25 3 6.4* ARDS 16 100 5 10.6* Barotrauma 3 18.75 0 0* Rhabdomyolysis 6 37.5 0 0* Bacteremia 7 43.75 1 6.25*
Specific pharmacological treatment
Steroid 14 87.5 43 91.5
Pulse steroid 4 25 4 8.5
IVIG 8 50 26 55.3
Figure 1. CART analysis of SARS patients with ARF
Figure 1. CART analysis of SARS patients with ARF (cont.)
Node 5 No myalgia Node 6 Not HBsAg carrier Node 8 Throat complaint Node 7 No Cough Intubation 25% (4/16) Intubation 75% (3/4) Intubation 11.1% (1/9) Intubation 37.5% (3/8) Intubation 0% (0/1)
+
-+
-+
-+
-Node 5 No myalgia Node 6 Not HBsAg carrier Node 8 Throat complaint Node 7 No Cough Intubation 25% (4/16) Intubation 75% (3/4) Intubation 11.1% (1/9) Intubation 37.5% (3/8) Intubation 0% (0/1)
+
-+
-+
-+
-Node 1 Plt≤92.5k Intubation 100% (4/4) Node 4 Neut≤58.7% Node 3 ALT≤34 Node 2 CPK≤82.5 Node 5 No myalgia Intubation 0% (4/4) Intubation 33.3% (4/12) Intubation 25% (1/4)
+
−
+
−
+
−
+
+
−
(…continued)
−
Node 1 Plt≤92.5k Intubation 100% (4/4) Node 4 Neut≤58.7% Node 3 ALT≤34 Node 2 CPK≤82.5 Node 5 No myalgia Intubation 0% (4/4) Intubation 33.3% (4/12) Intubation 25% (1/4)+
−
+
−
+
−
+
+
−
(…continued)
−
References
1. CDC. Preliminary Clinical Description of Severe Acute Respiratory Syndrome. MMWR 2003; 52;255-256.
2. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003;348:1986-94.
3. Tsang KW, Ho PL, Ooi GC, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003;348:1977-85.
4. Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003 Apr 19;361(9366):1319-25
5. Moira Chan-Yeung, W C Yu. Outbreak of severe acute respiratory syndrome in Hong Kong Special Administrative Region: case report. BMJ 2003;326:850-852 6. Poutanen SM, Low DE, Henry B, et al. Identification of severe acute respiratory
syndrome in Canada. N Engl J Med 2003 May 15;348(20):1995-2005.
7. JSM Peiris, CM Chu, VCC Cheng, et al. Prospective study of the clinical progression and viral load of SARS associated coronavirus pneumonia in a community outbreak. 8. Donnelly CA, Ghani AC, Leun GM, et al. Epidemiological determinants of spread of
causal agent of sever acute respiratory syndrome in Hong Kong. Lancet. May 7, 2003 (published on line).
9. WHO. Update 49 - SARS case fatality ratio, incubation period.
10. Ware LB, Conner ER, Matthay MA. Von Willebrand factor antigen is an indepndent marker of poor outcome in patients with early acute lung injury. Crit Care Med 2001;29:2325-31.
11. Cheng JW, Ware LB, Greene KE, Nuckton TJ, Eisner MD, Matthay MA. Prognostic value of surfactant proteins A and D in patients with acute lung injury. Crit Care Med 2003;31:20-7.
12. Imai Y, Parodo J, Kajikawa O, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 2003;289:2104-12.
13. Albertine KH, Soulier MF, Wang Z, et al. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory syndrome. Am J Pathol 2002;161:1783-96.
14. Shannon KC, Sinacore JM, Bennett SG, et al. Improving delivery of preventive health care with the comprehensive annotated reminder tool (CART). J Fam Pract. 2001 Sep;50(9):767-71.
15. Fann CSJ, Shugart Y, Lachman H, Collins A, Chang CJ: The Impact of Redefining Affection Status for Alcohilism on Affected Sib Pair Analysis. Genet. Epidemiology 17(suppl 1): S151-S156, 1999.
16. Chang CJ, Fann CSJ: Using Data Mining to Address Heterogeneity in the South Hampton Data. Genetic Epidemiology 21 (Suppl 1): S180-S185, 2001.