Kaohsiung J Med Sci June 2009 • Vol 25 • No 6 299
Metabolic syndrome (MS) is a complicated disorder comprising clinical features including obesity, hyper-glycemia, hypertension, dyslipidemia, and insulin re-sistance (IR). Atherosclerosis and type 2 diabetes
mellitus (DM), as major consequences of MS, are criti-cal, global health issues [1]. Current evidence suggests that the atherosclerotic process is regulated by interven-ing inflammatory mechanisms. IR, a key feature in the pathogenesis of MS, has been increasingly recognized as playing a key role in the inflammatory processes.
Hepatitis C virus (HCV) infection is another impor-tant global health issue. Approximately 170 million people suffer from HCV infection and it is one of the most important worldwide causes of cirrhosis and hepatocellular carcinoma. A number of metabolic
Received: Feb 25, 2009 Accepted: Apr 17, 2009 Address correspondence and reprint requests to: Dr Chia-Yen Dai, Hepatobiliary Division, Depart-ment of Internal Medicine, Kaohsiung Medical University Hospital, 100 Tzyou 1st Road, Kaohsiung 807, Taiwan.
E-mail: jf71218@gmail.com
H
EPATITIS
C V
IRUS
I
NFECTION AND
M
ETABOLIC
S
YNDROME
—A C
OMMUNITY
-
BASED
S
TUDY IN AN
E
NDEMIC
A
REA OF
T
AIWAN
Jee-Fu Huang,1,2Wan-Long Chuang,3,4Ming-Lung Yu,3,4Sung-Hua Yu,5Chung-Feng Huang,3 Ching-I Huang,3Ming-Lun Yeh,3Meng-Hsuan Hsieh,6Jeng-Fu Yang,7Zu-Yau Lin,3,4
Shinn-Chern Chen,3,4 Chia-Yen Dai,3,4and Wen-Yu Chang3
Departments of 1Internal Medicine, and 6Occupational and Environmental Medicine, Kaohsiung Municipal
Hsiao-Kang Hospital, Kaohsiung Medical University; 2Graduate Institute of Medicine, 4Faculty of Internal
Medicine, College of Medicine, and 5Department of Public Health, College of Health Science, Kaohsiung
Medical University; 3Hepatobiliary Division, Department of Internal Medicine, and 7Department of
Preventive Medicine, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan.
Metabolic syndrome (MS) is a complicated disorder associated with a high risk of future devel-opment of micro- and macrovascular complications. The extrahepatic manifestations of hepatitis C virus (HCV) infection can include multiple metabolic abnormalities. However, the extent, severity, and characteristics of MS in HCV-infected patients have rarely been investigated in community-based settings. This study aimed to determine the difference in prevalence and distribution of the components of MS between HCV-infected patients and healthy controls. Multipurpose mass screening of adults was conducted in an HCV-endemic area of Southern Taiwan. Clinical profiles in terms of anthropometric data and MS components, as well as viral hepatitis markers, were assessed. Two hundred and thirty-seven adults (94 males; mean age, 55.5± 10.8 years) were recruited. The prevalence of anti-HCV seropositivity was 39.2% (93/237). The prevalence of MS was higher in the HCV-infected individuals (24.7%, 23/93) than in the control, uninfected subjects (13.2%, 19/144, p= 0.02). In terms of MS components, HCV-infected subjects had a higher preva-lence of high waist circumference (51.6% vs. 25.7%, p< 0.001) and hypertension (58.1% vs. 36.8%, p= 0.001) than controls. Multivariate logistic regression analysis demonstrated that anti-HCV positivity was significantly associated with MS (odds ratio, 6.4; 95% confidence interval, 1.82–22.84; p= 0.004). HCV infection was associated with a higher prevalence of MS. Determination of MS in patients with HCV infection could therefore be indicated.
Key Words:hepatitis C virus, insulin resistance, metabolic syndrome (Kaohsiung J Med Sci 2009;25:299–305)
disturbances have been shown to be directly and indi-rectly associated with HCV infection. An association between HCV infection and lipid metabolism has been consistently reported [2–4]. Lower total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG) levels are commonly seen in patients with chronic HCV infection (CHC), compared with normal subjects. Hepatic steatosis is a common histo-logic feature of CHC and is observed in 30–70% of patients [5,6]. Many factors are known to increase the risk of hepatic steatosis, including DM, hyperlip-idemia, and obesity [7]. In addition to its hepatotropic characteristic, HCV infection is associated with the pathogenesis of IR, though the underlying biological mechanisms are diverse and multifactorial. Both experimental and clinical studies have shown that IR often emerges at a young age or during the early stages of histologic liver changes [8,9]. Furthermore, emerging lines of clinical evidence have revealed that several metabolic disturbances, such as obesity, IR, and hepatic steatosis, are significant risk factors for de-creased treatment response to combined pegylated interferon and ribavirin antiviral therapy in CHC patients [6,10–12]. IR and its related inflammatory processes thus appear to contribute not only to MS, but also to the metabolic disturbances associated with HCV infection. However, the characteristic fea-tures of MS in CHC patients have not been fully elu-cidated. Previous studies addressing the association between HCV infection and MS were mainly hospital-based and the extent, severity, and characteristics of MS in HCV-infected patients, compared with non-HCV subjects, have rarely been investigated in community-based settings.
This study aimed to determine the difference in the prevalence and distribution of MS between infected patients and healthy controls in an HCV-endemic area.
P
ATIENTS ANDM
ETHODSPatient selection
Tzukuan Township is located in southern Taiwan and is a hyper-endemic area for HCV infection, in both adults and adolescents. Our previous studies demon-strated that the prevalence of anti-HCV seropositiv-ity (anti-HCV+) in Tzukuan Township reached 41.6%
among adult residents, with an annual incidence of 4.5%. Moreover, about 90% of hepatocellular carcinoma cases in this township were HCV-related [9,13–15]. Based on occupational and geographic data, seven villages along the southwest coast were classified as maritime, while the other eight were non-maritime. Our previous study showed that the prevalence of HCV infection was markedly higher in the maritime area, compared with the non-maritime area. A multi-purpose health surveillance study was conducted in three hyper-endemic maritime villages in March 2007. A total of 396 adults participated in this study on a voluntary basis. After excluding those who refused examination (55, 13.9%), those who failed to com-plete the study (50, 12.6%), those who were very el-derly (46, 11.6%), and those who had received prior antiviral therapy (8, 2.0%), a total of 237 adults con-stituted the study population.
Study design
All subjects underwent a 12-hour overnight fast before blood tests. Blood was analyzed for HCV anti-body, fasting plasma glucose (FPG), TC, HDL-C, LDL-C, TG, and alanine aminotransferase (ALT) levels. Anthropometric data including body weight, height, and blood pressure were measured using standard-ized techniques. Research staff administered a ques-tionnaire covering medical history, drug history, possible parenteral risk history, and family history. Ver-bal or written informed consent for interviews, anthro-pometric measurements, blood sampling, and medical record reviews were obtained from patients prior to enrolment. The study was approved by the ethics com-mittee of Kaohsiung Medical University Hospital.
Definition of MS
MS was defined based on the updated National Cholesterol Education Program Adult Treatment Panel III criteria for Asian-Americans, modified by the cri-teria of obesity proposed for Asians by the Steering Committee of the Regional Office for the Western Pacific Region of WHO [1,16]. This requires the pres-ence of at least three of the following components: (1) waist circumference > 90 cm in men or > 80 cm in women; (2) TG > 150 mg/dL; (3) HDL-C < 40 mg/dL in men or < 50 mg/dL in women; (4) blood pressure > 130/85 mmHg or current use of antihypertensive medications; (5) FPG > 100 mg/dL or use of oral anti-diabetic agents or insulin.
Laboratory analyses
Hepatitis B surface antigen and anti-HCV antibody were detected using a third-generation, commercially available enzyme-linked immunosorbent assay kit (AxSYM 3.0; Abbott Laboratories, Chicago, IL, USA). Detection of serum HCV RNA was performed using a standardized automated qualitative reverse tran-scription-polymerase chain reaction assay (COBAS AMPLICOR Hepatitis C Virus Test, version 2.0; Roche, Branchburg, NJ, USA). The detection limit was 50 IU/ mL. HCV genotypes 1a, 1b, 2a, 2b, and 3a were deter-mined by the Okamoto method [17]. FPG, TC, TG, and ALT levels were measured using a multichannel auto-analyzer (Hitachi Inc., Tokyo, Japan). Fasting serum insulin levels were measured by radioimmunoassay (Diagnostic Products, Los Angeles, CA, USA).
IR and β-cell function were calculated based on FPG and insulin levels, according to the homeostasis model assessment (HOMA) method [18]. The formulae for the HOMA model are as follows: β-cell function (HOMA-%B)=fasting insulin level (μU/mL)×360/ [FPG (mg/dL)− 63]; IR (HOMA-IR) = FPG (mg/dL) ×
fasting insulin level (μU/mL)/405.
Statistical analysis
Frequencies were compared between groups using the χ2 test with Yates’s correction for categorical
variables and Student’s t test for continuous vari-ables. Results were expressed as mean±standard devi-ation. A p value < 0.05 was considered statistically significant. Univariate and multivariate logistic regres-sion analyses were conducted to explore the factors that were independently associated with MS. The strength of association was presented as odds ratio (OR) with 95% confidence intervals (CI) and p values. Quality control procedures, database processing and analyses were performed using the SPSS 12.0 statisti-cal package (SPSS Inc., Chicago, IL, USA).
R
ESULTSA total of 237 sex- and age-matched subjects (93 anti-HCV+ and 144 anti-HCV−) were recruited and their basic demographic characteristics are shown in Table 1. Fifty-six anti-HCV+ subjects (60.2%) were positive for HCV RNA (36 of genotype 1, 17 of genotype 2, and 3 of unclassified genotype infection). Forty-two subjects (17.7%) fulfilled the criteria for MS. The prevalence of anti-HCV+ was 39.2% (93/237). The anti-HCV+ subjects had a higher prevalence of hy-pertension, and higher waist circumference, ALT lev-els, insulin levlev-els, HOMA-IR, and HOMA-%B than the anti-HCV− subjects. The TC and LDL-C levels
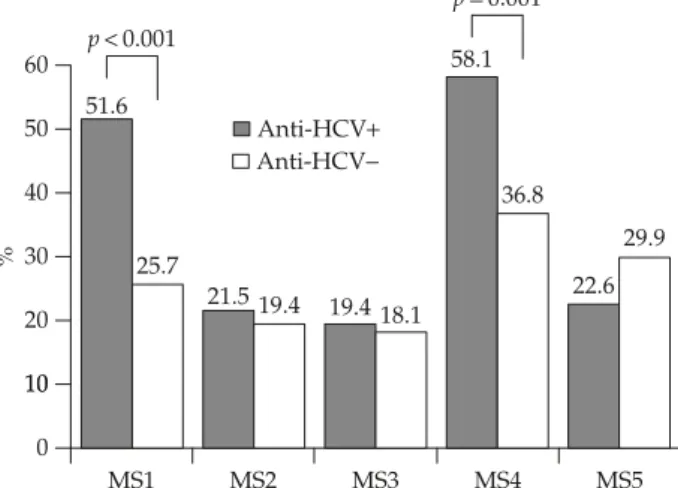
Table 1.Basic characteristics of anti-HCV-seropositive and -negative subjects*
Variables All subjects (n= 237) Anti-HCV p
Positive (n= 93) Negative (n= 144) Age (yr) 55.5± 10.8 57.1± 9.0 54.5± 11.7 NS Male 94 (39.7) 33 (35.5) 61 (42.4) NS Hypertension 107 (45.1) 54 (58.1) 53 (36.8) 0.001 BMI (kg/m2) 24.9± 3.7 25.4± 3.5 24.6± 3.9 NS Waist circumference (cm) 80.2± 11.0 82.5± 10.5 78.7± 11.1 0.01 ALT (U/L) 26.6± 26.6 36.3± 36.2 20.3± 15.0 <0.001 FPG (mg/dL) 101.6± 40.8 105.3± 48.3 95.9± 24.3 NS TC (mg/dL) 183.8± 32.9 176.1± 33.3 188.8± 31.7 <0.01 HDL-cholesterol 58.1± 15.0 56.1± 13.3 59.4± 15.9 NS LDL-cholesterol 120.6± 34.3 113.1± 35.6 125.4± 32.7 0.01 Triglycerides (mg/dL) 110.0± 64.6 102.9± 54.8 114.6± 69.9 NS Uric acid (mg/dL) 6.0± 1.5 6.2± 1.6 5.8± 1.5 NS Insulin (μU/mL) 10.3± 7.6 12.5± 9.7 8.8± 5.3 0.001 HOMA-IR 1.8± 0.1 2.2± 0.3 1.6± 0.2 0.02 HOMA-%B 103.2± 7.3 128.2± 13.4 87.1± 8.1 0.01 Metabolic syndrome 42 (17.7) 23 (24.7) 19 (13.2) 0.02
*Data presented as mean± standard deviation or n (%). HCV = hepatitis C virus; BMI = body mass index; ALT = alanine aminotrans-ferase; FPG = fasting plasma glucose; TC = total cholesterol; HDL = high-density lipoprotein; LDL = low-density lipoprotein; HOMA = homeostasis model assessment; IR = insulin resistance; %B = β-cell function.
were significantly lower in the anti-HCV+ subjects compared with the anti-HCV− subjects. Overall, the anti-HCV+ subjects had a higher prevalence of MS (24.7%, 23/93) than the anti-HCV− subjects (13.2%, 19/144, p= 0.02).
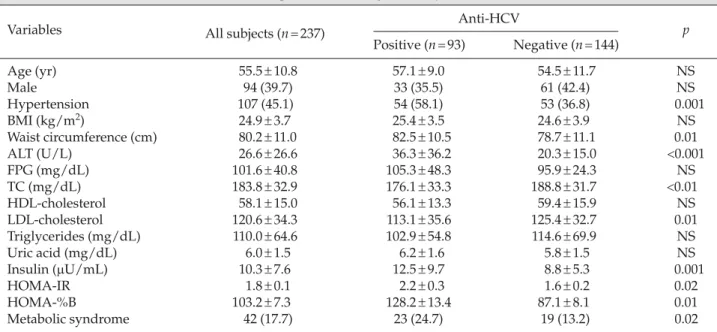
The prevalences of high waist circumference (51.6% vs. 25.7%, p<0.001) and hypertension (58.1% vs. 36.8%, p= 0.001) were significantly higher in anti-HCV+ sub-jects compared with anti-HCV− subjects (Figure). There were no significant differences between anti-HCV+ and anti-HCV− subjects in terms of high TG levels (21.5% vs. 19.4%, p= 0.7), low HDL-C levels (19.4% vs. 18.1%, p= 0.8), and DM (22.6% vs. 29.9%, p= 0.2).
Regarding the age-specific distribution of MS, the prevalence of MS among anti-HCV+ subjects aged
40–60 years was higher (25.5%, 14/55) than that of controls (10.6%, 10/94; p= 0.02). For those aged ≥ 60 years, the prevalence was not higher in anti-HCV+ subjects (25.7%, 9/35) compared with controls (18.9%, 7/37; p= 0.5).
Multivariate logistic regression analyses were con-ducted to clarify the independent factors associated with MS. Variables included age, sex, body mass index (BMI), ALT, creatinine, uric acid, FPG, HDL-C levels, and the presence of hypertension and IR. Anti-HCV+ was significantly associated with MS (OR, 6.4; 95% CI, 1.82–22.84; p= 0.004) as well as with hypertension, BMI, and HDL-C (Table 2).
D
ISCUSSIONThe unique HCV hyper-endemic geographic back-ground of the current case-control study allowed us to examine the association between HCV infection and MS. Our data demonstrated that HCV infection was associated with an increased prevalence of MS, compared with that in non-HCV-infected subjects. High waist circumference and hypertension were the common features of MS in subjects with HCV infec-tion. We also demonstrated that anti-HCV+ subjects had a higher prevalence of IR than anti-HCV− sub-jects. IR plays a key role in the emergence of MS, and our data may thus further suggest that HCV infec-tion contributes to the risk of developing MS.
Subjects with MS generally have higher rates of IR and are therefore also at increased risk of developing type 2 DM, as well as future micro- and macrovascu-lar complications [19,20]. Previous reports have indi-cated that metabolic abnormalities, including liver steatosis, obesity and DM, can worsen the course of CHC [21,22]. In addition, CHC has a direct steato-genic effect on liver cells and may be involved in the
Table 2.Multivariate logistic regression analyses of variables associated with metabolic syndrome
Variables* OR 95% CI p
Hypertension Positive= 1, Negative = 0 31.8 6.49–155.84 < 0.001
Anti-HCV+ Positive= 1, Negative = 0 6.4 1.82–22.84 0.004
BMI (kg/m2) Per 1 kg/m2increase 1.3 1.09–1.51 0.002
HDL-C (mg/dL) Per 1 mg/dL increase 0.9 0.88–0.98 0.009
*Variables included age, sex, body mass index (BMI), aminotransferase, creatinine, uric acid, fasting plasma glucose, high-density lipoprotein cholesterol (HDL-C) levels, and the presence of hypertension and insulin resistance. For the continuous variables, OR represents one unit increase in the value of the variable tested. OR = odds ratio; CI = confidence interval; HCV = hepatitis C virus.
Anti-HCV+ Anti-HCV− 51.6 58.1 60 p< 0.001 36.8 40 50 25.7 22.6 29.9 30 % 21.5 19.4 19.418.1 10 20 0 10 MS1 MS2 MS3 MS4 MS5 p= 0.001
Figure.Distribution of metabolic syndrome components in rela-tion to anti-hepatitis C virus (HCV ) seropositivity. MS1 = waist circumferences >90cm in men or >80cm in women; MS2 = triglyc-eride level >150 mg/dL; MS3 = high-density lipoprotein cholesterol
< 40 mg/dL in men or < 50 mg/dL in women; MS4 = blood
pres-sure > 130/85 mmHg or current use of antihypertensive medica-tions; MS5 = type 2 diabetes or use of oral antidiabetic agents or insulin.
development of type 2 DM [22–24]. However, the cor-relation between MS and HCV infection has rarely been investigated in clinical settings. We demonstrated that subjects with HCV infection were at increased risk of developing MS. This suggests that patients with HCV infection should be evaluated for the pres-ence of MS, while lifestyle changes directed at increas-ing physical activity, optimal weight maintenance, and diet composition should be emphasized.
The precise biological mechanisms whereby HCV infection leads to MS are not fully understood. HCV may induce a Th1 lymphocyte immune-mediated response, leading to activation of the tumor necrosis factor (TNF)-α system and elevation of interleukin-6 levels. Meanwhile, HCV directly causes liver steatosis. A combination of these events may result in the devel-opment of liver fibrosis. TNF-α system activation, liver steatosis, and fibrosis in turn contribute to the development of IR, which plays a pivotal role in the development of MS [25]. HCV-induced inflammatory changes may subsequently lead to increased oxida-tive stress and peroxidation, which evoke higher sys-temic inflammatory responses. Our results therefore imply that, in addition to the direct hepatotropic effects of HCV infection, MS should be considered as a possible extrahepatic manifestation of HCV [24,26–28]. The current study demonstrated that anti-HCV+ subjects had significantly lower TC and LDL-C levels and lower HDL-C and TG levels than anti-HCV− subjects. These data are in agreement with those from previous experimental and clinical studies that addressed the association between HCV infection and lipid metabolism [3,29]. HCV infection was shown to be associated with significantly lower cholesterol (TC, HDL-C and LDL-C) and TG levels compared with normal subjects [2,3] and a recent study demonstrated that TC, LDL-C and TG levels increased after suc-cessful eradication of HCV genotype-1 infection with antiviral therapy [30]. Although our study failed to show significant differences in TG and HDL-C levels between anti-HCV+ and anti-HCV− subjects, further long-term studies aimed at assessing the changing features of MS after antiviral therapy are warranted. In addition, the relative risk of atherosclerotic cardio-vascular disease in patients with MS and HCV infec-tion, compared with non-HCV subjects, deserves further investigation.
The increasing burden of obesity is the driving force behind the rising prevalence of MS. Body fat
distribution, particularly excess abdominal fat, plays an important role in the etiology of MS [1]. Regardless of the relative contributions of visceral fat and abdom-inal subcutaneous fat to IR, abdomabdom-inal (or upper-body) obesity correlates more strongly with IR and MS than does lower-body obesity [31]. Our previous study also demonstrated that the discrepancy in prevalence of MS between CHC patients and controls was inversely related to age, suggesting that HCV infection may contribute to the subtle development of glucose abnormalities at a younger age [9]. Intrigu-ingly, the current study showed that anti-HCV+ subjects had a significantly higher mean waist cir-cumference than anti-HCV− subjects. This may some-how reflect the common observation that IR is a general feature of HCV infection. However, the cross-sectional nature of the current study and incomplete coverage of all genotypes did not allow us to reach a definite conclusion on this issue. A large collabora-tive study comparing patients with different HCV genotypesis needed to further clarify any relation-ship between genotype and predisposition to or protection from MS. A well-designed longitudinal follow-up study is also warranted to further clarify if HCV infection predisposes to the development of upper-body obesity.
In conclusion, we demonstrated that HCV infection was associated with an increased prevalence of MS. High waist circumference and presence of hyperten-sion were the common features of MS in patients with HCV infection. Our data indicate a possible link between HCV infection and MS and suggest that assessment of MS in patients with HCV infection may therefore be warranted.
A
CKNOWLEDGMENTSThis study was partly supported by grants from the Kaohsiung Medical University Research Foundation and Kaohsiung Municipal Hsiao-Kang Hospital, Kaohsiung Medical University. The authors thank Taiwan Liver Research Foundation for secretarial assis-tance and help with serum processing. The Foundation was not involved in the study design or approval of the manuscript. The authors had full access to all of the data from the study and take full responsibility for the integrity of the data and the accuracy of the data analysis.
R
EFERENCES1. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112: 2735–52.
2. Dai CY, Huang JF, Hsieh MY, et al. Links between triglyceride levels, hepatitis C virus infection and dia-betes. Gut 2007;56:1167–8.
3. Dai CY, Chuang WL, Ho CK, et al. Associations between hepatitis C viremia and low serum triglyceride and cho-lesterol levels: a community-based study. J Hepatol 2008; 49:9–16.
4. Siagris D, Christofidou M, Theocharis GJ, et al. Serum lipid pattern in chronic hepatitis C: histological and viro-logical correlations. J Viral Hepat 2006;13:56–61. 5. Hsieh MH, Lee LP, Hsieh MY, et al. Hepatic steatosis
and fibrosis in chronic hepatitis C in Taiwan. Jpn J Infect
Dis 2007;60:377–81.
6. Watanabe S, Yaginuma R, Ikejima K, et al. Liver diseases and metabolic syndrome. J Gastroenterol 2008;43:509–18. 7. Sanyal AJ. Review article: non-alcoholic fatty liver dis-ease and hepatitis C—risk factors and clinical implica-tions. Aliment Pharmacol Ther 2005;22:48–51.
8. Shintani Y, Fujie H, Miyoshi H, et al. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology 2004;126:840–8.
9. Huang JF, Yu ML, Dai CY, et al. Reappraisal of the char-acteristics of glucose abnormalities in patients with chronic hepatitis C infection. Am J Gastroenterol 2008;103: 1933–40.
10. Camma C, Bruno S, Di Marco V, et al. Insulin resistance is associated with steatosis in nondiabetic patients with genotype 1 chronic hepatitis C. Hepatology 2006;43:64–71. 11. Bressler BL, Guindi M, Tomlinson G, et al. High body mass index is an independent risk factor for nonre-sponse to antiviral treatment in chronic hepatitis C.
Hepatology 2003;38:639–44.
12. Romero-Gomez M, Del Mar Viloria M, Andrade RJ, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology 2005;128:636–41.
13. Huang JF, Lu SN, Chue PY, et al. Hepatitis C virus infection among teenagers in an endemic township in Taiwan: epidemiological and clinical follow-up stud-ies. Epidemiol Infect 2001;127:485–92.
14. Lu SN, Chue PY, Chen IL, et al. Incidence of hepatitis C infection in a hepatitis C endemic township in south-ern Taiwan. Kaohsiung J Med Sci 1997;13:605–8.
15. Chuang WL, Yu ML, Dai CY, et al. Treatment of chronic hepatitis C in southern Taiwan. Intervirology 2006;49: 99–106.
16. Anuurad E, Shiwaku K, Nogi A, et al. The new BMI criteria for Asians by the regional office for the western pacific region of WHO are suitable for screening of
overweight to prevent metabolic syndrome in elder Japanese workers. J Occup Health 2003;45:335–43. 17. Okamoto H, Tokita H, Sakamoto M, et al.
Characteriza-tion of the genomic sequence of type V (or 3a) hepatitis C virus isolates and PCR primers for specific detection.
J Gen Virol 1993;74:2385–90.
18. Matthews DR, Hosker JP, Rudenski AS, et al. Homeosta-sis model assessment: insulin reHomeosta-sistance and beta-cell function from fasting plasma glucose and insulin con-centrations in man. Diabetologia 1985;28:412–9.
19. Carulli N. Metabolic syndrome—cardiovascular disease risk and more. Aliment Pharmacol Ther 2005;22:1–2. 20. Shaheen M, Echeverry D, Oblad MG, et al. Hepatitis C,
metabolic syndrome, and inflammatory markers: results from the Third National Health and Nutrition Examina-tion Survey [NHANES III]. Diabetes Res Clin Pract 2007;75:320–6.
21. Moucari R, Marcellin P, Asselah T. Steatosis during chronic hepatitis C: the role of insulin resistance and viral factors. Gastroenterol Clin Biol 2007;31:643–54. 22. Tarantino G, Conca P, Sorrentino P, et al. Metabolic
fac-tors involved in the therapeutic response of patients with hepatitis C virus-related chronic hepatitis. J
Gas-troenterol Hepatol2006;21:1266–8.
23. Sheikh MY, Choi J, Qadri I, et al. Hepatitis C virus infection: molecular pathways to metabolic syndrome.
Hepatology 2008;47:2127–33.
24. Huang JF, Dai CY, Hwang SJ, et al. Hepatitis C viremia increases the association with type 2 diabetes mellitus in a hepatitis B and C endemic area: an epidemiological link with virological implication. Am J Gastroenterol 2007; 102:1237–43.
25. Lecube A, Hernandez C, Genesca J, et al. Proinflamma-tory cytokines, insulin resistance, and insulin secretion in chronic hepatitis C patients: a case-control study.
Diabetes Care 2006;29:1096–101.
26. Huang JF, Chuang WL, Dai CY, et al. Viral hepatitis and proteinuria in an area endemic for hepatitis B and C infections: another chain of link? J Intern Med 2006;260: 255–62.
27. Huang JF, Chuang WL, Dai CY, et al. The role of thy-roid autoantibodies in the development of thythy-roid dys-function in Taiwanese chronic hepatitis C patients with interferon-alpha and ribavirin combination therapy.
J Viral Hepat 2006;13:396–401.
28. Hadziyannis SJ. The spectrum of extrahepatic manifesta-tions in hepatitis C virus infection. J Viral Hepat 1997; 4:9–28.
29. Monazahian M, Bohme I, Bonk S, et al. Low density lipoprotein receptor as a candidate receptor for hepati-tis C virus. J Med Virol 1999;57:223–9.
30. Tada S, Saito H, Ebinuma H, et al. Treatment of hepatitis C virus with peg-interferon and ribavirin combination therapy significantly affects lipid metabolism. Hepatol
Res 2009;39:195–9.
31. Jensen MD, Haymond MW, Rizza RA, et al. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest 1989;83:1168–73.