Endosonographic Features of Histologically Proven Gastric Ectopic Pancreas
Jen-Wei Chou1,2, Ken-Sheng Cheng1,2, Chun-Fu Ting2,
Chun-Lung Feng,2, Yu-Ta Lin2, Wen-Hsin Huang1,2
1School of Medicine, China Medical University, Taichung, Taiwan
2Division of Gastroenterology and Hepatology, Department of Internal Medicine, China
Medical University Hospital, Taichung, Taiwan
Correspondence and Reprints to: Dr. Yu-Ta Lin
Division of Gastroenterology and Hepatology, Department of Internal Medicine,
China Medical University Hospital, Taichung, Taiwan, No. 2, Yude Road, North District, Taichung 40447, Taiwan
Tel: +886-4-2205-2121 ext.2222, 2221; Fax: + 886-4-2202-3119. Email:
emissionspectrum@gmail.com
No conflicts of interest exist
Abstract
diagnosis is usually difficult by using a conventional biopsy forceps. From May 2006 to December 2013, we retrospectively reviewed patients who underwent endoscopic ultrasonography for gastric subepithelial tumors at China Medical University Hospital in Taiwan. Thirteen patients were histologically diagnosed with gastric ectopic pancreas. The endoscopic ultrasonographic features of these lesions were analyzed. These participants were 5 men (38%) and 8 women (62%), with a mean age of 40 years (range, 20–77 years). Eight lesions were located in the antrum, 4 lesions were in the body, and one was in the angulus. Central dimpling was noted in 7 lesions (53%). Nine lesions (69%) exhibited hypoechoic echogenicity and 4 (30%) exhibited mixed echoic echogenicity. Seven lesions (53%) were homogeneous. The borders were distinct in 10 lesions (76%). Anechoic tubular structures only appeared in one lesion (7.6%). Seven lesions (53%) originated in the fourth sonographic layer (muscularis propria), 4 lesions (30.7%) in the second layer (deep mucosa), and 2 lesions (15.3%) involved both the second and third layers (deep mucosa and submucosa). Gastric ectopic pancreas within the fourth sonographic layer (muscularis propria) could demonstrate hypoechoic, homogeneous echogenicity and distinct borders; they exhibit similar endoscopic ultrasonographic characteristics compared with gastrointestinal stromal tumors. Recognizing endoscopic ultrasonographic features, combining with deep biopsy, endoscopic ultrasound-guided fine needle aspiration/core needle biopsy, can prevent conducting unnecessary resection.proliferating ducts, exhibiting neither acini nor endocrine elements [9]. In our
present study, 5 lesions (38.4%) were Heinrich Type I and 8 (61.5%) were Type II.
The common site of ectopic pancreas is located in the GI tract: stomach (26%–38%), duodenum (28%–36%), and jejunum (16%) [10]. Lesions have also been reported in the colon, spleen, liver, Meckel’s diverticulum, gallbladder, bile ducts, or fallopian tubes [11]. The gross appearance of a typical ectopic pancreas in the stomach is a firm, round or oval subepithelial lesion. Central dimpling (also called central umbilication) caused by the opening of a duct may also be observed. This implies that ectopic pancreas can be presumptively diagnosed before obtaining histologic results. In previous reports, central dimpling was observed in 34.6%–90.0% of patients with ectopic pancreas [4, 5, 12]. In our present study, cenreal dimpling was present in 53.8% of patients. Anechoic cystic (or tubular) structure, known as the component of pancreas duct, is another specific EUS feature for ectopic pancreas [13]. However, anechoic area in EUS was only present in 1 lesion of all patients with ectopic pancreas (7.6%) in our present study.
Matsushita et al revealed useful EUS features for establishing a preoperative diagnosis of ectopic pancreas, namely, indistinct borders, heterogeneous echogenicity, the presence of an anechoic area, and a location within the second, third, and/or fourth layers [13]. Park et al described other characteristic EUS features of ectopic pancreas, such as lobulated margins, a mural growth pattern, and localization within 2 or more layers [4]. However, in our present study, 7 of 13 lesions (53.8%) were hypoechoic and homogeneous. The EUS findings
somewhat differ from some previous reports in the literature. This discrepancy might result from interobserver variation in interpreting the EUS images, and is suspected because of the hypoechoic homogeneous characteristics of the fourth sonographic layer ectopic pancreas in EUS. In our previous study by Chen in 2008, he also found a case of ectopic pancreas located within the fourth sonographic layer (muscularis propria) [5]. In our present study, the percentage of patients with ectopic pancreas exhibiting a fourth sonographic layer was larger compared with other studies. Seven lesions (53.8%) exhibited within the fourth layer. Six of these (85.7%) were hypoechoic and homogeneous. However, in previous reports by Matsushita et al and Park et al, they did not find any patients with gastric ectopic pancreas located within the fourth layer only In addition, according to the statistical analysis, the lesions in the fourth layer were more common to exhibit both hypoechoic and homogeneous feature than those not in the fourth layer (85.7% vs 16.6%, respectively, P = 0.029). It suggests that ectopic pancreas within the fourth layer exhibit various EUS features. In our present study, the border was distinct in 10 lesions (76.9%). The 7 lesions in the fourth layer all exhibited a distinct border (100%). This might be because the border in a hypoechoic lesion in the muscularis propria layer of the stomach is relatively easy to distinguish from the hyperechoic area in the submucosa and serosa layers. Although the statistical analysis showed no significant differences between lesions in the muscularis propria layer and not in the muscularis propria layer (100.0% vs 50.0%, respectively, P = 0.07) because of small
number of our cases, our results differed from the reports of Matsushita et al and Park et al, in which most ectopic pancreas exhibited indistinct borders. Despite of our new EUS findings of gastric ectopic pancreas, a smooth lesion in antrum or duodenum, with a central dimple, with a tubular structure within it, with mixed echogenicity and involving the submucosa and the muscularis propria would have a pretty high chance of being an ectopic pancreas.
The clinical presentations of patients with ectopic pancreas are usually asymptomatic or nonspecific symptoms such as abdominal pain, nausea, and vomiting. However, some rare complications of ectopic pancreas have been reported, including perforation, GI bleeding, gastric outlet obstruction, obstructive jaundice, intestinal obstruction, and intussusceptions [9,14]. Carcinoma developing in an ectopic pancreatic tissue was rarely reported in the literature [15,16]. Thus, resection is usually unnecessary for asymptomatic patients. In our present study, all patients underwent EGD because of dyspepsia or epigastralgia. Gastric subepithelial tumors were discovered and EUS was performed later. Eight patients (61.5%) underwent tumor resection due to the preoperative diagnosis of gastric GISTs by EUS. Thus, resection can be implemented following a misdiagnosis. Most GISTs are hypoechoic and homogeneous lesions, which exhibit distinct margins and typically originate within the fourth layer of the GI tract [17]. In our present study, gastric ectopic pancreas in the fourth layer shared similar features as GISTs. Thus, in addition to GISTs, ectopic pancreas is a possible diagnosis of a hypoechoic heterogeneous lesion originating from the gastric fourth layer with
distinct margins. Bite-on-bite biopsy, EUS-guided fine needle aspiration (EUS-FNA), and EUS-guided core needle biopsy are valuable modalities in evaluating these lesions [18]. Relevant pathological findings could prevent unnecessary resections.
Park et al classified the ectopic pancreas into 2 types based on the sonographic layer of origin: the superficial type and deep type. If symptoms are noted owning to ectopic pancreas, the superficial type is an optimal candidate for endoscopic resection. However, the deep type might require a surgical approach [4]. In the past, endoscopic mucosal resection (EMR) in treating gastric ectopic pancreas has been reported in the literature [19,20]. Recently, ESD is a newly developed method for removing subepithelial tumors of the GI tract [21,22]. Ryu et al performed ESD for 4 cases of gastric ectopic pancreas after failure of EMR [23]. Liu et al also reported 9 patients who underwent ESD and EMR for symptomatic ectopic pancreas [14]. In our present study, ESD was performed for 2 patients who exhibited deep type (the muscularis propria layer) ectopic pancreas without complications. Thus, ESD appears to be relatively safe for the complete resection of small (≤ 20 mm) gastric subepithelial tumors, including ectopic pancreas, originating from the muscularis propria layer [24].
In conclusions
The typical EUS features of ectopic pancreas include heterogeneous echogenicity, indistinct borders, and a location within 2 or more layers. However, it can also exhibit hypoechoic homogeneous echogenicity and a distinct border within the muscularis propria
layer similar to the EUS features of GISTs. Deep biopsy relevant EUS features by using EUS-guided FNA/core needle biopsy can make a differential diagnosis of gastric subepithelial tumors before resection. Surgical resection is the mainstay treatment for symptomatic gastric ectopic pancreas, but endoscopic resection using EMR or ESD technique provides an alternative method of removing superficial type and deep type gastric ectopic pancreas.
However, our present study involves certain limitations. First, the number of patients was small because pathological diagnosis is not always possible; thus, we could not exclude the possibility of selection bias. Second, because a pathological diagnosis was not made in all of the cases showing typical EUS findings for ectopic pancreas, a selection bias could exist.
References
1. Armstrong CP, King PM, Dixon JM, et al. The clinical significance of heterotopic pancreas in the gastrointestinal tract. Br J Surg 1981;68:384-387.
Gastroenterol 1999;28:144-147.
3. Tanaka K, Tsunoda T, Eto T, et al. Diagnosis and management of heterotopic pancreas. Int Surg 1993;78:32-35.
4. Park SH, Kim GH, Park do Y, et al. Endosonographic findings of gastric ectopic pancreas: a single center experience. J Gastroenterol Hepatol 2011;26:1441-1446.
5. Chen SH, Huang WH, Feng CL, et al. Clinical analysis of ectopic pancreas with endoscopic ultrasonography: an experience in a medical center. J Gastrointest Surg 2008;12:877-881.
6. Kim JY, Lee JM, Kim KW, et al. Ectopic pancreas: CT findings with emphasis on differentiation from small gastrointestinal stromal tumor and leiomyoma. Radiology 2009;252:92-100.
7. Jang KM, Kim SH, Park HJ, et al. Ectopic pancreas in upper gastrointestinal tract: MRI findings with emphasis on differentiation from submucosal tumor. Acta Radiol 2013; 54:1107-1116.
8. Yamada T and Ichikawa H. X-ray diagnosis of elevated lesions of the stomach. Radiology 1974;110:79-83.
9. Fukino N, Oida T, Mimatsu K, et al. Diffuse Peritonitis due to Perforated Gastric Ectopic Pancreas. Case Rep Gastroenterol 2012;6:689-694.
10. O'Malley RB, Maturen KE, Al-Hawary MM, et al. Case of the season: ectopic pancreas. Semin Roentgenol 2013;48:188-191.
11. Lee MJ, Chang JH, Maeng IH, et al. Ectopic pancreas bleeding in the jejunum revealed by capsule endoscopy. Clin Endosc 2012;45:194-197.
12. Zinkiewicz K, Juskiewicz W, Zgodzinski W, et al. Ectopic pancreas: endoscopic, ultrasound and radiological features. Folia Morphol (Warsz). 2003;62:205-209.
13. Matsushita M, Hajiro K, Okazaki K, et al. Gastric aberrant pancreas: EUS analysis in comparison with the histology. Gastrointest Endosc 1999;49:493-497.
14. Liu X, Wang G, Ge N, et al. Endoscopic Removal of Symptomatic Gastric Heterotopic Pancreas: A Report of Nine Cases. Surg Innov 2013;20:NP40-6.
15. Papaziogas B, Koutelidakis I, Tsiaousis P, et al. Carcinoma developing in ectopic pancreatic tissue in the stomach: a case report. Cases J 2008;1:249.
16. Fukumori D1, Matsuhisa T, Taguchi K, et al. Ectopic Gastric Pancreatic Cancer: Report
of a Case. Hepatogastroenterology 2011; 58 (107-108):740.
17. Jeon SW, Park YD, Chung YJ, et al. Gastrointestinal stromal tumors of the stomach: endosonographic differentiation in relation to histological risk. J Gastroenterol Hepatol 2007;22:2069-2075.
18. Hwang JH, Rulyak SD, Kimmey MB, et al. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology 2006;130:2217-2228.
19. Lee TH, Wang HP, Huang SF et al. Endoscopic mucosal resection for treatment of heterotopic pancreas in the stomach. J Formos Med Assoc 1999;98:643-645.
20. Khashab MA, Cummings OW, DeWitt JM. Ligation-assisted endoscopic mucosal resection of gastric heterotopic pancreas. World J Gastroenterol 2009;15:2805-2808. 21. Lee HL, Kwon OW, Lee KN, et al. Endoscopic histologic diagnosis of gastric GI
submucosal tumors via the endoscopic submucosal dissection technique. Gastrointest Endosc 2011;74:693-695.
22. Huang ZG, Zhang XS, Huang SL, Yuan XG. Endoscopy dissection of small stromal tumors emerged from the muscularis propria in the upper gastrointestinal tract: Preliminary study. World J Gastrointest Endosc 2012;4:565-570.
23. Ryu DY, Kim GH, Park do Y, et al. Endoscopic removal of gastric ectopic pancreas: an initial experience with endoscopic submucosal dissection. World J Gastroenterol
2010;16:4589-4593.
24. Chun SY, Kim KO, Park DS, et al. Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis propria layer: a preliminary analysis of appropriate indications. Surg Endosc 2013;27:3271-3279.
Tables
Table 1 Clinicopathological characteristics in 13 patients with gastric ectopic pancreas
Case Gender Age Symptom for EGD Location Gross shape* Dimpling Method of Pathological
confirmation
Pathological type†
1 F 40 Dyspepsia High body II Yes Biopsy II
2 M 36 Epigastric pain Antrum I No Biopsy II
3 F 30 Epigastric pain Antrum II No Biopsy I
4 F 20 Dyspepsia Antrum I Yes Biopsy II
5 M 43 Epigastric pain Middle body I Yes Biopsy II
6 M 30 Epigastric pain Antrum II No Laparotomy II
7 F 77 Dyspepsia Angulus I No Laparotomy I
8 M 39 Dyspepsia Middle body II Yes Laparotomy I
9 F 28 Epigastric pain Lower body I Yes Laparoscopy I
10 F 31 Dyspepsia Antrum II No ESD I
11 F 51 Epigastric pain Antrum II Yes ESD II
12 F 42 Dyspepsia Antrum II Yes ESD II
* By Yamada classification8
†By Heinrich classification9
EGD, esophagogastroduodenoscopy ESD, endoscopic submucosal dissection
Table 2 Endoscopic ultrasonographic features in 13 patients with gastric ectopic pancreas
Case EUS features
Size (mm) Layer Echogenicity Homogeneity Anechoic area of duct Border EUS classification#
1 12 4 Hypoechoic Homogenous No Distinct D-type
2 10 2 Mixed echoic Heterogeneous No Distinct S-type
3 12 2,3 Mixed echoic Heterogeneous No Indistinct S-type
4 11 2,3 Mixed echoic Heterogeneous No Distinct S-type
5 12 2 Mixed echoic Heterogeneous No Indistinct S-type
6 20 4 Hypoechoic Homogenous No Distinct D-type
7 22 4 Hypoechoic Homogenous No Distinct D-type
8 23 4 Hypoechoic Homogenous Yes Distinct D-type
9 20 4 Hypoechoic Homogenous No Distinct D-type
10 10 2 Hypoechoic Homogenous No Distinct S-type
11 20 4 Hypoechoic Heterogeneous No Distinct D-type
12 20 4 Hypoechoic Homogenous No Distinct D-type
13 21 2 Mixed echoic Heterogeneous No Indistinct S-type
Table 3 Endoscopic ultrasonographic features of 13 patients with gastric ectopic pancreas originating in the fourth sonographic layer or not
In the 4th layer Not in the 4th layer P-value
Gross shape (by Yamada classification 11 ) 1.000
I 3 2 II 4 4 Dimpling/umbilication 0.286 Present 5 2 Absent 2 4 Size (mm, mean SD) 19.5 ± 2.1 12.6 ± 2.7 0.008 Echogenicity 0.005 Mixed echoic 0 5 Hypoechoic 7 1 Homogenicity 0.015 Homogeneous 6 0 Heterogeneous 1 5
Hypoechoic and homogeneous 0.029
Yes 6 1
Border 0.070
Distinct 7 3
Indistinct 0 3
Anechoic duct-like structure 1.000
Present 1 0
Absent 6 6
Figure Legends
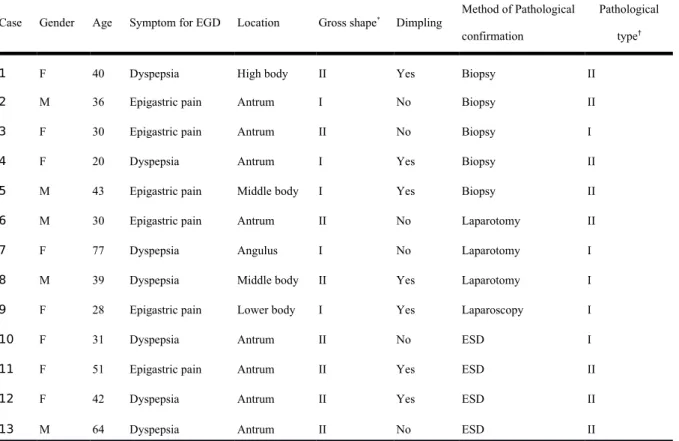
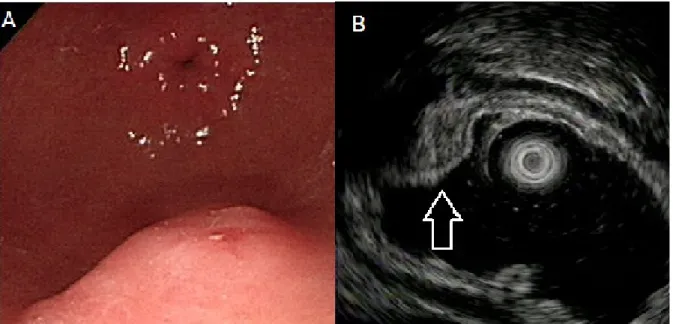
Figure 1. Ectopic pancreas originates from the second and third sonographic layers of the gastric wall. A, a subepithelial tumor with intact but uneven surface was identified in the
antrum. B, endoscopic ultrasonographic image obtained with a 12 MHz catheter probe. The tumor exhibits heterogeneous, mixed echoic echogenicity and indistinct margins, involving the second and third sonographic layers of the gastric wall (arrow).
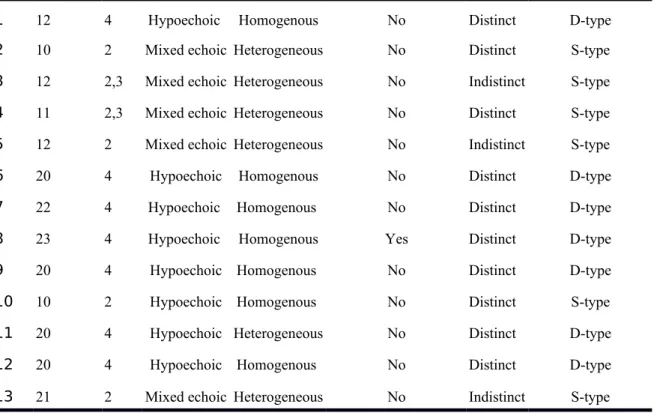
Figure 2. Ectopic pancreas originates from the second and third sonographic layers of the gastric wall. A, a subepithelial tumor with intact but uneven surface was identified in the antrum. B, endoscopic ultrasonographic image obtained with a 12 MHz catheter probe. The tumor exhibits heterogeneous, mixed echoic echogenicity and indistinct margins, involving the second and third sonographic layers of the gastric wall (arrow).
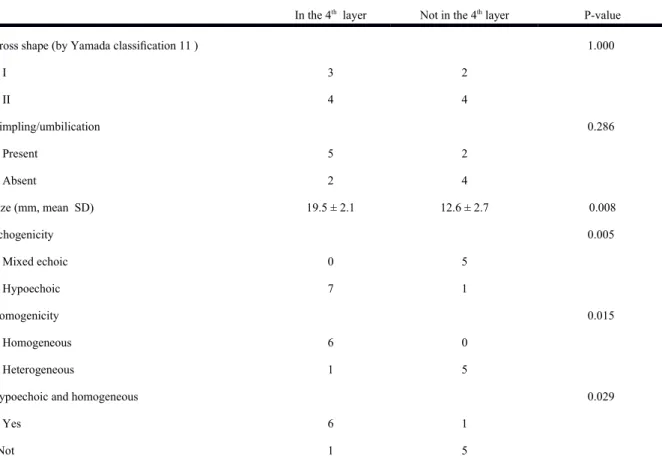
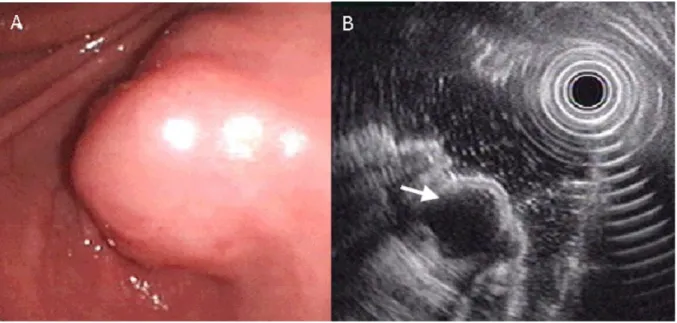
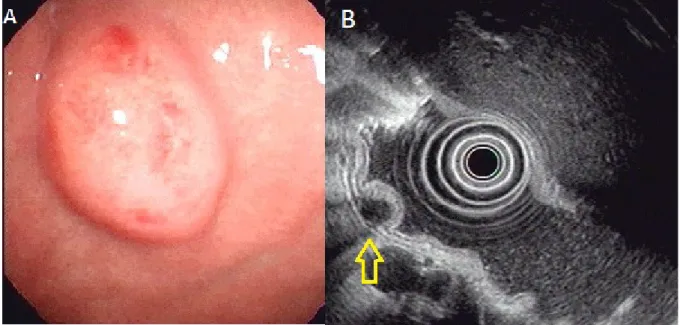
Figure 3. Ectopic pancreas originates from the fourth sonographic layers of the gastric wall. A, a subepithelial tumor with intact mucosa was identified at antrum. B, endoscopic ultrasonographic image obtained with a 12 MHz catheter probe. The tumor exhibits homogenous, hypoechoic echogenicity and distinct margin originating from the fourth sonographic layer of gastric wall (arrow).
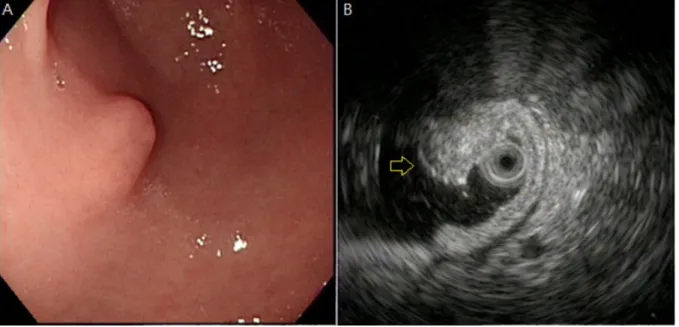
Figure 4. Ectopic pancreas originates from the fourth sonographic layers of the gastric wall. A, a subepithelial tumor with intact mucosa was identified at antrum. B, endoscopic ultrasonographic image obtained with a 12 MHz catheter probe. The tumor exhibits homogenous, hypoechoic echogenicity and distinct margin originating from the fourth sonographic layer of gastric wall (arrow).