行政院國家科學委員會專題研究計畫 成果報告
正常子宮肌細胞與腺肌症肌細胞中浸潤淋巴球之表現(2/2)
計畫類別: 個別型計畫 計畫編號: NSC93-2314-B-002-064- 執行期間: 93 年 08 月 01 日至 94 年 07 月 31 日 執行單位: 國立臺灣大學醫學院婦產科 計畫主持人: 楊友仕 共同主持人: 楊政憲 報告類型: 完整報告 處理方式: 本計畫可公開查詢中 華 民 國 94 年 10 月 21 日
中英文摘要 背景:曾有報告指出子宮內膜異位症患者的腹水中自然殺手(NK)細胞上殺手細胞抑制接 受器(KIR)有過度的表現。本研究嘗試探討子宮腺肌症 NK 及 T 細胞的 KIR 表現,以進 一步釐清 KIR 在子宮腺肌症形成過程中扮演的角色。 方法:收集 10 位罹患子宮腺肌症婦女(實驗組)及 12 位罹患子宮肌瘤婦女(對照組)的 子宮肌肉及子宮內膜,分離出 NK 及 T 細胞後,利用流式細胞儀測量細胞上 NKB1、GL183、 EB6、CD94 的表現。 結果:子宮腺肌症婦女的子宮內膜中NK細胞上的NKB1 及GL183 表現量明顯減少,但子宮 肌肉中NK細胞無此現象。而T細胞(包括CD4+及CD8+)上KIR的表現,在實驗組及對照組 之間無差異。 結論:子宮腺肌症的子宮內膜 NK 細胞上 KIR 的表現量明顯減少,這可能是一種補償作用, 藉此提高 NK 毒殺力,以消滅可能逸出的異常子宮內膜細胞。
BACKGROUND: Increased expression of killer cell inhibitory receptors (KIRs) has been found
on natural killer (NK) cells in peritoneal fluid in women with endometriosis. In this study, we tried to measure the expression of KIRs on NK and T cells in women with adenomyosis, in an attempt to find the possible role of KIRs in the development of adenomyosis.
METHODS: A total of 10 women with adenomyosis (study group) and 12 women with uterine
myoma (control group) were included in this study. The expression of KIRs, including NKB1, GL183, EB6, and CD94, on NK and T cells in myometrium and endometrium were examined by flow cytometry.
RESULTS: There was a decreased expression of NKB1 and GL183 on NK cells in the
endometrium, but not in the myometrium, in women with adenomyosis. However, the expression of KIRs on T cells, either CD4+ or CD8+, was not different in both myometrium and endometrium between women with and without adenomyosis.
CONCLUSIONS: The expression of KIRs on NK cells was decreased in eutopic endometrium
in women with adenomyosis. It may be a compensatory effect in which the NK cytotoxicity is activated in order to wipe out the abnormal endometrial cells that might go out of the eutopic site of endometrium.
目錄 頁次 報告內容 1 前言 1 研究目的 1 研究方法 1 結果與討論 3 參考文獻 5 計畫成果自評 7 附錄 8
報告內容
前言
Natural killer (NK) cells are known to kill virally infected or tumor cells while sparing normal self cells (Trinchieri, 1989; Moretta et al., 1994). This ability was found to depend on the interaction between killer cell inhibitory receptors (KIRs) expressed on NK cells and major histocompatibility complex (MHC) molecules expressed on normal cells, which leads to the inhibition of NK cell function (Lanier and Phillips, 1996; Gumperz et al., 1996; Rouas-Freiss et al., 1997). On the contrary, failure to express MHC molecules may render tumor or virus-infected cells susceptible to NK-mediated lysis (Ljunggren and Karre, 1990; Moretta et al., 1996; Moretta et al., 1992).
The decreased NK cell activity in peripheral blood and peritoneal fluid of women with endometriosis has been well established in recent years (Oosterlynck et al., 1991; Ho et al., 1995). It is thought to promote implantation of the endometrium as a tissue graft (Lefkowitz et al., 1988), and its cause is probably due to overexpression of KIRs. Our previous study demonstrated that increased expression of NKB1 and EB6 was found on NK cells in peritoneal fluid in women with advanced stage endometriosis (Wu et al., 2000). Another research achieved a similar result, in which the proportion of
KIR2DL1+ NK cells was increased in
peritoneal fluid and peripheral blood in women with endometriosis (Maeda et al., 2002). Moreover, the endometriotic tissue could also affect NK cells by an unknown mechanism to impair the NK cytotoxicity. Our previous studies demonstrated that NK
cytotoxicity in endometriosis could be affected by either cytokines or T cells (Ho et al., 1996a; Ho et al., 1997). The KIRs expressed on T cells might also play a role in the regulation of NK cytotoxicity.
研究目的
In contrast to that endometriosis is characterized by ectopic endometrium in the peritoneal cavity, adenomyosis is defined as the presence of endometrial glands within the myometrium. The only difference between adenomyosis and endometriosis is the site of ectopic endometriotic tissues, i.e. within or outside the uterus. In this study, we tried to measure different kinds of KIR expression on NK and T cells in different parts of uterus, as well as the expression of KIRs between women with and without adenomyosis, in an attempt to find the possible role of KIRs in the development of adenomyosis.
研究方法
Subjects and specimens
This study consisted of 10 women who suffered from adenomyosis (study group) and 12 women in whom uterine myoma was found (control group). These women underwent hysterectomy, either via abdominal or vaginal route, at our hospital due to intolerable symptoms. All the participating women were at pre-menopausal status, and they were free from recent infection and obvious clinical immunological diseases, such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and Hashimoto thyroiditis. The diagnosis of adenomyosis was made by histopathologic examination without exception, and this study protocol was approved by the institutional review boards at our hospital.
Peripheral venous blood, myometrium, and endometrium were obtained immediately after the uterus was removed away from the women in both groups. In study group, the myometrium was acquired from the tissue where there is coarsely trabeculated and diffusely hypertrophied myometrium stippled with foci of ectopic endometrium, while in the control group, the myometrium was obtained from the tissue other than uterine myoma. This tentative grouping determined by the naked eye was then found to be fully consistent with the final diagnosis provided by the pathologists. The cervical tissue was only derived in women without adenomyosis.
The aspirated blood was collected in glass tubes containing heparin, and was processed within 30 minutes. Peripheral blood mononuclear cells (PBMCs) were isolated by layering over Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) and centrifuged at 800 x g for 20 min. The isolated PBMCs were washed twice with RPMI-1640 medium (Life Technologies, Inc., Grand Island, NY, USA) to remove residual Ficoll-Hypaque solution, and were reconstituted to a final cell concentration of 1-2 x 106 cells/mL. The viability of PBMCs was verified with a trypan blue exclusion test.
Myometrial, endometrial, and cervical tissues were collected aseptically and separately in tissue flask containing RPMI-1640. Contaminated blood was removed after wash with RPMI-1640. Tissues were cut into tiny pieces (0.5 mm3) by surgical knife, and were suspended in 5 mL RPMI-1640. The suspensions were grinded and passed consecutively through different size of mechanical sieves (sieve size 150, 300 and 400), and were therefore overlaid on discontinuous (100%/50%/30%) Percoll
gradients (Sigma Chemical Co., St. Louis, MO, USA). After centrifuging at 800 x g for 45 min, mononuclear cells were obtained from the interface of 100% and 50% Percoll solution, and were then reconstituted to a final concentration of 1-2 x 106 cells/mL. The viability of mononuclear cells was verified with a trypan blue exclusion test.
Immunophenotypic analysis with three-color flow cytometry
The methods have been described in detail previously (Yang et al., 2000). In brief, monoclonal antibodies (mAbs) conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) were obtained (Becton Dickinson, San Jose, CA, USA). Mononuclear cells were incubated with mAbs at 4°C for 30 min and then were washed twice in phosphate-buffered saline (PBS) containing 2% fetal calf serum (FCS) and 0.1% sodium azide. These samples were fixed with 0.5% paraformaldehyde. Immunofluorescence and three-color flow cytometric analyses were done using a FACScan cytofluorimeter (Becton Dickinson) with computer interface to software (Hewlett-Packard Consort 32, Becton Dickinson) for full-list mode data storage, recovery and analysis.
The following combinations of mAbs were used: FITC-anti-CD45/PE-anti-CD14 (LeucoGATE), FITC-anti-IgG1/PE-anti- IgG2a (negative control), FITC-anti- CD3/PE-anti-CD19 (T cells), FITC-anti- CD3/PE-anti-CD56 (NK cells), FITC-anti- CD56/PE-anti-NKB1/PerCP-anti-CD3, FITC- anti-CD56/PE-anti-GL183/PerCP-anti- CD3, FITC-anti-CD56/PE-anti-EB6/PerCP-anti-CD 3, FITC-anti-CD56/PE-anti- CD94/PerCP-
anti-CD3, FITC-anti-CD4/PE-anti- NKB1/PerCP-anti-CD3, FITC-anti- CD4/PE-
anti-GL183/PerCP-anti-CD3, FITC-anti- CD4/PE-anti-EB6/PerCP-anti-CD3, FITC- anti-CD4/PE-anti-CD94/PerCP-anti-CD3,
FITC-anti-CD8/PE-anti- NKB1/PerCP-anti- CD3, FITC-anti-CD8/PE-anti-GL183/PerCP- anti-CD3, FITC-anti- CD8/PE-anti- EB6/PerCP-anti-CD3, and FITC-anti- CD8/PE-anti-CD94/PerCP-anti-CD3.
Leucogate was used to measure the proportion of lymphocytes in the sample being studied without any scatter gates. The gate was set around the lymphocytes (CD45+CD14−) to exclude other cells from analysis. The Simultest control (mouse FITC-anti- IgG1/PE-anti-IgG2a) was used for background control. The doublets, i.e. two cells either stuck together or very close in space, were strictly excluded from the calculation. In each cell suspension, 10,000 events in PBMCs as well as 2,000-5,000 events in tissue mononuclear cells acquired for gated lymphocytes were measured. The density of surface markers was expressed as the mean fluorescence intensity (MFI) of cells stained with specific monoclonal antibodies of KIRs.
Statistical analysis
All values are expressed as mean ± standard deviation (SD). As the data were not normally distributed, the Mann-Whitney U test for non-parametric data was used to compare the difference between the groups. A probability value less than 0.05 was considered statistically significant.
結果與討論
Ten women with and 12 women without adenomyosis were recruited in this study. The median age was 44 (range 38-49) for women with adenomyosis and 45 (range 37-51) for
those without adenomyosis. Among them, 5 out of 10 women with adenomyosis and 6 out of 12 women without adenomyosis were at follicular phase, while the others were at luteal phase. Since our previous study (Ho et al., 1996b) revealed that the NK cell populations and the NK cell activation markers were similar in different phases of menstrual cycle, the following data are analyzed no matter the phases of menstrual cycle.
The mean numbers of total lymphocytes per gram of tissue were 2.6 (range 0.1-6.5) x 104 in the myometrium and 1.9 (0.7-4.4) x 106 in the endometrium in women with adenomyosis, similar to those obtained in the myometrium [1.8 (0.3-5.4) x 104] and endometrium [2.5 (0.1-7.5) x 106] in women without adenomyosis. The mean lymphocyte number in the cervix was 1 (0.3-3.4) x 104 in women without adenomyosis.
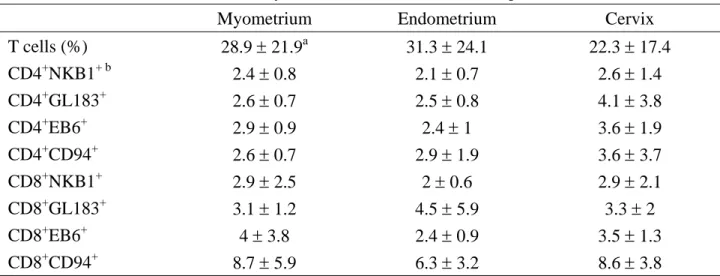
Among myometrium, endometrium, and cervix in women without adenomyosis, there was no difference in the fraction of NK and T cells. The MFI of CD94 was significantly higher in the subpopulations of CD56+ (114.5 ± 99) and CD56+
CD94+ (175.1 ± 113) cells in the endometrium, compared with those in the myometrium (38.5 ± 29.9 and 94.2 ± 48.5) and cervix (44.6 ± 33.8 and 84.8 ± 38). The
MFI of GL183 in the CD56+GL183+
subpopulation in endometrium was 157.4 ± 97.8, much higher than that in cervix (80.9 ± 52). On the other hand, the NKB1 and EB6 were similarly expressed on NK cells among different parts of uterus (Table I). The difference was also not prominent in the expression of various kinds of KIRs on CD4+
and CD8+ T cells among different
compartments of the uterus (Table II).
In the comparison between women with and without adenomyosis, NK cell
populations were not different. The MFI of NKB1 in the CD56+ subpopulation (3.2 ± 1), as well as the percentage of CD56+NKB1+ in CD56+ cells (12.2% ± 12.7%), in eutopic endometrium in women with adenomyosis were significantly lower than those in women without adenomyosis (5.8 ± 2.8 and 26.1% ± 10.4% respectively). The MFI of GL183 in the CD56+ subpopulation (8.3 ± 2) in endometrium in women with adenomyosis was also lower than that in women without adenomyosis (27.4 ± 23.6). On the other hand, the KIR expression on NK cells was not different in the myometrium and peripheral blood between women with and without adenomyosis (Table III). Also, the expression of KIRs on T cells, either CD4+ or CD8+, was similar in endometrium, myometrium, and peripheral blood between those with and without adenomyosis (Table IV).
The functions of uterine NK cells are not yet well known by far. Because they are present in abundance at the time of implantation, they may play a role in the implantation process and the subsequent orderly growth and development of the placenta (King et al., 1998). Previous results revealed that uterine NK cells have a similar repertoire of KIRs that are also found on NK cells in the blood (Verma et al., 1997). These uterine NK cell KIRs have identical structural characteristics in the excellular domains, transmembrane region and cytoplasmic tail as those of peripheral blood NK cell receptors, indicating that the two cell populations may function in the same way. However, the proportions of NK cells expressing a particular KIR and the antigenic density are not the same between peripheral blood and uterine NK cells (Verma et al., 1997).
Although the heterogeneity in expression
of KIR between blood and uterus was found, there has not been any report describing the KIR expression in different parts of the uterus. Our results revealed that there was an increased CD94 expression on NK cells in the endometrium than that in the myometrium and cervix. It might imply that the NK activity is by nature depressed in the endometrium compared with that in the myometrium and cervix because the increased expression of KIR generally represented a decreased NK activity (Wu et al., 2000). This naturally depressed NK cytotoxicity in the endometrium might therefore be one of the possible mechanisms that account for the development of endometriosis and/or adenomyosis in women of reproductive age. Without doing functional studies using class I HLA expressing target cells, however, we cannot confirm that the increased expression of CD94 could inevitably result in inhibition of NK activity in this study.
We also demonstrated decreased expression of NKB1 and GL183 on endometrial NK cells in women with adenomyosis compared with that in women without adenomyosis. It may be a compensatory effect, in which the NK cytotoxicity is activated in women with adenomyosis in order to wipe out the abnormal endometrial cells that might go out of the eutopic site of endometrium. It also implies that the “abnormal” endometrial cells, rather than the impaired NK cell function, account for the development of adenomyosis. This various immunological expression of KIRs in the eutopic endometrium is supported by a previous report (Braun et al., 2002), in which a reduction in apoptosis of endometrial cells was found in the eutopic endometrium in women with endometriosis due to reduced
macrophage trafficking into the eutopic endometrium. However, GL183 recognizes both inhibitory and activatory forms of KIRs, which could result in either inhibition or activation of the NK-mediated cytolytic activity (Moretta et al., 1995). Since Western blotting and reverse transcription polymerase chain reaction (RT-PCR) were not done in this study, the possibility remains that inhibitory as well as activatory forms of GL183 may both be decreased. Similarly, an alternative explanation for the decreased NK cytotoxicity seen in endometriosis is that there may be decreased expression of activatory receptors rather than increased inhibitory KIR expression. As K562 target cells generally lack class I MHC expression (Rouas-Freiss et al., 1997), any changes in levels of inhibitory or activatory KIRs should not necessarily affect NK cytotoxicity once K562 were employed as the target cells.
Our results did not reveal different KIR expression on the myometrial NK cells between women with and without adenomyosis. It is unlike the finding that has been achieved in endometriosis, in which there was an increased KIR expression on the peritoneal NK cells in women with endometriosis (Wu et al., 2000; Maeda et al., 2002). As a result, the local immunological appearance in response to the ectopic endometrium might be different between adenomyosis and endometriosis.
Unlike the different expression of KIRs on NK cells, we demonstrated that KIRs were similarly expressed on both CD4+ and CD8+ T cells among various uterine tissues and between women with and without adenomyosis (Table II and IV). Agreeing with previous reports (Moretta et al., 1996; Mingari et al., 1996), we also found that KIRs were
more frequently expressed on CD8+ T cells than on CD4+ T cells. One reasonable explanation for the advantage of this event is that the CD8+ T cells have NK-like activity, and would thus be deleterious to normal cells if they did not express KIRs. Their defective expression could be involved in autoimmune diseases caused by autoreactive cytotoxic T cells (Mingari et al., 1998).
We demonstrated a decreased expression of KIRs on NK cells in eutopic endometrium in women with adenomyosis compared with that in women without adenomyosis. It might be a compensatory effect in which the NK cytotoxicity is activated in order to wipe out the abnormal endometrial cells that might go out of the eutopic site of endometrium.
參考文獻
1. Braun, D.P., Ding, J., Shen, J., Rana, N., Fernandez, B.B. and Dmowski, W.P. (2002) Relationship between apoptosis and the number of macrophages in eutopic endometrium from women with and without endometriosis. Fertil. Steril.,
78, 830-835.
2. Gumperz, J.E., Valiante, N.M., Parham, P., Lanier, L.L. and Tyan, D. (1996) Heterogeneous phenotypes of expression of the NKB1 natural killer cell class I receptor among individuals of different human histocompatibility leukocyte antigens types appear genetically regulated, but not linked to major histocompatibililty complex haplotype. J. Exp. Med., 183, 1817-1827.
3. Ho, H.N., Chao, K.H., Chen, H.F., Wu, M.Y., Yang, Y.S. and Lee, T.Y. (1995) Peritoneal natural killer cytotoxicity and CD25+ CD3+ lymphocyte subpopulation are decreased in women with stage III-IV
endometriosis. Hum. Reprod., 10,
2671-2675.
4. Ho, H.N., Wu, M.Y., Chao, K.H., Chen, C.D., Chen, S.U., Chen, H.F. and Yang, Y.S. (1996a) Decrease in interferon gamma production and impairment of T-lymphocyte proliferation in peritoneal fluid of women with endometriosis. Am. J. Obstet. Gynecol., 175, 1236-1241. 5. Ho, H.N., Chao, K.H., Chen, C.K., Yang,
Y.S. and Huang, S.C. (1996b) Activation status of T and NK cells in the endometrium throughout menstrual cycle and normal and abnormal early pregnancy. Hum. Immunol., 49, 130-136. 6. Ho, H.N., Wu, M.Y., Chao, K.H., Chen,
C.D., Chen, S.U. and Yang, Y.S. (1997) Peritoneal interleukin-10 increases with
decrease in activated CD4+ T
lymphocytes in women with endometriosis. Hum. Reprod., 12,
2528-2533.
7. King, A., Burrows, T., Verma, S., Hiby, S. and Loke, Y.W. (1998) Human uterine lymphocytes. Hum. Reprod. Update, 4, 480-485.
8. Lanier, L.L. and Phillips, J.H. (1996) Inhibitory MHC class I receptors on NK cells and T cells. Immunol. Today, 17, 86-91.
9. Lefkowitz, M., Kornbluth, J.,
Tomaszewski, J.E. and Jorkasky, D.K. (1988) Natural killer-cell activity in cyclosporine-treated renal allograft recipients. J. Clin. Immunol., 8, 121-127. 10. Ljunggren, H.G. and Karre, K. (1990) In
search of the 'missing self': MHC molecules and NK cell recognition. Immunol. Today, 11, 237-244.
11. Maeda, N., Izumiya, C., Yamamoto, Y., Oguri, H., Kusume, T. and Fukaya, T.
(2002) Increased killer inhibitory receptor KIR2DL1 expression among natural killer cells in women with pelvic endometriosis. Fertil. Steril., 77,
297-302.
12. Mingari, M.C., Schiavetti, F., Ponte, M., Vitale, C., Maggi, E., Romagnani, S., Demarest, J., Pantaleo, G., Fauci, A.S. and Moretta, L. (1996) Human CD8+ T lymphocyte subsets that express HLA class I-specific inhibitory receptors represent oligoclonally or monoclonally expanded cell populations. Proc. Natl. Acad. Sci. USA, 93, 12433-12438.
13. Mingari, M.C., Moretta, A. and Moretta, L. (1998) Regulation of KIR expression in human T cells: a safety mechanism that may impair protective T-cell responses. Immunol. Today, 19, 153-157. 14. Moretta, A., Sivori, S., Vitale, M., Pende,
D., Morelli, L., Augugliaro, R., Bottino, C. and Moretta, L. (1995) Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. J. Exp. Med., 18, 875-884.
15. Moretta, A., Bottino, C., Vitale, M., Pende, D., Biassoni, R., Mingari, M.C. and Moretta, L. (1996) Receptors for HLA class-I molecules in human natural killer cells. Annu. Rev. Immunol., 14, 619-648.
16. Moretta, L., Ciccone, E., Moretta, A., Hoglund, P., Ohlen, C. and Karre, K. (1992) Allorecognition by NK cells: nonself or no self? Immunol. Today, 13, 300-306.
17. Moretta, L., Ciccone, E., Mingari, M.C., Biassoni, R. and Moretta, A. (1994) Human natural killer cells: origin, clonality, specificity, and receptors. Adv.
Immunol., 55, 341-380.
18. Oosterlynck, D.J., Cornillie, F.J., Waer, M., Vandeputte, M. and Koninckx, P.R. (1991) Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil. Steril., 56, 45-51. 19. Rouas-Freiss, N., Goncalves, R.M.,
Menier, C., Dausset, J. and Carosella, E.D. (1997) Direct evidence, to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc. Natl. Acad. Sci. USA, 94, 11520 –11525.
20. Trinchieri, G. (1989) Biology of natural killer cells. Adv. Immunol., 47, 187-376. 21. Verma, S., King, A. and Loke, Y.W.
(1997) Expression of killer cell inhibitory receptors on human uterine natural killer cells. Eur. J. Immunol., 27, 979-983. 22. Wu, M.Y., Yang, J.H., Chao, K.H.,
Hwang, J.L., Yang, Y.S. and Ho, H.N. (2000) Increase in the expression of killer cell inhibitory receptors on peritoneal natural killer cells in women with endometriosis. Fertil. Steril., 74, 1187-1191.
23. Yang, J.H., Chen, C.D., Wu, M.Y., Chao, K.H., Yang, Y.S. and Ho, H.N. (2000) Hormone replacement therapy reverses the decrease in natural killer cytotoxicity but does not reverse the decreases in the T cell subpopulation or interferon gamma production in postmenopausal women. Fertil. Steril., 74, 261-267. 計畫成果自評 本 研 究 內 容 與 原 計 畫 百 分 之 九 十 相 符,已達成預期目標,並已發表於著名的學 術 期 刊 Hum Reprod 2004;19:1974-1978 (Impact factor = 3.365)。在應用價值方面, 現有針對子宮腺肌症的治療方式,不論藥物 或手術,很難讓罹患子宮腺肌症婦女同時達 到懷孕與症狀緩解的目的,進一步瞭解其免 疫變化及致病機轉有助於未來尋求更妥善 的臨床處理方法。
附錄
Table I. KIR expression on NK cells in different portions of uterus from women with uterine myoma (n =
12). The myometrium was obtained from the tissue other than uterine myoma in these women.
Myometrium Endometrium Cervix
NK cells (%) 22.9 ± 16.6a 11.6 ± 9.9 19.3 ± 16.7 NKB1 MFI of CD56+ cellsb 5.8 ± 2.8 5.8 ± 2.8 8.6 ± 11.5 % CD56+NKB1+ cellsc 22.8 ± 12.1 26.1 ± 10.4 29.3 ± 20.9 MFI of CD56+NKB1+ cellsd 73.3 ± 37.8 71.1 ± 44.2 55.5 ± 40.8 GL183 MFI of CD56+ cells 15.2 ± 9.3 27.4 ± 23.6 21.7 ± 21.9 % CD56+GL183+ cells 45.5 ± 18 46.3 ± 21.1 52.9 ± 17.7 MFI of CD56+GL183+ cells 100.8 ± 57.1 157.4 ± 97.8e 80.9 ± 52e
EB6 MFI of CD56+ cells 6.3 ± 3.7 5.2 ± 3.2 7.9 ± 4.5
% CD56+EB6+ cells 31.2 ± 24 27 ± 19.7 33 ± 18.4
MFI of CD56+EB6+ cells 26.5 ± 8.1 36.2 ± 26.1 28 ± 7.5
CD94 MFI of CD56+ cells 38.5 ± 29.9f 114.5 ± 99fg 44.6 ± 33.8g
% CD56+CD94+ cells 67.5 ± 17.1 67.7 ± 23.6 73.7 ± 16
MFI of CD56+CD94+ cells 94.2 ± 48.5h 175.1 ± 113hi 84.8 ± 38i KIR = killer cell inhibitory receptor, NK = natural killer cell, MFI = mean fluorescence intensity
a Values are mean ± standard deviation b
Calculated as the mean fluorescence intensity of NKB1 on CD56+ cells
c
Calculated as the percentage of CD56+NKB1+ in CD56+ cells
d
Calculated as the mean fluorescence intensity of NKB1 on CD56+NKB1+ cells
e, f, g, h, i
P < 0.05 by Mann-Whitney U test
Table II. Mean fluorescence intensity of KIRs on T cells in different portions of uterus (n = 12).
Myometrium Endometrium Cervix
T cells (%) 28.9 ± 21.9a 31.3 ± 24.1 22.3 ± 17.4 CD4+NKB1+ b 2.4 ± 0.8 2.1 ± 0.7 2.6 ± 1.4 CD4+GL183+ 2.6 ± 0.7 2.5 ± 0.8 4.1 ± 3.8 CD4+EB6+ 2.9 ± 0.9 2.4 ± 1 3.6 ± 1.9 CD4+CD94+ 2.6 ± 0.7 2.9 ± 1.9 3.6 ± 3.7 CD8+NKB1+ 2.9 ± 2.5 2 ± 0.6 2.9 ± 2.1 CD8+GL183+ 3.1 ± 1.2 4.5 ± 5.9 3.3 ± 2 CD8+EB6+ 4 ± 3.8 2.4 ± 0.9 3.5 ± 1.3 CD8+CD94+ 8.7 ± 5.9 6.3 ± 3.2 8.6 ± 3.8
KIR = killer cell inhibitory receptor
a Values are mean ± standard deviation b
Table III. KIR expression on NK cells in women with and without adenomyosis Adenomyosis (n=10) Control (n=12)
Blood Myometrium Endometrium Blood Myometrium Endometrium
NK cells (%) 23.5 ± 10.3a 20.2 ± 13.3 12.3 ± 9.2 26.4 ± 10.2 22.9 ± 16.6 11.6 ± 9.9 NKB1 MFI of CD56+ cellsb 11 ± 12.6 5.7 ± 6.4 3.2 ± 1e 9.1 ± 9.2 5.8 ± 2.8 5.8 ± 2.8e % CD56+NKB1+ cellsc 22.3 ± 23.1 19.4 ± 21.8 12.2 ± 12.7f 28.7 ± 18.8 22.8 ± 12.1 26.1 ± 10.4f MFI of CD56+NKB1+ cellsd 214.6 ± 139.6 63.7 ± 50.6 41.6 ± 16.6g 207.8 ± 116.7 73.3 ± 37.8 71.1 ± 44.2g GL183 MFI of CD56+ cells 22 ± 19.6 16.4 ± 14.8 8.3 ± 2h 34.8 ± 33.5 15.2 ± 9.3 27.4 ± 23.6h % CD56+GL183+ cells 39.1 ± 24.5 42 ± 22.4 32.7 ± 14.9 57.1 ± 17.9 45.5 ± 18 46.3 ± 21.1 MFI of CD56+GL183+ cells 165 ± 69.6 84.7 ± 39 117.2 ± 55.2 201.4 ± 72.6 100.8 ± 57.1 157.4 ± 97.8
EB6 MFI of CD56+ cells 7.9 ± 7.6 6.8 ± 2.3 4.6 ± 1.4 4.3 ± 2.2 6.3 ± 3.7 5.2 ± 3.2 % CD56+EB6+ cells 25.6 ± 20.3 31.7 ± 14.8 19.5 ± 14.5 17.8 ± 11.2 31.2 ± 24 27 ± 19.7
MFI of CD56+EB6+ cells 75.7 ± 29.7 26.9 ± 6.5 24.3 ± 6.4 57.5 ± 31.4 26.5 ± 8.1 36.2 ± 26.1
CD94 MFI of CD56+ cells 70.2 ± 45.2 45.1 ± 25.3 102.3 ± 79.4 76.8 ± 30.5 38.5 ± 29.9 114.5 ± 99
% CD56+CD94+ cells 82.8 ± 10 73.9 ± 15.7 79.7 ± 16.7 87.2 ± 6.1 67.5 ± 17.1 67.7 ± 23.6
MFI of CD56+CD94+ cells 110.2 ± 58.6 110.5 ± 48.3 226 ± 114.8 115 ± 40.8 94.2 ± 48.5 175.1 ± 113
KIR = killer cell inhibitory receptor, NK = natural killer cell, MFI = mean fluorescence intensity
a Values are mean ± standard deviation b
Calculated as the mean fluorescence intensity of NKB1 on CD56+ cells
c
Calculated as the percentage of CD56+NKB1+ in CD56+ cells
d
Calculated as the mean fluorescence intensity of NKB1 on CD56+NKB1+ cells
e, f, g
P < 0.05 by Mann-Whitney U test
h
P < 0.005 by Mann-Whitney U test
Table IV. Mean fluorescence intensity of KIR expressed on CD4+ and CD8+ T lymphocytes in women with and without adenomyosis
Adenomyosis (n=10) Control (n=12)
Blood Myometrium Endometrium Blood Myometrium Endometrium
T cells (%) 59.9 ± 9.9a 19.3 ± 14.1 47.4 ± 18 56.5 ± 10.8 28.9 ± 21.9 31.3 ± 24.1 CD4+NKB1+ b 1.7 ± 0.4 2.1 ± 0.4 2.2 ± 0.6 1.6 ± 0.4 2.4 ± 0.8 2.1 ± 0.7 CD4+GL183+ 2 ± 0.4 2.5 ± 0.4 3 ± 0.9 1.7 ± 0.4 2.6 ± 0.7 2.5 ± 0.8 CD4+EB6+ 2 ± 0.4 2.9 ± 0.6 2.9 ± 1 1.7 ± 0.3 2.9 ± 0.9 2.4 ± 1 CD4+CD94+ 1.8 ± 0.4 2.6 ± 1.3 2.5 ± 0.7 1.7 ± 0.5 2.6 ± 0.7 2.9 ± 1.9 CD8+NKB1+ 1.9 ± 0.6 2.2 ± 0.8 2.5 ± 1.4 1.8 ± 0.4 2.9 ± 2.5 2 ± 0.6 CD8+GL183+ 2.5 ± 0.6 2.9 ± 0.8 2.9 ± 0.7 2.1 ± 0.6 3.1 ± 1.2 4.5 ± 5.9 CD8+EB6+ 2.1 ± 0.6 3.2 ± 1.4 2.6 ± 0.8 1.8 ± 0.4 4 ± 3.8 2.4 ± 0.9 CD8+CD94+ 5 ± 3.3 8.3 ± 3.8 5.4 ± 2.8 4 ± 2.1 8.7 ± 5.9 6.3 ± 3.2
KIR = killer cell inhibitory receptor
a Values are mean ± standard deviation b