Acid-Free Synthesis of Mesoporous Silica Using Triblock Copolymer
as Template with the Aid of Salt and Alcohol
Shih-Yuan Chen and Soofin Cheng*
Department of Chemistry, National Taiwan UniVersity, Taipei 106, Taiwan ReceiVed January 25, 2007. ReVised Manuscript ReceiVed March 24, 2007
Under acid-free conditions using tetraethyl orthosilicate (TEOS) as the silica source and triblock copolymer P123 as the template, mesoporous silica materials of either ultra-large mesoporous cellular foam (MCF) or two-dimensional (2D)-hexagonal p6mm pore arrangement were synthesized with the aid of salt and alcohol. The pore structure was varied with the concentrations of P123 and NaCl and the presence of alcohol in the synthesis solution. The porous network of the resulting MCF structure obtained in the aqueous solutions containing TEOS, P123, and NaCl was changed from closed window cell to open window cell when the concentrations of P123 and NaCl were increased. On the other hand, when a proper amount of ethanol was added as cosolvent, mesoporous silica with 2D-hexagonal pore arrangement similar to that of SBA-15 and MSU-H was obtained. The resultant materials possessed high surface area
(626-896 m2/g), mesopores (6.3-6.6 nm in diameter) of reasonable pore size distribution (2.5-3.0
nm), and large porosity (0.91-1.13 cm3/g). On the basis of the29Si magic angle spinning NMR spectra,
the mesoporous materials synthesized under acid-free conditions have better cross-linked silica framework than those prepared in an acidic environment such as SBA-15. The pore ordering was retained even after boiling the material in water for 72 h. Moreover, the pore size distribution was narrowed down to 1.0-1.7 nm, attributed to the re-organization of the silica framework during the hydrothermal treatment.
Introduction
Mesoporous materials have been widely studied since the
first report by the researchers at Mobil,1 because of their
potential applications in sorption, separation, catalysis,
nanocasting, and electro-optical devices.2-4 Generally, the
materials of ordered mesopores are synthesized by self-assembly of the surfactant-type templates and inorganic precursors through electrostatic forces or hydrogen bond-ing.1,5,6Materials of relatively large pores have been obtained
by using amphiphilic triblock copolymers as the pore-directing agents under strong acidic condition. Among them, two-dimensional (2D)-hexagonal SBA-15 synthesized with
Pluronic P123 (EO20PO70EO20) as the pore directing agent
has received great attention because of its relatively large
pores and high hydrothermal stability.6-8 For the purpose
of further expanding the pore diameter, swelling agents such as trimethylbenzene (TMB) which dissolves in the hydro-phobic core of P123 micelles are usually added into the synthesis mixture. When the TMB/P123 mass ratio was greater than 0.3, ultra-large mesoporous cellular foam
(designated as MCF) was obtained,7c which could also be
made using hard templates such as polymer nanospheres.8
Both SBA-15 and MCF materials have shown higher adsorption capacities and faster adsorption rates toward large
molecules, in comparison to those of the M41S family.7e,f
However, the synthesis conditions for SBA-15 and MCF are usually in strong acids and environmentally unfriendly. The mineral acids are considered to play a key role in assembly
of the surfactant and silica precursor through a N0H+X-S+
type interaction,6where N0is the neutral surfactant, H+is
the proton, X-is the counteranion, and S+is the protonated
silicate species. Although several reports have shown that * Corresponding author. Fax: +886-2-23636359. E-mail: chem1031@
ntu.edu.tw.
(1) (a) Kresge, C. T.; Leonowicz, M. E.; Roth, W. J.; Vartuli, J. C.; Beck, J. S. Nature 1992, 359, 710. (b) Beck, J. S.; Vartuli, J. C.; Roth, W. J.; Leonowicz, M. E.; Kresge, C. T.; Schmitt, K. D.; Chu, C. T.-W.; Olson, D. H.; Sheppard, E. W.; McCullen, S. B.; Higgins, J. B.; Schlenker, J. L. J. Am. Chem. Soc. 1992, 114, 10834. (c) Lu, A.-H.; Schuth, F. AdV. Mater. 2006, 18, 1793.
(2) (a) Feng, X.; Fryxell, G. E.; Wang, L. Q.; Kim, A. Y.; Liu, J.; Kemner, K. M. Science 1997, 276, 92. (b) Brown, J.; Mercier, L.; Pinnavaia, T. J. Chem. Commun. 1999, 69.
(3) (a) Corma, A. Chem. ReV. 1997, 97, 237. (b) Wang, X.; Chen, C. C.; Chen, S. Y.; Mou, Y.; Cheng, S. Appl. Catal. A 2005, 281, 47. (c) Crudden, C. M.; Sateesh, M.; Lewis, R. J. Am. Chem. Soc. 2005, 127, 10045.
(4) (a) Baskaran, S.; Liu, J.; Domansky, K.; Kohler, N.; Li, X.; Coyle, C.; Fryxell, G. E.; Thevuthasan, S.; Williford, R. E. AdV. Mater. 2000, 12, 291. (b) Wang, Y.; Yang, C. M.; Schmidt, W.; Spliethoff, B.; Bill, E.; Schu¨th, F. AdV. Mater. 2005, 17, 53. (c) Yang, P. D.; Wirnsberger, G.; Huang, H. C.; Cordero, S. R.; McGehee, M. D.; Scott, B.; Deng, T.; Whitesides, G. M.; Chmelka, B. F.; Buratto, S. K.; Stucky, G. D. Science 2000, 287, 465.
(5) Tanev, P. T.; Pinnavaia, T. J. Science 1995, 267, 865.
(6) (a) Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G. H.; Chmelka, B. F.; Stucky, G. D. Science 1998, 179, 548. (b) Zhso, D.; Huo, Q.; Feng, J.; Chmelka, B. F.; Stucky, G. D. J. Am. Soc. Chem. 1998, 120, 6024. (c) Melosh, N. A.; Davidson, P.; Chmelka, B. F. J.
Am. Chem. Soc. 2000, 122, 823. (d) Yang,C. M.; Zibrowius, B.; Schmidt, W.; Schuth, F. Chem. Mater. 2004, 16, 2918.
(7) (a) Lukens, W. W.; Schmidt-Winkel, P.; Zhao, D.; Feng, J.; Stucky, G. D. Langmuir 1999, 15, 5403. (b) Lukens, W. W.; Yang, P.; Stucky, G. D. Chem. Mater. 2001, 13, 28. (c) Lettow, J. S.; Han, Y. J.; Schmidt-Winkel, P.; Yang, P.; Zhao, D.; Stucky, G. D.; Ying, J. Y. Langmuir 2000, 16, 8291. (d) Han, Y.-J.; Watson, J. T.; Stucky, G. D.; Butler, A. J. Mol. Catal. B: Enzym. 2002, 17, 1. (e) Han, Y.; Lee, S. S.; Ying, J. Y. Chem. Mater. 2006, 18, 643. (f) Schmidt-Winkel, P.; Lukens, Jr., W. W.; Yang, P.; Margolese, D. I.; Lettow, J. S.; Ying, J. Y.; Stucky, G. D. Chem. Mater. 2000, 12, 686. (8) Fan, J.; Yu, C.; Wang, L.; Tu, B.; Zhao, D.; Sakamoto, Y.; Terasaki,
O. J. Am. Chem. Soc. 2001, 123, 12113.
3041 Chem. Mater. 2007, 19, 3041-3051
10.1021/cm070232y CCC: $37.00 © 2007 American Chemical Society Published on Web 04/28/2007
Downloaded by NATIONAL TAIWAN UNIV on August 5, 2009
the final synthesis gels of SBA-15 can be adjusted to near neutral by neutralizing the acidified surfactant solutions with weak bases or sodium silicate, the synthesis procedures have
been very complicated and uncontrollable.9-12
Our laboratory has developed a method to synthesize well-ordered mesoporous SBA-15 materials incorporated with
large amounts of tetravalent cations such as Zr4+and Sn4+
without the addition of hazardous mineral acids.13The acidity
generated from the hydrolysis of the metal salts in water was considered high enough to catalyze the tetraethyl orthosilicate (TEOS) hydrolysis. In addition, the long-range ordering of the mesopores in the materials was significantly improved by the addition of mineral salts such as NaCl, KCl,
K2(SO4), and NaI. It has been suggested that salts can
decrease the critical micelle temperature (CMT) of the surfactants, increase the ionic strength of synthesis solutions, and thus facilitate the self-assembly of the micelles and
inorganic presursors.13-19We have shown recently in a short
communication the success of preparing pure siliceous mesoporous materials using triblock copolymer as
pore-directing agent without adding hazardous mineral acids.20
Herein a detailed study on the preparation condition of the mesostructured silica materials under acid-free condition using triblock copolymer as template was presented. The materials were characterized with various techniques, includ-ing small-angle X-ray diffraction (XRD), thermogravimetric (TG) analysis, elemental analysis, nitrogen sorption isotherm,
pore size analysis, solid state 29Si magic angle spinning
(MAS) NMR, and scanning and transmission electron microscopies (SEM and TEM). Moreover, the resultant mesostructured silica materials were found to have high hydrothermal stability because of the better cross-linking of the silica framework.
Experimental Methods
Synthesis. No mineral acids and swelling agents were used in the synthesis procedures. The reactants in the synthesis gel were amphiphilic triblock copolymer (P123) as pore-directing agent, TEOS as the silica source, NaCl salt, and sometimes the alcohol
as cosolvent. Typically, 0.20-0.60 g of P123 ((EO)20(PO)70(EO)20, Aldrich, Mn ) 5800) and 0.59 g of NaCl (Acros, 99%) were dissolved in 40 g of deionized water at 35°C, and the solution was stirred for 2 h, followed by the addition of 2.10 g of TEOS (Acros, 98%). The mixture was sealed in a polypropylene (PP) bottle and stirred for 24 h and then hydrothermally treated in the static condition at 90°C for another 24 h. The solid precipitate was separated by filtering, washing with a 500 mL of deionized water, and drying at 50°C overnight. Calcination of the samples was performed at 500°C in air for 12 h with the ramp rate of 1°C /min. The molar ratios of the reactants in the synthesis gel were 0.0034-0.0103:0.50-5.0:1:221 P123/NaCl/TEOS/H2O. In other words, the concentrations of P123 and NaCl were varied in the range of 0.5-1.5 wt % and 0.13-1.27 M, respectively, in the synthesis gel. To study the effect of cosolvent, alcohol, such as absolute methanol, ethanol, and 1-propanol, was also added in the synthesis solution. The reactant compositions including ethanol were varied in the molar ratio of 0.0086:1:1:221:10.8-64.7 P123/NaCl/ TEOS/H2O/EtOH. The molar yields of silica materials based on the residues of TG analyses were around 86-93% without alcohols and 85-90% with alcohols.
Hydrothermal Stability. To examine the hydrothermal stability of the synthesized materials, about 0.30 g of calcined sample dispersed in 50 mL of deionized water was sealed in a PP bottle and heated at 100°C under the static condition for 72 h. The solid was then filtered and dried at 50°C overnight.
Characterization. XRD patterns were recorded using a Philip X’pert Pro diffractometer with Cu KR radiation operated at 40 mA and 45 kV. The pore structures of mesoporous silica materials were analyzed by nitrogen physisorption at liquid nitrogen temperature (77 K) using a Micrometerics TriStar 3000 system. Prior to the experiments, the materials were degassed at 200°C for 6-8 h under vacuum (10-3Torr). The specific surface areas were evaluated using the Brunauer-Emmett-Teller (BET) method in the P/P0range of 0.05-0.3. Pore size distribution (PSD) curves were calculated using the desorption branch of the isotherms and the Barrett-Joyner-Halenda (BJH) method,21and pore sizes were obtained from the peak positions of the distribution curves. The pore volume was accumulated up to P/P0) 0.990. The PSD of MCF was analyzed by using a modified BdB-FHH method with spherical pore model.7a The SEM photographs were taken on a Hitachi S-800 field emission scanning electron microscope. The energy dispersed X-ray (EDX) emission spectra were taken with a Hitachi S-2400 scanning electron microscope. Sample preparation was similar to that for SEM experiment except the sample was stuck on the holder made by carbon instead of copper and without coating a gold-palladium alloy layer. The EDX data were the average result of more than 20 spots. The TEM was performed using a Hitachi H-7100 transmis-sion electron microscope operating at 75 kV. The resin solidified samples cut into thin slices of 90-110 nm thickness were used for TEM studies. TG analyses were carried out on a Dupont TA 951 instrument with a ramping rate of 10°C/min in an air flow of 50 mL/min. The inductively coupled plasma-atomic emission spec-troscopy (ICP-AES) spectra of the samples dissolved in mixed HF-HNO3solutions were taken using an ELAN 5000 instrument. The solid state29Si MAS NMR spectra were measured using a Bruker MSL-300 spectrometer with 7 mm zirconia rotors spun at 5 kHz. Data were acquired at 59.6 MHz, 20 µs pulse width, and 60 s recycle delay. The Q4/(Q2 + Q3) area ratios were obtained by deconvolution of the peaks in the spectra using WinFit software. Chemical shifts were externally referenced to trimethylsilane. The
(9) (a) Kim, S.-S.; Pauly, T. R.; Pinnavaia, T. J. Chem. Commun. 2000, 1661. (b) Kim, S. S.; Karkamkar, A.; Pinnavaia, T. J.; Kruk, M.; Jaronec, M. J. Phys. Chem. B 2001, 105, 7663.
(10) Kim, J. M.; Han, Y. J.; Chmelka, B. F.; Stucky, G. D. Chem. Commun. 2000, 2437.
(11) Sierra, L.; Lopez, B.; Gil, H.; Guth, J. L. AdV. Mater. 1999, 11, 307. (12) Song, H.; Rioux, R. M.; Hoefelmeyer, J. D.; Komor, R.; Niesz, K.; Grass, M.; Yang, P.; Somorjai. G. A. J. Am. Chem. Soc. 2006, 128, 3027.
(13) (a) Chen, S. Y.; Jang, L. Y.; Cheng, S. Chem. Mater. 2004, 16, 4174. (b) Chen, S. Y.; Cheng, S.; Chung, W. T.; Lee, J. J.; Chiang, Y. P.; Tang, C. Y.; Lin, C. Y. Stud. Surf. Sci. Catal. 2006, 162, 369. (14) (a) Yu, C.; Tian, B.; Fan, J.; Stucky, G. D.; Zhao, D. J. Am. Chem.
Soc. 2002, 124, 4556. (b) Yu, C.; Fan, J.; Tian, B.; Zhao, D.; Stucky, G. D. AdV. Mater. 2002, 14, 1742.
(15) Flodstrom, K.; Alfredsson, V.; Kallrot, N. J. Am. Chem. Soc. 2003, 125, 4402.
(16) Guo, W.; Park, J. Y.; Oh, M. O.; Jeong, H. W.; Cho, W. J.; Kim, I.; Ha, C. S. Chem. Mater. 2003, 15, 2295.
(17) Wang, Y. Q.; Yang, C.-M.; Zibrowius, B.; Spliethoff, B.; Linden, M.; Schuth, F. Chem. Mater. 2003, 15, 5029.
(18) Karkamkar, A.; Kim, S. S.; Pinnavaia, T. J. Chem. Mater. 2003, 15, 11.
(19) (a) Das, D.; Tsai, C. M.; Cheng, S. Chem. Commun. 1999, 473. (b) Chen, S. Y.; Jang, L. Y.; Cheng, S. J. Phys. Chem. B 2006, 110, 11761. (20) Chen, S. Y.; Cheng, S. Stud. Surf. Sci. Catal. 2005, 156, 89.
(21) Barrett, E. P.; Joyner, L. G.; Hanlenda, P. C. J. Am. Chem. Soc. 1951, 73, 373.
Downloaded by NATIONAL TAIWAN UNIV on August 5, 2009
ζ potential of the P123 solution was measured by using a Malvern Zetasizer 3000 HS instrument.
Results and Discussion
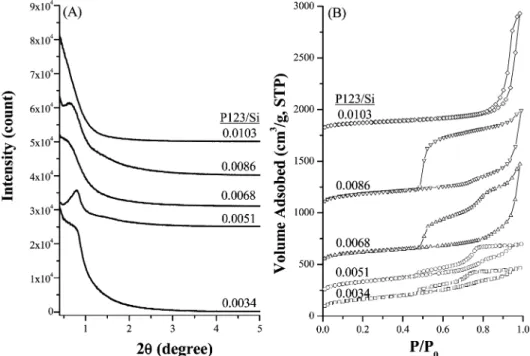
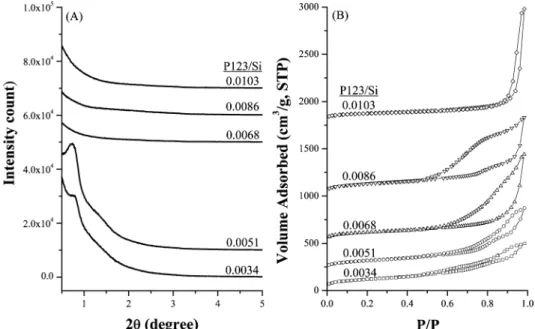
The addition of proper concentrations of NaCl salt and P123 pore-directing agent was found to be essential to obtain silica materials of ordered mesostructures in acid-free condi-tions. Figure 1a shows the small-angle XRD patterns of calcined mesoporous silica materials prepared without adding hazardous mineral acids. When the NaCl/Si molar ratio was
around 1, a broad diffraction peak around 2θ∼ 1°was seen
for the samples prepared with the reactant molar composition
varied in 0.0034-0.0086:1:221 P123/Si/H2O. These results
indicate that mesoporous silica materials with slightly ordered pore structures could be prepared in acid-free conditions when the reactant compositions are in the range of
0.0034-0.0086:1:1:221 P123/NaCl/TEOS/H2O.
Elemental analysis by either ICP-AES or the EDX technique shows that only a trace amount of sodium ions was left in the solid material (Na/Si molar ratio < 0.003). The quantities of chloride ions measured by EDX were also negligible. These results revealed that both sodium and chloride ions were hardly incorporated into the silica mesostructure during the self-assembly of P123 micelles and silica precursors. It has been shown that the presence of non-adsorbed ions, such as chloride ions, in the solution would render the hydrophobic portion of the surfactant toward the
more hydrophobic, so-called salting-out property.13,17-20
Moreover, the condensation and hydrolysis of silica have been found to increase by adding electrolytes in the synthesis solution. In the present study, similar effect was also observed with other salts including NaBr, NaSCN, tetramethylammo-nium chloride (TMACl), tetrapropylammtetramethylammo-nium bromide (TPABr), tetrabutylammonium bromide (TBABr), and ben-zyltrimethylammonium chloride (BTMACl).
The nitrogen sorption isotherms of calcined mesoporous silica materials are shown in Figure 1b. The adsorption-desorption hysteresis loops were found to vary with the concentrations of NaCl salt and P123 copolymer in the mother solution. Most of the hysteresis loops covered a wide
range of P/P0between 0.5 and 1.0, indicating that pores from
mesoporous to ultra-large sizes are probably present in the
materials.7For the applications in adsorption and catalysis
where the diffusion of molecules was concerned, porous materials containing both macro- and mesoporous structures were reported to be better than those with only one kind of
pore structure.6,22
The amount of nitrogen adsorbed in the capillary conden-sation region seems to increase with the P123/Si molar ratios. For the materials prepared with a P123/Si ratio within 0.0034-0.0051, the hysteresis loops were small in adsorbed
volume but covered a wide P/P0range, indicating that the
pore structures were complicated. When the P123/Si ratios were raised to 0.0068-0.0086, the hysteresis loops expanded;
the adsorption branches shifted to higher P/P0values whereas
the desorption branches retained in similar P/P0 region.
Further increase in the P123/Si ratio shifted both the adsorption and desorption branches of the isotherm to the
very high P/P0 region. According to the analyses by
Karkamkar and co-workers18 who studied the change of
hysteresis loops in mesocellular silica foam during the hydrothermal process, the materials prepared with P123/Si ratios within 0.0068-0.0086 correspond to an MCF-contain-ing closed window cells while that prepared with P123/Si ratio of 0.0103 is a MCF-containing open window cell. The cell and window diameters were calculated on the basis of the adsorption and desorption branches of the loops,
respec-(22) (a) Gagea, B. C.; Liang, D.; Tendeloo, G. V.; Martens, J. A.; Jacobs, P. A. Stud. Surf. Sci. Catal. 2006, 162, 259. (b) Yuan, Z. Y.; Ren, T. Z.; Azioune, A.; Pireaux, J. J.; Su, B. L. Chem. Mater. 2006, 18, 1753. Figure 1. (A) Small-angle XRD patterns and (B) N2adsorption-desorption isotherms of mesocellular silica foams synthesized in an acid-free
0.0034-0.0103: 1:1:221 P123/NaCl/Si/H2O system.
Acid-Free Synthesis of Mesoporous Silica Chem. Mater., Vol. 19, No. 12, 2007 3043
Downloaded by NATIONAL TAIWAN UNIV on August 5, 2009
tively. The differences between the cell width (63-76 nm) and window diameter (5-6 nm) are greater for the closed window cells but smaller (66 and 30 nm, respectively) for the open window cells. The expansion of the cell and window diameters was also observed by increasing the NaCl con-centration in the synthesis solution when the P123/Si ratio was fixed at 0.0086. These results show that silica materials with mesocellular pore structures can be prepared in aqueous solutions of NaCl salt and P123 copolymer without adding hazardous mineral acids and swelling agent.
The textural properties of the mesocellular silica materials are summarized in Table 1. For the sample synthesized with low P123/Si molar ratio of 0.0034, the surface area and pore
volume were 580 m2/g and 0.7 cm3/g, respectively, which
were slightly lower than those of SBA-15 synthesized in a
strong acidic condition.6When the P123/Si molar ratios were
within 0.0051-0.0086, the pore volume enlarged to
0.8-1.2 cm3/g, and the surface areas were within 595-758 m2/
g, which were close to those of SBA-15 but slightly greater than those of MCF materials synthesized using TMB as
swelling agents in strong acidic conditions.7When the P123/
Si ratio was further raised to 0.0103, the pore volume was
retained around 1.2 cm3/g. However, the surface area
decreased to 436 m2/g. It is noticeable that the sizes of the
window and cell in the present MCF materials were larger than those synthesized under acidic conditions in previous
reports.6-11It suggests that the average aggregation number
of P123 molecules in a micelle in the neutral brine is probably larger than that in the acidic solution. Recently,
Song and co-workers12 also reported that the pore size of
SBA-15 synthesized from neutralizing the acidic synthesis solution with a weak base was slightly greater than that from a strong acidic environment (2 M HCl). The micropore surface area and volume of the present MCF materials are
around 110-185 m2/g and 0.04-0.08 cm3/g, slightly lower
than that of SBA-15 (Smicro) 168-200 m2/g and Vmicro)
0.06-0.12 cm3/g).6
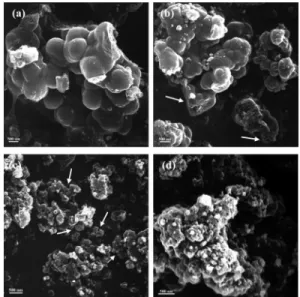
The morphologies of mesocellular silica materials
syn-thesized with NaCl/Si∼ 1 and various P123 concentrations
were examined by SEM, and the photographs are shown in Figure 2. The silica materials are aggregates of spherical particles. The particle sizes seem to decrease with the increase of P123/Si molar ratio. Figure 2b,c shows that some pores of 50-250 nm in diameter can be seen in the cross sections of the broken particles prepared with a P123/Si molar ratio of 0.0051 and smaller pores of 50-100 nm in diameter on those prepared with P123/Si ratio of 0.0068.
For the material prepared with P123/Si ratio of 0.0103, the pore structures are hardly seen because of the very small particle size (Figure 2d).
The pore structures of the MCF materials synthesized with
NaCl/Si∼ 1 and various P123 concentrations were examined
by TEM microscopy. Figure 3 shows that the three-dimensional MCFs of diameter ranging in 50-200 nm can be clearly seen. Moreover, the walls of the foams synthesized with low P123/Si molar ratios in 0.0034-0.0051 contain nanopores of approximately 5-10 nm in diameter, which is consistent with the result of modified BdB-FHH pore size analysis. On the other hand, the mesocellular silica synthe-sized with P123/Si ratios greater than 0.0068 contain solid-framework foams of approximately 50-100 nm in diameter and without nanopores on the walls (Figure 3b). When the P123/Si ratio was increased to 0.0103, the walls of the foams seem to break into nanoslices with approximately 15-30 nm openings (Figure 3c). These results on porous structures are consistent with the variation observed in nitrogen sorption hysteresis loops and the SEM photographs. The wall thick-ness of present MCF materials, based on the TEM photo-graphs, is around 8-10 nm and much thicker than those of
SBA-15 (Wt ) 3-4 nm) and MCF (Wt ) 4-6 nm)
synthesized in acidic conditions reported in the literature.6a,7f
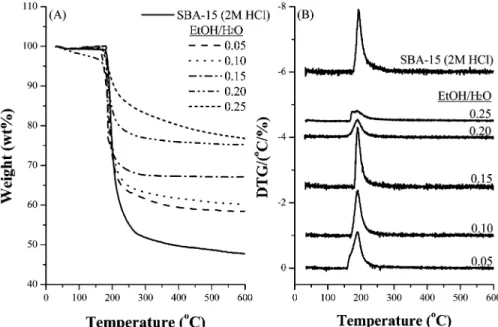
The interaction between the P-123 triblock copolymer and the framework of MCF materials was examined by the thermal analysis technique, and the results are compared with that of SBA-15 synthesized in 2 M HCl (Supporting Information). All the MCF materials showed a single-step
weight loss at 185-195 °C. Moreover, the weight losses
varied in 27-44 wt % are generally proportional to the concentration of P123 in the synthesis solutions. On the other hand, the weight loss due to decomposition of pore directing agent P123 in SBA-15 was 53 wt % with the maximum
differential thermogravimetric (DTG) temperature at 194°C.
The similarity in decomposition temperatures of pore-directing agent P123 indicates that the interaction between P123 and the silica framework of MCF materials is similar in strength to that in SBA-15. It also implies that the triblock Table 1. Structural Properties of Mesocellular Silica Foams
Synthesized under Acid-Free Conditions with Reactant Compositions within 0.0034-0.0103:1:1:221 P123/NaCl/Si/H2O
P123/Si (molar ratio) pH (gel) d (nm) SBET (m2/g) Smicro (m2/g) Vtotal (cm3/g) Vmicro (cm3/g) Dca (nm) Dwa (nm) 0.0103 4.40 11.1 436 141 1.2 0.06 66 30 0.0086 4.43 11.1 758 185 1.2 0.08 76 5 0.0068 4.46 13.8 595 189 1.0 0.04 63 5 0.0051 4.58 13.6 649 134 0.8 0.04 31 6 0.0034 4.99 n.d. 580 110 0.7 0.03 27 5
aThe diameters of cell (D
c) and window (Dw) determined from the adsorption and desorption branches, respectively, by using a modified BdB-FHH method with a spherical model.
Figure 2. SEM photographs of mesocellular silica foams synthesized with
a NaCl/Si ratio of 1 and various P123/Si ratios of (a) 0.0034, (b) 0.0051, (c) 0.0068, and (d) 0.0103.
Downloaded by NATIONAL TAIWAN UNIV on August 5, 2009
copolymer P123 is incorporated into the mesocellular silica foams through the self-assembly process.
Figure 4 shows the solid state29Si MAS NMR spectra of
as-made MCF synthesized in acid-free conditions, in com-parison to that of conventional SBA-15. Three peaks with
chemical shifts at -91, -99, and -108 ppm are assigned to
the Q2, Q3, and Q4bands of Si(OH)2(OSi)2, Si(OH)(OSi)3,
and Si(OSi)4silicate species, respectively.23The Q4/(Q2+
Q3) area ratios obtained by deconvolution of the spectra are
also shown in Figure 4. The Q4/(Q2+ Q3) value of as-made
SBA-15 in the present study is 1.36, similar to that of 1.28
reported in the literature.6On the other hand, Schmidt-Winkel
et al.7fhave shown that the Q
4/Q3ratio of MCF prepared in
2 M HCl was around 2.22. The Q4/Q3ratio increased to 2.50
when NH4F was also applied in the synthesis. Comparatively,
the Q4/(Q2+ Q3) values of 2.57-2.98 obtained on the present
mesocellular silica materials are much higher. It reveals that the silanol condensation is more complete under the
acid-free synthesis condition. The Q4/(Q2 + Q3) values of
mesoporous silica materials synthesized by basic and acidic
routes are very distinct. The Q4/(Q2 + Q3) ratios were in
2.2-2.5 for MCM-41 synthesized at a pH around 10, and the ratios are much higher than those of conventional
SBA-15 synthesized in a strong acidic environment.6,23However,
the main factor which enhances the silanol condensation in this study is probably the presence of salt instead of the pH values of the synthesis solutions, which were within 4.4-5.
Wang and co-workers23ahave shown that the Q
4/(Q2+ Q3) (23) (a) Wang, X.; Lin, K. S. K.; Chan, J. C. C.; Cheng, S. J. Phys. Chem. B 2005, 109, 1763. (b) Wouters, B. H.; Chen, T.; Dewilde, M.; Grobet, P. J. Microporous Mesoporous Mater. 2001, 44-45, 453. (c) Luhmer, M.; d’Espinose, J. B.; Hommel, H.; Legrand, A. P. Magn. Reson. Imaging 1996, 14, 911.
Figure 3. TEM photographs of mesocellular silica foams synthesized with a NaCl/Si ratio of 1 and various P123/Si ratios of (a) 0.0051, (b) 0.0068, and
(c) 0.0103, respectively.
Figure 4. Solid state 29Si MAS NMR of as-made siliceous SBA-15
mesoporous material synthesized in the 2 M HCl system and mesocellular silica foams synthesized in an acid-free 0.0034-0.0103:1:1:221 P123/NaCl/ Si/H2O system.
Acid-Free Synthesis of Mesoporous Silica Chem. Mater., Vol. 19, No. 12, 2007 3045
Downloaded by NATIONAL TAIWAN UNIV on August 5, 2009
ratio of propylamine-functionalized SBA-15 prepared in 2 M HCl with the aid of NaCl was around 2.2 and much higher than that of conventional SBA-15.
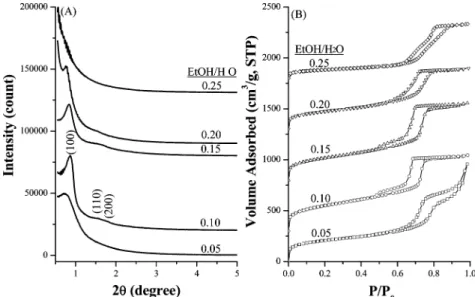
By adding a proper amount of alcohols, such as methanol, ethanol, and 1-propanol, as cosolvent into the acid-free synthesis gel of TEOS, P123, and NaCl, mesoporous silica materials possessing ordered pore arrangement were ob-tained. The pH values of the synthesis gels did not vary significantly as alcohols were present. The small-angle XRD patterns of mesoporous silica materials synthesized with reactant compositions of 0.008:1:1:221:10.8-64.7 P123/
NaCl/TEOS/H2O/EtOH are shown in Figure 5a. A strong
diffraction peak at 2θ around 0.86°and two weak peaks at
1.56 and 1.75° were observed when the EtOH/H2O molar
ratios were in the range of 0.10-0.20. The diffraction peaks were more intense and well-resolved than those of meso-cellular silica foams prepared without EtOH as cosolvent. The locations of diffraction peaks are consistent with 2D-hexagonal p6mm structure, similar to that of SBA-15 material. Comparatively, the d-spacings of these mesoporous silica materials are slightly smaller than those of MCF. It implies that the aggregation number of P123 in micelles probably decreased when ethanol was added in the synthesis
solution. When the EtOH/H2O molar ratios were out of
0.10-0.20, only one broad diffraction peak or no peak was observed in the XRD patterns. The results indicate that mesoporous silica materials with fairly ordered p6mm pore arrangement can be synthesized with the reactant
composi-tions of 0.008:1:1:221:21.6-43.1 P123/NaCl/TEOS/H2O/
EtOH. Moreover, similar results were observed when NaCl
was replaced by other salts or ethanol was replaced by methanol or propanol.
The nitrogen sorption isotherms of the mesoporous silica materials synthesized with EtOH as cosolvent are shown in
Figure 5b. With EtOH/H2O molar ratio in 0.05-0.25, the
silica materials show type IV isotherms with H1hysteresis
loops, very similar to that of SBA-15 prepared in 2 M HCl.6
The corresponding textural properties are summarized in Table 2. All the materials prepared with EtOH as cosolvent,
except the one with EtOH/H2O ratio as high as 0.25, have
relatively high surface areas (626-896 m2/g), mesopores of
6.3-7.0 nm in diameter, and large pore volumes (0.9-1.2
cm3/g). It is also noticeable that the shape of the hysteresis
loops varied slightly with the amount of ethanol added in the synthesis gel. The steepest hysteresis loops were observed
at P/P0between 0.6 and 0.75 for the material synthesized
with a EtOH/H2O molar ratio within 0.10-0.15, implying
that the PSDs for these samples are relatively narrow. The PSD values determined from the peak widths at half-maximum of the BJH PSD profiles were 2.5-3.0 nm (Table 2), which are similar to that of SBA-15. However, a small quantity of smaller mesopores of approximately 3.7 nm in diameter was also observed on these materials. These pores are likely attributed to the defects formed in the structure. The TG and DTG analyses of ordered mesoporous silica materials synthesized under acid-free condition with a P123/
Si molar ratio kept at 0.0086 and EtOH/H2O ratios within
0.05-0.25 are compared with those of SBA-15 synthesized with 2 M HCl, and the profiles are shown in Figure 6. The decomposition of pore directing agent P123 was found to Figure 5. (A) Small-angle XRD patterns and (B) N2adsorption-desorption isotherms of ordered mesoporous silica materials synthesized with various
EtOH/H2O ratios of 0.05-0.20.
Table 2. Textual Properties of Ordered Mesoporous Silica Materials Synthesized with EtOH/H2O Ratios in 0.05-0.25 EtOH/H2O (molar ratio) pH (gel) d(100) (nm) a0 (nm) SBET (m2/g) Smicro (m2/g) Vtotal (cm3/g) Vmirco (cm3/g) Dpa (nm) Wtb (nm) PSDc (nm) 0.05 4.54 12.0 13.8 728 178 1.2 0.08 7.0 6.8 5.0 0.10 4.59 10.5 12.1 896 81 1.1 0.03 6.3 5.8 2.5 0.15 4.73 10.4 12.0 776 74 1.2 0.03 6.5 5.5 3.0 0.20 4.82 11.7 13.5 626 85 0.9 0.03 6.6 6.9 4.8 0.25 5.13 n.d. n.d. 299 46 0.8 0.02 6.0 n.d 5.0
aThe peak positions of the distribution curves by BJH.bWall thickness.cThe peak width at half-maximum height of the BJH peak.
Downloaded by NATIONAL TAIWAN UNIV on August 5, 2009
occur around 190°C. It implies that the interactions between the pore directing agent P123 and the silica framework in these mesoporous silica materials are similar. Because the pH values of the synthesis solutions in the present study were within 4.4-5.1, which were independent of the presence of ethanol or not, silicate should remain negatively charged. The isoelectric point of a 5.8 wt % P123 aqueous solution was determined to be around 6.1 (Supporting Information). Hence, the P123 micelles should be partially protonated in the synthesis solutions with pH within 4.4-5. Therefore, the self-assembly of P123 and silica is proposed to proceed through a weakly electrostatic force or hydrogen bonding. The weight losses due to the decomposition of P123 in the ordered mesoporous silica materials were within 24-42 wt %, less than that of SBA-15. Moreover, the weight loss decreased as the amount of ethanol added in the synthesis solution increased. Because better ordering of the pore structures was observed in the presence of alcohol, alcohol should help the self-assembly of P123 micelles and silicate species. It has been proposed that alcohols probably change the volume ratio of poly(ethylene oxide) (PEO)/poly-(propylene oxide) (PPO) and P123 micelles of uniform sizes are formed in the solutions containing alcohols as
cosol-vent.17,24-27However, no weight losses corresponding to the
desorption or decomposition of ethanol were detected in the TG profiles, possibly due to the easy vaporization of alcohols during the drying process.
The TEM photographs of mesoporous silica materials
synthesized in acid-free conditions with the EtOH/H2O molar
ratios in the range of 0.05-0.25 are shown in Figure 7. For
the material prepared with EtOH/H2O ratio smaller than 0.05,
most of the structures are MCFs. When the EtOH/H2O ratio
is raised to 0.1-0.2, the ordered hexagonal pore arrangement
and well-aligned nanochannels can be clearly seen in the cross section of the particles. The diameter of the nanochan-nels is about 5-6 nm, slightly smaller than that estimated by BJH pore size analysis. Although a small quantity of worm-like and vesicular pores are also seen, to the best of our knowledge, this is the first report on the synthesis of pure silica material with ordered hexagonal mesopores using triblock copolymer template under acid-free conditions. The vesicular structures have also been observed in the gelled
state of M41S material.28 The formation of vesicular
structures was suggested to be due to the strong absorption of ions, such as silicate oligomers and counteranions, or swelling agent on the surfactant micelles. On the basis of the TEM results, the aggregation number of P123 in micelles in the brine is probably decreased when ethanol is present as cosolvent, as a result of the variation in the polarity of the solvent.
The solid state 29Si MAS NMR spectra of as-made
mesoporous silica materials synthesized with ethanol as cosolvent under acid-free conditions are shown in Figure 8. Similar to the MCF silica prepared without ethanol, the degree of silicate condensation in the mesoporous silica materials synthesized with ethanol is more complete than
that of SBA-15. However, the Q4/(Q2+ Q3) values in the
range of 1.80-2.39 are lower than those of MCF silica
prepared without ethanol. Moreover, the Q4/(Q2+ Q3) value
decreases with the increase in ethanol concentration in the synthesis solution, implying that ethanol may disturb the silanol condensation.
The structure and ordering of triblock copolymer micelles in aqueous solution have been found to change with the
electrolytes, cosolvents, and swelling agents.7,13-20,23-27
Recently, Kim and co-workers25found that the introduction
of butanol to the synthesis gel of silica with triblock copolymer as pore-directing agent could tune the mesos-tructure efficiently. Butanol has been proposed to locate (24) Lin, H. P.; Mou, C. Y. Acc. Chem. Res. 2002, 35, 927.
(25) Kim, T.-W.; Kleitz, F.; Paul, B.; Ryoo, R. J. Am. Chem. Soc. 2005, 127, 7601.
(26) Wang, Y.; Wang, Y.; Yang, C.-M.; Lu, G.; Schuth, F. Langmuir 2006, 22, 5491.
(27) Tang, J. W.; Yu, C. Z.; Zhou, X. F.; Zhao, D. Y. Chem. Commun. 2004, 2240.
(28) Pevzner, S.; Regev, O.; Lind, A.; Linden, M. J. Am. Chem. Soc. 2003, 125, 652.
Figure 6. (A) TG analysis and (B) DTG profiles of ordered mesoporous silica materials synthesized with various EtOH/H2O ratios of 0.05-0.20.
Acid-Free Synthesis of Mesoporous Silica Chem. Mater., Vol. 19, No. 12, 2007 3047
Downloaded by NATIONAL TAIWAN UNIV on August 5, 2009
mainly at the hydrophilic-hydrophobic interface of PEO and PPO blocks, stabilize the micellar aggregates, and determine the surface curvature of the micelles. In the present study, alcohols probably have similar effect. On the other hand, the appearance of white precipitate in the present acid-free conditions was found to slow down when ethanol was present as the cosolvent. It implies that alcohols can change the polarity of synthesis solutions and reduce the hydrolysis and condensation rates of silicate species.
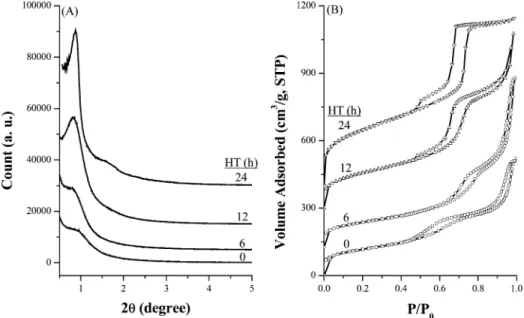
To investigate the formation mechanism of mesoporous silica material with ordered hexagonal pores, the gels before and after hydrothermal treatment (HT) for different periods
were collected. The XRD patterns and N2sorption isotherms
of calcined mesoporous silica are shown in Figure 9. A broad
XRD peak at 2θ∼ 1°is seen on the material before HT. As
the HT period lengthened, the peak becomes sharper and shifted slightly toward the low-angle region, and another
diffraction peak grows gradually at 1.3-2.0°. These results
indicate that the ordering of mesoporous structure is
im-proved under HT. The corresponding N2sorption isotherms
show consistent variation. The type IV isotherm with a weak
hysteresis loop covering a wide P/P0region was found on
the material before HT, and it transformed gradually toward
a sharp hysteresis loop covering a narrower P/P0region as
HT prolonged. These results indicate that the arrangement of mesopores becomes more ordered accompanied by the pore size narrowing when the HT period is lengthened. On
the other hand, the hysteresis loop extended to the high P/P0
region (around 0.85-0.90) for the materials before HT is probably attributed to the porous texture from the aggregated particles in sub-micrometer sizes. This high-pressure loop becomes less pronounced as the HT is prolonged and disappears after the material is hydrothermally treated for 24 h. A similar phenomenon has also been reported by Han
and Ying29who studied mixed surfactant system containing
fluorocarbon cationic surfactant and amphiphilic triblock
copolymer. The adsorption step in the high P/P0region has
also been attributed to the inter-particle porosity.
The SEM and TEM photographs of the mesoporous silica materials synthesized with ethanol as cosolvent after various HT periods are displayed in Figure 10. Small particles with a size less than 100 nm are observed for the material before HT. Its TEM photograph shows aggregates of nanosized particles and mainly inter-particle pores as well as worm-like or tubular-worm-like pore structure in the silica particles. After HT for 12 h, the material forms large particles with diameter
greater than 1µm, which are however the aggregates of small
particles and therefore look loose. In its TEM photograph, worm-like pore structures with pore diameter around 4 nm arranged in slightly ordered patterns can be found in a relatively large domain. After HT for 24 h, solid particles with smooth surface were obtained. The TEM photograph
(29) Han, Y.; Ying, J. Y. Angew. Chem., Int. Ed. 2005, 44, 288. Figure 7. TEM photographs of ordered mesoporous silica materials
synthesized with EtOH/H2O ratios of (a) 0.05 and (b, c) 0.10.
Figure 8. Solid state 29Si MAS NMR of as-made siliceous SBA-15
mesoporous material synthesized in a 2 M HCl system and ordered mesoporous silica materials synthesized with various EtOH/H2O ratios of
0.05-0.20.
Downloaded by NATIONAL TAIWAN UNIV on August 5, 2009
reveals that the material contains mesopores of hexagonal-ordered arrangement and the pore size has been expanded to 5-6 nm. These results are well consistent with those of
N2sorption isotherms. The P123 micelles in the neutral salt
solution containing approximately 10 mol % ethanol are probably in short cylindrical or elliptic shape of ap-proximately 4 nm in diameter. The silicate condensated around the micelles forms worm-like pores before HT. The HT seems to expand the micelles and enhance the silicate condensation and results in silica materials containing
hexagonal-arranged mesopores of 5-6 nm in diameter, similar to those of SBA-15 and MSU-H materials.
In the literature, it has been reported that ethanol has good
solubility toward both PEO and PPO groups.30,31Addition
of ethanol in aqueous solution has great influence on the self-assembly of block copolymer, such as the increase in CMT and the decrease in the aggregation number of block
copolymer in the micelle. Ganguly and co-workers30have
shown that the size of prolate ellipsoid P123 micelles in the aqueous solution containing 10 wt % of P123 was reduced by adding ethanol. The core radius of P123 micelles in the presence of 10 wt % ethanol has also been estimated to be approximately 5 nm, which is close to the pore diameter of the ordered mesoporous silica material synthesized in the present study.
The hydrothermal stabilities of the ultra-large MCF and hexagonal-arranged mesoporous silica materials prepared in
acid-free conditions were tested by boiling in 100°C water
for 72 h. No detectable changes in the pore structures were seen on the TEM photographs (Supporting Information). The XRD patterns of MCF show that only the samples synthe-sized with a P123/Si ratio within 0.0034-0.0051 still display
a weak diffraction peak at 2θ∼ 0.75°after boiling in water
(30) Ganguly, R.; Aswal, V. K.; Hassan, P. A.; Gopalakrishnan, I. K.; Yakhmi, J. V. J. Phys. Chem. B 2005, 109, 5653.
(31) Soni, S. S.; Brotons, G.; Bellour, M.; Narayanan, T.; Gibaud, A. J. Phys. Chem. B 2006, 110, 15157.
Figure 9. (A) Small-angle XRD patterns and (B) N2adsorption-desorption isotherms of ordered mesoporous silica materials synthesized with a EtOH/H2O
ratio of 0.1 and varied hours of HT.
Figure 10. SEM and TEM photographs of mesoporous silica materials
prepared with HT of (a, b) 0 h; (c, d) 12 h; and (e, f) 24 h.
Table 3. Hydrothermal Stability of MCF Materials after Boiling in Water for 72 h P123/Si (molar ratio) SBET (m2/g) Smicro (m2/g) Vtotal (cm3/g) Vmicro (cm3/g) Dca (nm) Dwa (nm) 0.0103 266 45 2.08 0.019 68 42 0.0086 462 88 1.56 0.038 93 76 0.0068 407 96 1.77 0.043 78 11 0.0051 415 65 1.08 0.027 125 25 0.0034 422 63 0.79 0.027 90 19
aThe diameters of cell (D
c) and window (Dw) determined from the
adsorption and desorption branches, respectively, by using a modified BdB-FHH method with a spherical model.
Acid-Free Synthesis of Mesoporous Silica Chem. Mater., Vol. 19, No. 12, 2007 3049
Downloaded by NATIONAL TAIWAN UNIV on August 5, 2009
for 72 h (Figure 11a). The corresponding N2 sorption isotherms show that the desorption branches of the hysteresis
loops shift toward higher P/P0 region and the adsorbed
volumes decrease (Figure 11b). This phenomenon is eluci-dated by that the nanopores in the silica framework of MCF disappear and the window of the foams is enlarged, as a result of further condensation of the silanol groups in the boiling water. Table 3 shows that the surface areas and pore
volumes of the mesocellular silica foams after boiling in water are all reduced.
The small-angle XRD patterns and N2sorption isotherms
of the mesoporous materials synthesized with ethanol as cosolvent after boiling in water for 72 h are shown in Figure 12a,b, respectively. The XRD peaks remain well-resolved and intense, indicating that the ordered mesostructures were
retained. Consistently, the N2sorption isotherms still preserve
Figure 11. (A) Small-angle XRD patterns and (B) N2sorption isotherms of mesocellular silica foams after boiling in water for 72 h.
Figure 12. (A) Small-angle XRD patterns and (B) N2sorption isotherms of mesoporous silica materials synthesized with the EtOH/H2O ratios of 0.05-0.20
and treated by boiling water for 72 h.
Table 4. Hydrothermal Stability of Ordered Mesoporous Silica Materials Synthesized with Ethanol Cosolvent after Boiling in Water for 72 h
EtOH/H2O (molar ratio) d(100)(nm) SBET (m2/g) Smicro (m2/g) Vtotal (cm3/g) Vmirco (cm3/g) Dpa (nm) Wtb (nm) PSDc (nm) 0.05 11.0 372 57 0.98 0.024 9.0 2.0 2.0 0.10 9.8 467 68 0.98 0.028 6.9 2.9 1.0 0.15 10.2 478 68 1.1 0.028 7.4 2.8 1.1 0.20 11.6 464 71 0.89 0.030 7.5 4.1 1.7 0.25 n.d. 265 41 0.83 0.017 12.8 n.d. 9.6
aThe peak positions of the distribution curves by BJH.bWall thickness.cThe peak width at half-maximum height of the BJH peak.
Downloaded by NATIONAL TAIWAN UNIV on August 5, 2009
the type IV isotherms with H1hystersis loops. However, the
loops shift slightly toward higher P/P0region. The BJH pore
size analysis shows that the pore sizes are enlarged from 6.0-7.0 nm to 6.9-13 nm (Table 4). It is also noteworthy that the defect pores of approximately 3.7 nm in diameter observed in the original materials disappear. These results suggest that the silica framework around the defects probably re-constructs in boiling water. Accordingly, although the BET
surface areas decrease from 600-900 m2/g to 460-480 m2/
g, the pore sizes increase and the PSD narrows down from 2.5-3.0 nm to 1.0-1.7 nm for the materials synthesized with
EtOH/H2O molar ratio within 0.10-0.20.
Conclusions
In the absence of hazardous acids or swelling agent, mesoporous silica materials with tunable pore structures including ultra-large mesocellular foam and ordered hex-agonal pore arrangement were successfully synthesized using amphiphilic triblock copolymer as template with the aids of salt and alcohol. The pore structure of resultant materials could be finely controlled by varying the concentrations of triblock copolymer, salt, and cosolvent in the synthesis solution. The mesocellular silica foams with ultra-large cell size, closed window, and nanopores on the walls were obtained in a low P123/Si ratio of 0.0034-0.0051 and NaCl/ Si ratio around 1. The porous network changed from a closed window foam to an open one when the P123/Si ratio was over 0.0103 or the NaCl/Si ratio was raised to 2. It is particularly noteworthy that mesoporous silica material with
hexagonal-arranged pore structure, similar to that of SBA-15 and MSU-H materials, could be made by adding 10-20 mol % of alcohol in the synthesis solution. This is the first report on synthesis of large mesoporous silica material with ordered hexagonal-arranged pores in neutral conditions. The ordered mesoporous silica synthesized with alcohol as cosolvent was hydrothermally stable up to 72 h. Moreover, as a result of the re-construction of the silica framework during HT, the materials had less defects and narrower PSD.
Acknowledgment. The financial support from the National Science Council and the Ministry of Education, Taiwan, is gratefully acknowledged. Acknowledgment is also extended to C.-Y. Tang and C.-Y. Lin of National Taiwan University for TEM and SEM experiments, C.-N. Ke of National Tsing-Hua University, Taiwan, for the ICP-AES experiment, and S.-Y. Sun
of National Cheng Kung University, Taiwan, for the29Si MAS
solid-state NMR experiment. S.-Y.C. would like to thank Prof. G. D. Stucky of University of California at Santa Barbara, U.S.A., and Dr. W. Lukens of Lawrence Berkeley National Laboratory, U.S.A., for the helpful discussion and for providing the modified BdB-FHH program to analyze the pore structure and PSD of mesocellular silica foams.
Supporting Information Available: TG and DTG profiles of MCF materials synthesized in acid-free conditions,ζ potential vs pH profile for a P123 aqueous solution, and TEM photographs (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
CM070232Y
Acid-Free Synthesis of Mesoporous Silica Chem. Mater., Vol. 19, No. 12, 2007 3051
Downloaded by NATIONAL TAIWAN UNIV on August 5, 2009