Introduction
Schizophrenia is a common severe neuropsy-chiatric disorder that affects 1% of the general population. Although the pathogenesis of schizo-phrenia remains unclear, immune alterations asso-ciated with this disease have been studied for de-cades [1]. Tumor necrosis factor alpha (TNF-α) is
one of the major cytokines that mediates primary host response during infl ammation. It has been proposed that TNF-α is related to the pathogenesis of schizophrenia through the processes of neuro-development and neurodegeneration [2]. Signifi cant increases in the plasma concentration of TNF-α have been reported in patients with schizophrenia [3].
The TNF-α gene is located in the region
Objectives: Tumor necrosis factor alpha (TNF-α) has been related to the
pathogenesis of schizophrenia through its involvement in the processes of neuro-development and neurodegeneration. We examined the association between the TNF-α -308G/A polymorphism and schizophrenia in Taiwanese samples.
Meth-ods: The association analysis was performed using both a case-control and a
fami-ly-based association design. The case-control sample comprised 124 patients and 119 controls; the family-based sample included 80 parent-offspring trio families.
Results: No signifi cant difference was found in genotype or allele frequencies
be-tween patients with schizophrenia and controls. No signifi cant transmission distor-tion of the polymorphism was found in the family sample. Conclusion: Our results suggest that -308 G/A polymorphism in the TNF-α gene does not confer increased susceptibility to schizophrenia in this population of Han Chinese ethnicity from Taiwan.
A Negative Study of the Association between
Tumor Necrosis Factor Alpha-308G/A
Polymorphism and Schizophrenia among
Taiwanese Patients
Lung-Cheng Huang, M.D.
1,2, Chih-Min Liu, M.D.
3, Hai-Gwo Hwu, M.D.
31
Department of Psychiatry, National Taiwan University Hospital Yun-Lin Branch 2
Institute of Medicine, Kaohsiung Medical Uni-versity 3
Department of Psychiatry, National Taiwan University Hospital and National Taiwan University College of Medicine Received: August 3, 2007; accepted: September 9, 2007
Address correspondence to: Dr Chih-Min Liu, Department of Psychiatry, National Taiwan University Hospital, No. 7, Chung-Shan South Road, Taipei 100, Taiwan
Key words: association, polymorphism, schizophrenia, tumor necrosis factor alpha (Taiwanese J Psychiatry 2008;22:141-7)
6p21.1-21.3, near the HLA region, a locus associ-ated with genetic susceptibility to schizophrenia [4]. For the TNF-α gene promoter region, there is a functional single-nucleotide polymorphism (SNP) located at nucleotide position -308, which involves a common variant with guanine (G) (TNF1) and a less common one with adenine (A) (TNF2). Higher transcriptional activity has been revealed for the -308A allele in comparison to the -308G variant [5]. Recent studies found an associ-ation between this SNP and schizophrenia, with a signifi cantly increased -308A allele frequency in patients with schizophrenia compared to controls [6,7]. In contrast, other studies have demonstrated that the frequency of the 308A allele was signifi -cantly lower in schizophrenia [8-10], while no positive results were observed in other samples [11-15]. Given these inconsistent fi ndings, we sought to clarify the possible role of TNF-α in the etiopathogenesis of schizophrenia by examining the association between the TNF-α -308G/A poly-morphism and schizophrenia in Taiwanese sam-ples using both a case-control and a family-based association design.
Materials and Methods
Approval was obtained from the Ethics Committee for Human Research of National Taiwan University Medical Center. After listening to a complete description of the project, patients, their relatives, and controls provided informed consent prior to their inclusion in the study. A sample of 10 ml whole blood was collected for DNA extraction from all recruited subjects.
The Case-control sample
A total of 124 patients with schizophrenia (68 males, mean age ± SD: 30.0 ± 6.8 years, mean age of onset ± SD: 21.9 ± 5.5 years) and 119
nor-mal controls (50 nor-males, mean age ± SD: 32.8 ± 7.2 years) participated in this study. All of the subjects were genetically unrelated Han Chinese. The pa-tients with schizophrenia were all participants in a prospective study of schizophrenia in Northern Taiwan called the multidimensional psychopatho-logical group research projects (MPGRP). Briefl y, from August 1, 1993 to June 30, 1997, all patients consecutively admitted to the acute wards of three hospitals, National Taiwan University Hospital, Taipei City Psychiatric Center, and Taoyuan Psychiatric Center were recruited if they met the DSM-IV criteria for schizophrenia. Patients with other axis I diagnoses (substance abuse, organic mental disorders, affective disorders), neurologi-cal illness (epilepsy), or systemic illness were ex-cluded. A clinical interview using the Positive and Negative Syndrome Scale (PANSS) [16] was con-ducted within one week after patients were admit-ted due to acute exacerbation. Among the 234 pa-tients recruited at the index admission, only 124 signed informed consent for blood collection and were entered into this study. No signifi cant differ-ences were found between patients entering this study and those who were excluded in terms of gender distribution, age, age at onset and the scores of total symptoms, positive subscale, nega-tive subscale and general psychopathology sub-scale of the PANSS (data not shown).
The normal control subjects were mostly re-cruited from hospital staff (n=104) and patients visiting the physical examination ward (n=15) of National Taiwan University Hospital without matching for cases. After informed consent was obtained, control subjects underwent a screening interview schedule to rule out major psychiatric illness and substance abuse. Because most of the controls were within the characteristic age range of schizophrenia onset, we could not exclude po-tential cases completely. However, considering
the disease prevalence of schizophrenia was 0.3% in our population [17], the number of potential cases was likely too small to infl uence the associa-tion results.
The family sample
Schizophrenic probands were recruited from the same hospitals using the same inclusion and exclusion criteria as the case-control sample. The parents and siblings of each proband were also re-cruited. All of the patients and their fi rst-degree relatives were interviewed with the Chinese ver-sion of the Diagnostic Interview for Genetic Studies (DIGS-C) [18]. This family sample com-prised 80 parent-offspring trio families, including 80 schizophrenic probands (41 males) with a mean age of 30 years old, 149 of their parents (72 males) with a mean age of 58 years old, and 42 of their healthy siblings (21 males) with a mean age of 31 years old. Most of the families (n=69) were com-plete trios while 11 families had only one parent.
Genotyping
Genomic DNA was extracted from whole blood using standard procedures. The SNP at po-sition -308 in the TNF-α promoter region was screened by polymerase chain reaction (PCR) using the methods described by Wilson et al [19]. Oligonucleotides primers were (forward) 5’-AGGCAATAGGTTTTGAGGGCCAT-3’ and (reverse) 5’-TCCTCCCTGCTCCGATTCCG-3’. Conditions for PCR were as previously de-scribed with minor modifi cations [6]. The digested samples of amplifi ed DNA with NcoI restriction enzyme (New England Biolabs, MA, USA) were electrophoresed on 4% MetaPhor agarose gel and stained with ethidium bromide. The product size after digestion was: TNF1 allele (-308G) = 87bp/ 20bp, TNF2 allele (-308A) =107 bp.
The statistical analysis
The presence of Hardy-Weinberg equilibri-um was examined by the chi-square (χ2
) test for goodness of fi t. Allele and genotype frequencies in each group were compared using the χ2
test and Fisher’s exact test. Between-genotype differences in continuous variables were evaluated using the Student’s t-test or analysis of variance (ANOVA). The critical p-value was set at 0.05 (two-tailed). All data for the case-control sample were analyzed using SPSS version 10.0 (SPSS, IL, USA). Family-based association analysis was performed using the TDT/S-TDT program [20].
Results
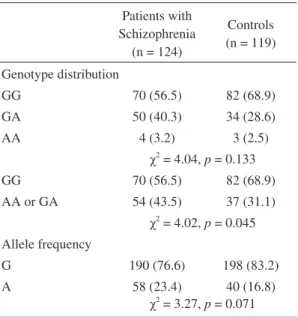
Case-control sampleThe genotype distributions and allele fre-quencies of the SNP for the patients and controls are compared in Table 1. The genotype distribu-tion of the SNP for patients and controls was in Hardy-Weinberg equilibrium. Although the fre-quency of the minor allele (-308A) was somewhat higher in the schizophrenics compared to the con-trols, the genotype and allele distributions in these patients were not signifi cantly different from those in the controls. However, under the dominant he-reditary model, there were signifi cantly more A/A or G/A genotypes in the patients than in the con-trols (p = 0.045).
Among the genotypes, there were no differ-ences in clinical variables, such as onset age (F = 0.50, p = 0.611; data not shown), PANSS-positive subscale, negative subscale, general psychopa-thology subscale, and total scores (F = 0.21, p = 0.809; F = 0.84, p = 0.433; F = 0.40, p = 0.673; F = 0.68, p = 0.508, respectively; data not shown). No evidence of association was found between this SNP and the severity of clinical symptoms in the acute exacerbation stage.
The family sample
The genotype distribution of the SNP in the family sample was in Hardy-Weinberg equilibri-um. Table 2 shows the results of the family-based association analysis using TDT/S-TDT. No signif-icant transmission distortion of the SNP was found in the family sample.
Discussion
This study failed to detect a positive associa-tion between -308 G/A polymorphism in the pro-moter region of the TNF-α gene and schizophre-nia in a Taiwanese sample. Only a borderline signifi cant difference in genotype distribution was found when the dominant model was applied. We could not replicate the previous fi ndings of asso-ciation between -308 G/A and patients with schizophrenia in samples of Italians [6], Brazilians [7], Polish [8], Germans [10], and Chinese Singaporeans [9]. However, this result is similar to fi ndings from studies of other Asian samples [11-15]. In addition, the odds ratio of -308A ver-sus -308G obtained in our study is close to that re-ported by Tsai et al. [13] in a previous study of an-other Taiwanese sample (1.51; 1.53, respectively). These fi ndings suggest that an ethnic difference may account for the variation in etiological signif-icance of this SNP in patients with schizophrenia from different populations.
It is possible that this study generated a false negative result due to the small genetic effect of the -308G/A polymorphism of the TNF-α gene in the Taiwanese population, or because of the rela-tively small sample size and inadequate statistical power. The statistical power of this study was 0.20 with an alpha of 0.05 and the minor allele frequen-cy difference between cases and controls was 0.066. With the assumption of adequate power of 0.8, the estimated required sample size for cases and controls would both be 606. For our family sample, with the assumptions of disease frequency of 0.003 [17], disease allele frequency of 0.234, alpha of 0.05, the power would reach 0.8 when the allelic odds ratio reaches 2.15. This implies that the study had inadequate power for detecting of gene effect below 2.15.
Table 1. Genotype distribution and allele frequencies of the TNF-α gene polymorphism at position -308 among patients with schizophre-nia and controls
Patients with Schizophrenia (n = 124) Controls (n = 119) Genotype distribution GG 70 (56.5) 82 (68.9) GA 50 (40.3) 34 (28.6) AA 4 (3.2) 3 (2.5) χ2 = 4.04, p = 0.133 GG 70 (56.5) 82 (68.9) AA or GA 54 (43.5) 37 (31.1) χ2 = 4.02, p = 0.045 Allele frequency G 190 (76.6) 198 (83.2) A 58 (23.4) 40 (16.8) χ2 = 3.27, p = 0.071 Odds ratio (-308A vs. -308G) = 1.511 (95%CI = 0.964-2.368)
Table 2. Family-based association analysis using TDT/S-TDT program 1.1 Allele N Z P-value A 38 1.161 0.2456-G 1.161 0.2456+
-: under-transmitted to affected individuals +: over-transmitted to affected individuals N: Number of informative families
Clinical implication
1. Our study does not support the notion that -308 G/A polymorphism in the TNF-α gene plays a major role in the susceptibility to schizophrenia among the Han Chinese popu-lation of Taiwan.
2. Further study with a larger sample size is needed in order to confi rm these fi ndings.
Acknowledgements
This work was supported by Grant NHRI-EX91-9113PT from the National Health Research Institute, Taiwan, ROC.
References
1. Muller N, Riedel M, Gruber R, et al.: The immune system and schizophrenia. An integrative view. Ann NY Acad Sci 2000;917:456-67.
2. Marx CE, Jarskog LF, Lauder JM, et al.: Cytokine effects on cortical neuron MAP-2 immunoreactiv-ity: Implications for schizophrenia. Biol Psychiatry 2001;50:743-9.
3. Kowalski J, Blada P, Kucia K, et al.: Neuroleptics normalize increased release of interleukin-1 beta and tumor necrosis factor-alpha from monocytes in schizophrenia. Schizophr Res 2001;50:169-75. 4. Wright P, Nimgaonkar VL, Donaldson PT, et al.:
Schizophrenia and HLA: a review. Schizophr Res 2001;47:1-12.
5. Wilson AG, Symons JA, McDowell TL, et al.: Effects of a polymorphism in the human tumor ne-crosis factor alpha promoter on transcriptional acti-vation. Proc Natl Acad Sci USA 1997;94:3195-9. 6. Boin F, Zanardini R, Pioli R, et al.: Association
between G -308A tumor necrosis factor alpha gene polymorphism and schizophrenia. Mol Psychiatry 2001;6:79-82.
7. Meira-Lima IV, Pereira AC, Mota GF, et al.: Analysis of a polymorphism in the promoter
re-gion of the tumor necrosis factor alpha gene in schizophrenia and bipolar disorder: further sup-port for an association with schizophrenia. Mol Psychiatry 2003;8:718-20.
8. Rybakowski JK, Czerski PM, Borkowska A, et al.: Tumor necrosis factor-alpha promoter gene poly-morphism: association with schizophrenia and eye movement disturbances. Biol Psychiatry 2002; 51:26S.
9. Tan EC, Chong SA, Tan CH, et al.: Tumor ne-crosis factor-alpha gene promoter polymor-phisms in chronic schizophrenia. Biol Psychiatry 2003;54:1205-11.
10. Schwab SG, Mondabon S, Knapp M, et al.: Association of tumor necrosis factor alpha gene -G308A polymorphism with schizophrenia. Schizophr Res 2003;65:19-25.
11. Handoko HY, Nancarrow DJ, Hayward NK, et al.: Tumor necrosis factor haplotype analysis amongst schizophrenia probands from four distinct popula-tions in the Asia-Pacifi c region. Am J Med Genet 2003;121:1-6.
12. Pae CU, Chae JH, Bahk WM, et al.: Tumor necro-sis factor-alpha gene polymorphism at position − 308 and schizophrenia in the Korean population. Psychiatry Clin Neurosci 2003;57:399-403. 13. Tsai SJ, Hong CJ, Yu YW, et al.: No association of
tumor necrosis factor alpha gene polymorphisms with schizophrenia or response to clozapine. Schizophr Res 2003;65:27-32.
14. Hashimoto R, Yoshida M, Ozaki N, et al.: Association analysis of the -308G>A promoter polymorphism of the tumor necrosis factor alpha (TNF-alpha) gene in Japanese patients with schizo-phrenia. J Neural Transm 2004;111:217-21. 15. Duan S, Xu Y, Chen W, et al.: No association
between the promoter variants of tumor necro-sis factor alpha (TNF-alpha) and schizophre-nia in Chinese Han population. Neurosci Lett 2004;366:139-43.
16. Kay SR, Fiszbein A, Opler LA: The positive and negative syndrome scale (PANSS) for schizophre-nia. Schizophr Bull 1987;13:261-76.
17. Hwu HG, Yeh EK, Chang LY: Prevalence of psy-chiatric disorders in Taiwan defi ned by the Chinese Diagnostic Interview Schedule. Acta Psychiatr Scand 1989;79:136-47.
18. Nurnberger JI Jr, Blehar MC, Kaufmann CA, et al.: Diagnostic interview for genetic studies: rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry 1994;51:849-59. 19. Wilson AG, di Giovine FS, Blakemore AI, et al.:
Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by Ncol restriction of PCR product. Hum Mol Genet 1992;1:353.
20. Spielman RS, Ewens WJ: A sibship test for link-age in the presence of association: the sib trans-mission/disequilibrium test. Am J Hum Genet 1998;62:450-8.
目的:研究腫瘤壞死因子 (TNF-a) 基因 之 -308G/A 單核酸多型性與精神分裂症之相 關性。ПݲȈ本研究採用病例 – 對照相關研 究法,及家系關聯性分析研究法來分析 TNF-a 基因 -308G/A 單核酸多型性與精神分裂症之 相關性。病例 – 對照相關研究組包括 124 名 病人及 119 名對照組;家系關聯性分析研究 組包括 80 名病人,149 名父母,及 42 名健 康手足。結果:病例 – 對照相關研究的結果 1台大醫院雲林分院精神科 2高雄醫學大學醫學研究所 3台大醫院精神部暨台大醫院精神科 受理日期:2007年8月3日;接受日期:2007年9月9日 通信作者地址:劉智民,100台北市中正區中山南路7號 台大醫院精神部 關鍵詞:關聯研究,單核酸多型性,精神分裂症,腫瘤壞死因子 ( 台灣精神醫學 2008;22:141-7 ) 顯示,在 TNF-a 基因之 -308G/A 單核酸多型 性上,精神分裂症與對照組之間其對偶基因 頻率或基因型頻率並沒有任何差異;在家系 關聯性分析研究中,亦未發現此一單核酸多 型性有傳遞不平衡之現象。๖፣Ȉ本研究顯 示 TNF-a 基 因 之 -308G/A 單 核 酸 多 型 性 在 台灣精神分裂症的病源學上並不扮演決定性 角色。