Molecular (Sub)Grouping of Endosymbiont Wolbachia Infection
Among Mosquitoes of Taiwan
KUN-HSIEN TSAI,1JIH-CHING LIEN,2CHIN-GI HUANG,1WEN-JER WU,1
ANDWEI-JUNE CHEN3
J. Med. Entomol. 41(4): 677Ð683 (2004)
ABSTRACT Wolbachia are maternally inherited bacteria that infect a wide range of arthropods as well as Þlarial worms. The infection usually results in reproductive distortions of the host, primarily cytoplasmic incompatibility, parthenogenesis, and feminization. This study showed that Wolbachia infection (15/29; 51.72%) was prevalent among Þeld-caught mosquitoes in Taiwan. Three mosquito species were identiÞed as having Wolbachia A infection, eight species as having Wolbachia B, and four other species were dually infected by both groups. Each Wolbachia isolate from different mosquitoes was further divided into a speciÞc subgroup. However, there were still some isolates that did not belong to any known subgroup, suggesting that more subgroups remain to be identiÞed. Investigation of tissue tropism in either Aedes albopictus (Skuse) or Armigeres subalbatus (Coquillett) revealed that
Wol-bachiawere extensively distributed within the host, although the ovary was most susceptible to infection. This report provides preliminary features of molecular relationships among Wolbachia groups of mosquitoes from Taiwan.
KEY WORDS endosymbiont, mosquito, tissue tropism, Wolbachia
THE ENDOSYMBIONTWolbachiaare intracellular rickett-sia-like bacteria that are maternally inherited and nor-mally harbored in a number of arthropods (Rigaud and Juchault 1993, 1995; Breeuwer and Jacobs 1996; OÕNeill et al. 1997; Werren 1997; Bourtzis and OÕNeill 1998). According to previous reports, 16.9% of Neo-tropical insects (Werren et al. 1995a), 35.7% of dipter-ans (Werren et al. 1995a, Bourtzis et al. 1996), 35%Ð 40% of mites (Breeuwer and Jacobs 1996), and up to 46.3% of terrestrial isopods (Bouchon et al. 1998) are infected with Wolbachia. Moreover, it was recently found that Wolbachia could infect various nematode Þlarial worms (Sironi et al. 1995). Undoubtedly,
Wol-bachiais one of the most ubiquitous endosymbionts described to date (Werren et al. 1995a). Wolbachia causes reproductive distortions, primarily cytoplasmic incompatibility (CI), feminization of genetic males, and induction of parthenogenesis in infected arthro-pods (Werren 1997). Of these, CI is the most common feature occurring in the mosquito. The reproductive distortions caused by Wolbachia infection are known to result in embryonic death and subsequent failure of egg hatch due to disruptions during early events of fertilization (Hoffmann and Turelli 1997).
It is believed that Wolbachia is a monophyletic as-semblage belonging to the alpha-subdivision of the Proteobacteria (OÕNeill et al. 1992, Werren et al.
1995b, Zhou et al. 1998). The type species, Wolbachia
pipientis,was Þrst identiÞed in the mosquito Culex
pipiens(L.) complex (Hertig and Wolbach 1924). It has been found that Wolbachia infects almost 100% of wild-caught mosquitoes of the Cx. pipiens complex (Sinkins et al. 1995). Wolbachia is normally main-tained in nature through vertical transmission (Wer-ren 1997). However, horizontal transmission of
Wol-bachiahas been mentioned; this apparently sheds light on mosquito control because Wolbachia is expected to drive a deleterious gene into and to reduce the density of natural populations of mosquitoes (Beard et al. 1993, Sinkins et al. 1997, Beard et al. 1998, Curtis and Sinkins 1998).
IdentiÞcation of Wolbachia has been convention-ally dependent on electron microscopy because of difÞculties with in vitro culture (Wright and Barr 1980, Hayes and Burgdorfer 1981, Louis and Nigro 1989). Polymerase chain reaction (PCR) ampliÞcation and sequencing are routine techniques used in the iden-tiÞcation of Wolbachia. At present, various genes, in-cluding wsp (Wolbachia outer surface protein) (Zhou et al. 1998), 16S rDNA (Breeuwer et al. 1992, OÕNeill et al. 1992, Rousset et al. 1992, Bensaadi-Merchermek et al. 1995, Clark et al. 2001), ftsZ (Holden et al. 1993, Clark et al. 2001), and dnaA protein sequence (Bourtzis et al. 1994) are extensively used for the purpose of Wolbachia detection and have shown tre-mendous advantages in its phylogenic analysis.
Wolbachiaoccurs commonly in natural mosquito populations (Sinkins et al. 1995, Kittayapong et al. 2000). As mentioned, Wolbachia may play a role in the control of disease vectors, especially the mosquito,
1Department of Entomology, National Taiwan University, Taipei 106, Taiwan.
2Institute of Preventive Medicine, National Defense Medical Cen-ter, San-Hsia, Taipei 237, Taiwan.
3Department of Public Health and Parasitology, College of Med-icine, Chang Gung University, Kwei-San, Tao-Yuan 333, Taiwan.
due to its potential role in reducing the Þtness of the host population (Beard et al. 1993, 1998). Thus, it is worthwhile to extensively investigate the occurrence and distribution of Wolbachia in mosquitoes in nature. Herein, we describe the status of Wolbachia infection among mosquitoes in Taiwan based on molecular data derived from PCR-based techniques, including DNA sequencing.
Materials and Methods
Mosquitoes. Larvae of 29 mosquito species belong-ing to nine genera were collected in the Þeld from various parts of Taiwan and then reared in the labo-ratory as described previously (Chen et al. 2000). Mosquito identiÞcation was carried out on the basis of morphological characteristics illustrated by Lien (1978). To cure mosquitoes of the Wolbachia infec-tion, newly eclosed larvae were reared in water con-taining diluted tetracycline as described by Yen and Barr (1973).
DNA Extraction and PCR. DNA was extracted from tissues dissected from at least Þve mosquitoes of each test, except for Mansonia uniformis (Theobald) and
Uranotaenia novobscuraBarraud, by using a DNA ex-traction kit (Viogene, Sunnyvale, CA). Twenty mi-croliters of reaction mix contained 1l of extracted DNA, 12.5l of double distilled H2O, 2l of 10⫻
buffer (Klen Tag), 2l of 25 mM MgCl2, 0.5 l of
dNTPs (10 mM each), 1l each of 2 pM forward and reverse primers, and 1 U of Tag DNA polymerase (Klen Tag). All reactions in this study were run in an ERICOMP thermocycler (PowerBlock System, San Diego, CA) under the following settings: one cycle (1 min at 94⬚C, 1 min at 55⬚C, and 3 min at 72⬚C), 35 cycles (15 s at 94⬚C, 1 min at 55⬚C, and 3 min at 72⬚C), and one cycle (15 s at 94⬚C, 1 min at 55⬚C, and 10 min at 72⬚C). The universal primers (12SAI-forward, 5⬘-AAACTAGGATTAGATACCCTATTAT-3⬘; 12SBI-re-verse, 5⬘-AAGAGCGACGGGCGATGTGT-3⬘) were used to amplify a cDNA fragment of insect mtDNA (of ⬇400 bp) (Simon et al. 1991). This primer pair served as a control to assess the quality of the template DNA extracted from mosquito tissues. In each test, tissues from Wolbachia-infected and tetracycline-treated
Armigeres subalbatus(Coquillett) were used as the positive and negative controls, respectively.
Primer Pairs Used to Amplify Wolbachia-Specific Genes. Three primer pairs were used to amplify
Wol-bachia-speciÞc genes in this study. The 99 F/994R primers (99 F, 5⬘-TTG TAG CCT GCT ATG GTA TAA CT-3⬘; 994R, 5⬘-GAA TAG GTA TGA TTT TCA TGT-3⬘) were designed from the hypervariable V1 and V6 regions of the 16S rRNA gene of Wolbachia pipiens; from which we anticipated to obtain a 900-bp cDNA fragment (OÕNeill et al. 1992). The fragment (⬇955Ð 957 bp) ampliÞed from the ftsZ gene was obtained by using the primer pair ftsZ F/R (Holden et al. 1993, Sinkins et al. 1995), which consisted of ftsZA (forward, CTCAAGCACTAGAAAAGTCG-3⬘; reverse: 5⬘-TTAGCTCCTTCGCTTACCTG-3⬘) for group A and
ftsZB (forward,
5⬘-CCGATGCTCAAGCGTTAGAG-3⬘; reverse, 5⬘-CCACTTAACTCTTTCGTTTG-3⬘) for group B. Another primer pair was wsp81 F/691R (wsp 81F, 5⬘-TGGTCCAATAAGTGATGAAGAAAC-3⬘; wsp691R, 5⬘-AAAAATTAAACGCTACTCCA-3⬘) from which 590Ð632 bp of cDNA was synthesized (Zhou et al. 1998).
Subgrouping of Wolbachia. The wsp gene fragment was used as the target for further division of each group of Wolbachia. In this experiment, all PCR prod-ucts obtained from the wsp 81F/691R primer pair were used as the template to amplify a shorter gene frag-ment that served as the gene marker speciÞc to sub-groups, including AlbA, Aus, Haw, Mel, Mors, Pap, Riv, and Uni for group A and CauB, Con, Dei, and Pip for group B. The PCR products ampliÞed from secondary PCR ranged from 379 to 556 bp in length (Zhou et al. 1998).
Visualization of PCR Products. PCR products of the
wspgene fragment derived from 81F/691R primers were separated on a 2% (wt:vol) agarose gel and then stained with ethidium bromide. Banding patterns on the gel were photographed with a Polaroid camera. A recheck was carried out with higher concentrations of the DNA template of negative cases. All results, except those for which the 12S insect-speciÞc gene was not successfully detected, were subjected to further anal-ysis. The band of the target cDNA fragment was sub-sequently excised from the gel and puriÞed using a gel extraction kit (Viogene). The puriÞed cDNA derived three individuals of each species were directly se-quenced using a Prism automated DNA sequencing kit from the Applied Biosystems (Foster City, CA).
Wolbachia Detection from Mosquito Tissues. Tis-sues, including muscles, guts, testes, salivary glands, and ovaries were dissected from Þve mosquito larvae or 2-d-old adults (unfed males and females) of either
Ae. albopictus or Ar. subalbatus. Individual tissues were Þrst rinsed with phosphate-buffered saline (pH 7.4) to avoid contamination between tissues and were then processed for DNA extraction as described above. The presence of Wolbachia in tissues was dem-onstrated by ampliÞcation of a speciÞc gene fragment by using the primer pair of wsp 81F/691R (Zhou et al. 1998). Three replicates have been applied in the de-tection of this study.
Results
Identification of Wolbachia Infection in Ae. albop-ictus. Wolbachia infection was deÞnitively demon-strated in Ae. albopictus by checking ovaries through a series of gene ampliÞcations with various primer pairs, including the universal Wolbachia-speciÞc 16S rDNA (900 bp), the group A-speciÞc 16S rDNA (259 bp), the group B-speciÞc 16S rDNA (259 bp), the group A-speciÞc ftsZ gene (955Ð957 bp), the group B-speciÞc ftsZ gene (955Ð957 bp), the universal
Wol-bachia-speciÞc wsp gene (⬇590Ð632 bp), the group A-speciÞc wsp gene (556 bp), and the group B-speciÞc
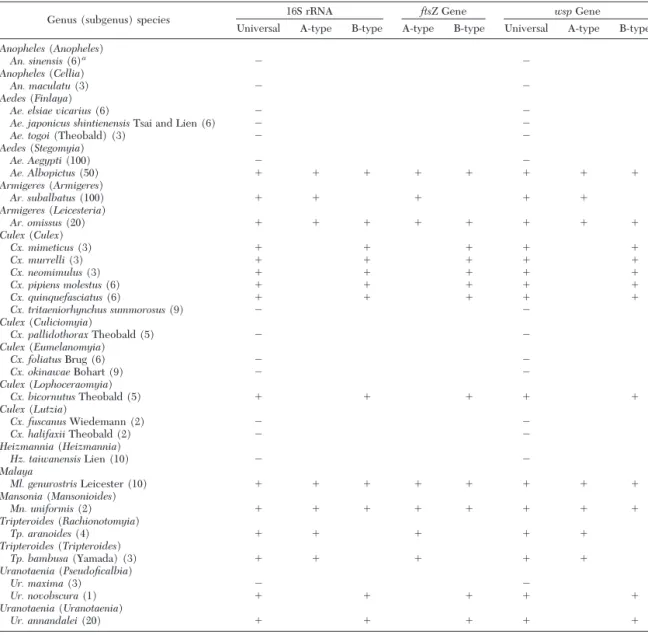
Wolbachia Infection among Field-Caught Mosqui-toes. Field-caught mosquitoes were identiÞed to nine genera. Except for Anopheles and Heizmannia,
Wol-bachiainfection was seen in seven other mosquito genera, including Aedes, Armigeres, Culex, Malaya,
Mansonia, Tripteroides,and Uranotaenia with infection rates of 20% (1/5), 100% (2/2), 50% (6/12), 100% (1/1), 100% (1/1), 100% (2/2), and 66.67% (2/3), respectively. Of the 29 mosquito species tested, 15 (51.72%) were infected with Wolbachia by showing speciÞc wsp fragments (Table 1); all wsp fragments (except those in Culex mimeticus Noe´ and Mn.
uni-formis) were sequenced and shown to be Wolbachia-speciÞc (accession nos. AY462852Ð5, and AY462857Ð 67). Of these species, Ar. subalbatus, Tripteroides
aranoides (Theobald), and Triperoides bambusa (Yamada) were infected with group A (3/15; 20%), whereas Cx. mimeticus, Culex murrelli Lien, Culex
neo-mimulusLien, Culex pipiens molestus Forskal, Culex
quinquefasciatus Say, Culex bicornutus Theobald,
Uranotaenia novobscuraBarraud, and Uranotaenia
an-nandaleiBarraud were infected with group B (8/15; 53.33%). Four species, Ae. albopictus, Armigeres
omissus(Edwards), Malaya genurostris Liecester, and
Mn. uniformiswere dually infected with both group A and B (4/15; 26.67%) (Table 1).
Subgroups of Wolbachia. Wolbachia A harbored in
Ae. albopictus belonged to the subgroup AlbA, whereas that in Ml. genurostris and Mn. uniformis be-longed to subgroups Pap and Riv, respectively.
Wol-bachiaB harbored in Cx. neomimulus, Cx. pipiens
mo-lestus, Cx. quinquefasciatus, Cx. bicornutus, Tp. aranoides, Ae. albopictus,and Ar. omissus were iden-tiÞed as the subgroup Pip, whereas that in Ur.
novob-scura, Ur. annandalei,and Mn. uniformis were identi-Þed to the subgroup Con (Table 2). However,
Wolbachia harbored in Cx. mimeticus, Cx. murrelli (group B), Ar. subalbatus, Tp. bambusa (group A), Ar.
omissus, and one subgroup of Ml. genurostris (dual
infection) could not be further classiÞed to any known subgroup (Table 2).
Tissue Tropism of Wolbachia within the Mosquito. Tissues, including the pharynx, salivary gland, esoph-agus, cecum, trachea, stomach, Malpighian tubules, anterior hindgut, rectum, testis, accessory gland, and ovary, were dissected out from either Ae. albopictus or
Ar. subalbatus mosquitoes (adults and larvae). A cDNA fragment of ⬇590Ð632 bp ampliÞed from the
wspgene occurred in most tissues from both mosqui-toes (Table 3). The strongest band representing the
wspgene fragment usually occurred in the preparation of ovaries.
Discussion
This study showed that 51.72% (15/29) of mosquito species collected in Taiwan were positive for
Wol-bachiainfection. In addition to 26.67% of mosquitoes simultaneously infected by groups A and B, more mosquito species were shown to be infected by group B (53.33%) than group A (20%). Recently, eight spe-cies of mosquitoes were recorded as being Wolbachia-infected in Taiwan but not in other Southeast Asian countries (Kittayapong et al. 2000), implying that
Wolbachiainfection, especially group B, has become extensively established among mosquitoes of Taiwan. In addition, Þve species of Culex were all identiÞed to be infected by Wolbachia B, whereas two species of
Tripteroideswere infected by Wolbachia A.
This study also identiÞed dual infection with
Wolbachiagroups A and B in Ae. albopictus, Ar.
omis-sus, Ml. genurostris,and Mn. uniformis. This is consis-tent with the fact that an insect may naturally harbor more than one strain of Wolbachia (superinfection) (Breeuwer et al. 1992, Rousset et al. 1992, Rousset and Solignac 1995). In fact, more than one-fourth of spe-cies identiÞed with Wolbachia may have actually been superinfected (Werren et al. 1995a,b). A stable triple
Fig. 1. PCR-ampliÞed products from the ovary of Ae. albopictus. Lanes 1 and 12, 100-bp DNA ladder; lane 2, insect 12S
rRNA gene (400 bp); lane 3, 16S rDNA, A-speciÞc Wolbachia (259 bp); lane 4, 16S rDNA, B-speciÞc Wolbachia (259 bp); lane 5, universal Wolbachia 16S rDNA (900 bp); lane 6, ftsZ gene, A-speciÞc Wolbachia (⬇955Ð957 bp); lane 7, ftsZ gene, B-speciÞc Wolbachia (⬇955Ð957 bp); lane 8, the universal Wolbachia wsp gene (⬇590Ð632 bp); lane 9, the wsp gene, A-speciÞc
infection of Wolbachia in Drosophila has even been generated by an experimental injection (Rousset et al. 1999). However, it is still not clear whether compe-tition between different bacteria may actually limit the persistence of dual or multiple infections (Rousset et al. 1999). Until recently, any individual host in-fected by more than two Wolbachia strains had not been described in nature (Nirgianaki et al. 2003). New molecular markers may be more efÞcient at diagnos-ing multiple infections.
Wolbachiawere detected in seven of nine genera of mosquitoes examined in this study. The absence of
Wolbachiafrom Anopheles and Heizmannia is believed to be the result of differential intracellular environ-ments, such as competition from other bacterial en-dosymbionts of these mosquitoes (West et al. 1998, Kittayapong et al. 2000). In addition, some disease
vectors, including Culex tritaeniorhynchus Dyar and
Aedes aegypti(L.) have never been identiÞed as har-boring Wolbachia (Sinkins et al. 1997, Kittayapong et al. 2000). One explanation for this phenomenon is that these mosquitoes are presumed to be unsuit-able for supporting Wolbachia reproduction. Never-theless, eubacteria-speciÞc genes, including 16S and 23S rDNAs, were recently identiÞed from Anopheles
gambiaeGiles (Brown et al. 2001). This implies that a eubacterial symbionts other than Wolbachia may re-side in Anopheles mosquitoes (Brown et al. 2001) and possibly in other currently “Wolbachia-free” species as well.
The wsp gene is known to possess ⬇10 times the variability of the ftsZ gene (Werren et al. 1995b, Zhou et al. 1998, van Meer and Stouthamer 1999) and is thus more suitable for elucidation of evolutionary
relation-Table 1. Infection of Wolbachia in mosquitoes of Taiwan
Genus (subgenus) species 16S rRNA ftsZGene wspGene
Universal A-type B-type A-type B-type Universal A-type B-type
Anopheles(Anopheles)
An. sinensis(6)a ⫺ ⫺
Anopheles(Cellia)
An. maculatu(3) ⫺ ⫺
Aedes(Finlaya)
Ae. elsiae vicarius(6) ⫺ ⫺
Ae. japonicus shintienensisTsai and Lien (6) ⫺ ⫺
Ae. togoi(Theobald) (3) ⫺ ⫺
Aedes(Stegomyia) Ae. Aegypti(100) ⫺ ⫺ Ae. Albopictus(50) ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ Armigeres(Armigeres) Ar. subalbatus(100) ⫹ ⫹ ⫹ ⫹ ⫹ Armigeres(Leicesteria) Ar. omissus(20) ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ Culex(Culex) Cx. mimeticus(3) ⫹ ⫹ ⫹ ⫹ ⫹ Cx. murrelli(3) ⫹ ⫹ ⫹ ⫹ ⫹ Cx. neomimulus(3) ⫹ ⫹ ⫹ ⫹ ⫹ Cx. pipiens molestus(6) ⫹ ⫹ ⫹ ⫹ ⫹ Cx. quinquefasciatus(6) ⫹ ⫹ ⫹ ⫹ ⫹ Cx. tritaeniorhynchus summorosus(9) ⫺ ⫺ Culex(Culiciomyia) Cx. pallidothoraxTheobald (5) ⫺ ⫺ Culex(Eumelanomyia) Cx. foliatusBrug (6) ⫺ ⫺ Cx. okinawaeBohart (9) ⫺ ⫺ Culex(Lophoceraomyia) Cx. bicornutusTheobald (5) ⫹ ⫹ ⫹ ⫹ ⫹ Culex(Lutzia) Cx. fuscanusWiedemann (2) ⫺ ⫺ Cx. halifaxiiTheobald (2) ⫺ ⫺ Heizmannia(Heizmannia) Hz. taiwanensisLien (10) ⫺ ⫺ Malaya Ml. genurostrisLeicester (10) ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ Mansonia(Mansonioides) Mn. uniformis(2) ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ Tripteroides(Rachionotomyia) Tp. aranoides(4) ⫹ ⫹ ⫹ ⫹ ⫹ Tripteroides(Tripteroides) Tp. bambusa(Yamada) (3) ⫹ ⫹ ⫹ ⫹ ⫹ Uranotaenia(Pseudoficalbia) Ur. maxima(3) ⫺ ⫺ Ur. novobscura(1) ⫹ ⫹ ⫹ ⫹ ⫹ Uranotaenia(Uranotaenia) Ur. annandalei(20) ⫹ ⫹ ⫹ ⫹ ⫹
ships among Wolbachia isolates. Even so, some wsp-positive Wolbachia could not be appropriately placed into any known subgroup, suggesting that some
Wolbachiaisolates actually belonged to a subgroup not described previously (Zhou et al. 1998). It would be interesting to determine whether coinfection of two subgroups of Wolbachia also could cause CI or
other reproductive distortions in a host. In insects infected by Wolbachia, host Þtness can actually be enhanced by increased host fertility (Wade and Chang 1995, Poinsot and Merc¸ot 1997, Hariri et al. 1998). However, such infection in some cases may decrease host Þtness through reduced female fecun-dity (Hoffmann et al. 1990) or deleterious effects under speciÞc conditions (Hoffmann et al. 1990). More observations are needed to elucidate why CI is only seen in Culex, Aedes, and Armigeres mosquitoes (Yen and Barr 1973, Wright and Barr 1980, Jamnon-gluk et al. 2000, Dobson and Rattanadechakul 2001).
Wolbachiais highly infective to reproductive tissues and may be deleterious to the reproduction of infected hosts in certain cases (OÕNeill et al. 1997, Werren 1997, Bourtzis and OÕNeill 1998). However, heavy infection of Wolbachia in nervous and muscle tissues has been shown in Drosophila (Min and Benzer 1997) and other insects (Dobson et al. 1999), indicating that nonre-productive tissues also are susceptible to Wolbachia (Min and Benzer 1997). The current study showed that a variety of somatic tissues of Ae. albopictus and
Ar. subalbatuswere actually infected by Wolbachia, although the intensities of infection were varied. Among these tissues sampled, the ovary seemed to be the most suitable site for Wolbachia infection. In con-trast, Wolbachia-infected salivary glands were only infected at a moderate intensity. The relevance of this observation will be better understood once it is de-termined whether Wolbachia is involved in interfer-ence with arboviral replication or even transmission by a mosquito vector. Furthermore, the broad spec-trum of tissue tropism renders it possible to use
Wol-bachiato manipulate host populations as long as it can express antipathogen proteins in mosquito vectors (Beard et al. 1993, Sinkins et al. 1995, Beard et al. 1998).
Acknowledgments
This work was in part supported by a grant (NSC92-2815-C-182-029-B) from the National Science Council, Taiwan, Republic of China.
References Cited
Beard, C. B., R. V. Durvasula, and F. F. Richards. 1998.
Bacterial symbiosis in arthropods and the control of dis-ease transmission. Emerg. Infect. Dis. 4: 581Ð591.
Beard, C. B., S. L. O’Neill, R. B. Tesh, F. F. Richards, and S. Aksoy. 1993. ModiÞcation of arthropod vector
com-petence via symbiotic bacteria. Parasitol. Today 9: 179Ð 183.
Bensaadi-Merchermek, N., J. C. Salvado, C. Cagnon, S. Karama, and C. Mouches. 1995. Characterization of
the unlinked 16S rDNA and 23SÐ5S rRNA operon of
Wolbachia pipientis,a prokaryotic parasite of insect go-nads. Gene 165: 81Ð86.
Bouchon, D., T. Rigaud, and P. Juchault. 1998. Evidence for
widespread Wolbachia infection in isopod crustaceans: molecular identiÞcation and host feminization. Proc. R. Soc. Lond. Ser. B Biol. Sci. 265: 1081Ð1090.
Bourtzis, K., and S. L. O’Neill. 1998. Wolbachia infections
and arthropod reproduction: Wolbachia can cause
cyto-Table 2. Subgroups of Wolbachia based on the wsp gene among mosquitoes of Taiwan
Species Wolbachiasubgroup
a A B Single infection Armigeres(Armigeres) Ar. subalbatus * Culex(Culex) Cx. mimeticus * Cx. murrelli * Cx. neomimulus Pip
Cx. pipiens molestus Pip
Cx. quinquefasciatus Pip Culex(Lophoceraomyia) Cx. bicornutus Pip Tripteroides(Rachionotomyia) Tp. aranoides * Tripteroides(Tripteroides) Tp. bambusa * Uranotaenia(Pseudoficalbia)
Ur. novobscura Con
Uranotaenia(Uranotaenia)
Ur. annandalei Con
Dual infection
Aedes(Stegomyia)
Ae. albopictus AlbA Pip
Armigeres(Leicesteria)
Ar. omissus * Pip
Malaya
Ml. genurostris Pap *
Mansonia
Mn. uniformis Riv Con
aGene fragments for identiÞcation of Wolbachia A included Mel, AlbA, Mors, Riv, Uni, Haw, Pap,and Aus, whereas those for Wolbachia B included Con, Dei, Pip, and CauB.
*The species was not subgrouped.
Table 3. Tissue tropism of Wolbachia within Ae. albopictus and
Ar. subalbatus, based on amplification of a wsp gene fragment
Tissue Ae. albopictus Ar. subalbatus
Larva Pharynx ⫹ ⫹ Salivary gland ⫹ ⫹ Esophagus ⫺ ⫹ Cecum ⫹ ⫹ Trachea ⫹ ⫹ Stomach ⫹ ⫹ Malpighian tubules ⫹ ⫹ Anterior hindgut ⫹ ⫹ Rectum Adult ⫹ ⫹ Testis ⫹ ⫹ Accessory gland ⫺ ⫺ Ovary ⫹ ⫹ Salivary gland ⫹ ⫹ Anterior midgut ⫹ ⫹ Stomach ⫺ ⫺ Malpighian tubules ⫹ ⫺ Anterior hindgut ⫹ ⫹ Rectum ⫹ ⫹
plasmic incompatibility, parthenogenesis, and feminiza-tion in many arthropods. Bioscience 48: 287Ð293.
Bourtzis, K., A. Nirgianaki, G. Markakis, and C. Savakis. 1996. Wolbachia infection and cytoplasmic
incompati-bility in Drosophila species. Genetics 144: 1063Ð1073.
Bourtzis, K., A. Nirgianaki, P. Onyango, and C. Savakis. 1994.
A prokaryotic dnaA sequence in Drosophila melanogaster:
Wolbachia infection and cytoplasmic incompatibility among laboratory strains. Insect Mol. Biol. 3: 131Ð142.
Breeuwer, J.A.J., and G. Jacobs. 1996. Wolbachia:
intra-cellular manipulators of mite reproduction. Exp. Appl. Acarol. 20: 421Ð434.
Breeuwer, J.A.J., R. Stouthamer, S. M. Barns, D. A. Pelletier, W. G. Weisburg, and J. H. Werren. 1992. Phylogeny of
cytoplasmic incompatibility microorganisms in the para-sitoid wasp genus Nasonia (Hymenoptera: Pteromalidae) based on 16S ribosomal DNA sequences. Insect Mol. Biol. 1: 25Ð36.
Brown, C. A., F. N. Gyang, D. A. Boakye, and M. D. Wilson. 2001. IdentiÞcation and molecular characterization of
bacterial symbionts of Anopheles gambiae sensu stricto. Am. J. Trop. Med. Hyg. 65 (Suppl.): 182.
Chen, W. J., C. F. Dong, L. Y. Chiu, and W. L. Chuang. 2000.
Potential role of Armigeres subalbatus (Diptera: Culici-dae) in the transmission of Japanese encephalitis virus in the absence of rice culture on Liu-Chiu islet, Taiwan. J. Med. Entomol. 37: 108Ð112.
Clark, T. L., L. J. Meinke, S. R. Skoda, and J. E. Foster. 2001.
Occurrence of Wolbachia in selected Diabroticite (Coleoptera: Chrysomelidae) beetles. Ann. Entomol. Soc. Am. 94: 877Ð885.
Curtis, C. F., and S. P. Sinkins. 1998. Wolbachia as a possible
means of driving genes into populations. Parasitology 116 (Suppl.): 111Ð115.
Dobson, S. L., and W. Rattanadechakul. 2001. A novel
tech-nique for removing Wolbachia infections from Aedes
albopictus(Diptera: Culicidae). J. Med. Entomol. 38: 844Ð 849.
Dobson, S. L., K. Bourtzis, H. R. Braig, B. F. Jones, W. Zhou, F. Rousset, and S. L. O’Neill. 1999. Wolbachia infections
are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29: 153Ð160.
Hariri, A. R., J. H. Werren, and G. S. Wilkinson. 1998.
Dis-tribution and reproductive effects of Wolbachia in stalk eyed ßies (Diptera: Diopsidae). Heredity 81: 254Ð260.
Hayes, S. F., and W. Burgdorfer. 1981. Ultrastructural
com-parisons of Wolbachia-like symbiotes of ticks (Acari: Ixodidae), pp. 281Ð289. In W. Burgdorfer and R. L. Anacker [eds.], Rickettsiae and rickettsial disease. Academic, New York.
Hertig, M., and S. B. Wolbach. 1924. Studies on
rickettsia-like microorganisms in insects. J. Med. Res. 44: 329Ð374 (cited in Werren, 1997).
Hoffmann, A. A., and M. Turelli. 1997. Cytoplasmic
incom-patibility in insects, pp. 42Ð80. In S. L. OÕNeill, A. A. Hoffmann and J. H. Werren [eds.], Inßuential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, Oxford, United Kingdom.
Hoffmann, A. A., M. Turelli, and L. G. Harshman. 1990.
Factors affecting the distribution of cytoplasmic incom-patibility in Drosophila simulans. Genetics 126: 933Ð948.
Holden, P. R., P. Jones, and J. F. Brookfield. 1993. Evidence
for a Wolbachia symbiont in Drosophila melanogaster. Genet. Res. 62: 23Ð29.
Jamnongluk, W., P. Kittayapong, K. J. Baisley, and S. L. O’Neill. 2000. Wolbachia infection and expression of
cy-toplasmic incompatibility in Armigeres subalbatus (Diptera: Culicidae). J. Med. Entomol. 37: 53Ð57.
Kittayapong, P., K. J. Baisley, V. Baimai, and S. L. O’Neill. 2000. Distribution and diversity of Wolbachia infections
in Southeast Asian mosquitoes (Diptera: Culicidae). J. Med. Entomol. 37: 340Ð345.
Lien, J. C. 1978. The ecology and control of mosquitoes in
Taiwan, pp. 37Ð69. In C. C. Su, F. T. Lin, and D. F. Yen [eds.], Proceedings of the Seminar on Insect Ecology and Control. Academia Sinica, Taipei, Taiwan.
Louis, C., and L. Nigro. 1989. Ultrastructural evidence of
Wolbachiarickettsiales in Drosophila simulans and their relationships with unidirectional cross-incompatibility. J. Invertebr. Pathol. 54: 39Ð44.
Min, K. T., and S. Benzer. 1997. Wolbachia, normally a
sym-biont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. U.S.A. 94: 10792Ð 10796.
Nirgianaki, A., G. K. Banks, D. R. Frohlich, Z. Veneti, H. R. Braig, T. A. Miller, I. D. Bedford, P. G. Markham, C. Savakis, and K. Bourtzis. 2003. Wolbachia infections
of the whiteßy Bemisia tabaci. Curr. Microbiol. 47: 93Ð101.
O’Neill, S. L., R. Giordano, A.M.E. Colbert, T. L. Karr, and H. M. Robertson. 1992. 16S rRNA phylogenetic analysis
of the bacterial endosymbionts associated with cytoplas-mic incompatibility in insects. Proc. Natl. Acad. Sci. U.S.A. 89: 2699Ð2702.
O’Neill, S. L., M. M. Pettigrew, S. P. Sinkins, H. R. Braig, T. G. Andreadis, and R. B. Tesh. 1997. In vitro cultivation
of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol. Biol. 6: 33Ð39.
Poinsot, D., and H. Merc¸ot. 1997. Wolbachia infection in
Drosophila simulans:does the female host bear a physi-ological cost? Evolution 51: 180Ð186.
Rigaud, T., and P. Juchault. 1993. Conßict between
femi-nizing sex ratio distorters and an autosomal masculifemi-nizing gene in the terrestrial isopod Armadillidium vulgare (Latr.). Genetics 133: 247Ð252.
Rigaud, T., and P. Juchault. 1995. Success and failure of
horizontal transfer of feminizing Wolbachia endosymbi-onts in woodlice. J. Evol. Biol. 8: 249Ð255.
Rousset, F., and M. Solignac. 1995. Evolution of single and
double Wolbachia symbiosis during speciation in the
Drosophila simulanscomplex. Proc. Natl. Acad. Sci. U.S.A. 92: 6389Ð6393.
Rousset, F., H. R. Braig, and S. L. O’Neill. 1999. A stable
triple Wolbachia infection in Drosophila with nearly ad-ditive incompatibility effects. Heredity 82: 620Ð627.
Rousset, F., D. Vautrin, and M. Solignac. 1992. Molecular
identiÞcation of Wolbachia, the agent of cytoplasmic in-compatibility in Drosophila simulans, and variability in relation with host mitochondrial types. Proc. R. Soc. Lond. Ser. B Biol. Sci. 247: 163Ð168.
Simon, C., A. Frank, and A. Martin. 1991. Polymerase chain
reaction: DNA extraction and ampliÞcation, pp. 329Ð355.
InG. M. Hewitt, A.W.B. Johnston, and J.P.W. Young [eds.], Molecular techniques in taxonomy. Springer, Ber-lin, Germany.
Sinkins, S. P., H. R. Braig, and S. L. O’Neill. 1995. Wolbachia
superinfections and the expression of cytoplasmic incom-patibility. Proc. R. Soc. Lond. Ser. B Biol. Sci. 261: 325Ð330.
Sinkins, S. P., C. F. Curtis, and S. L. O’Neill. 1997. The
potential application of inherited symbiont systems to pest control, pp. 155Ð208. In S. L. OÕNeill, A. A. Hoffmann, and J. H. Werren [eds.], Inßuential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, Oxford, United Kingdom.
Sironi, M., C. Bandi, L. Sacchi, B. Di Sacco, G. Damiani, and C. Genchi. 1995. Molecular evidence for a close relative
of the arthropod endosymbiont Wolbachia in a Þlarial worm. Mol. Biochem. Parasitol. 74: 223Ð227.
van Meer, M.M.M., and R. Stouthamer. 1999. Cross-order
transfer of Wolbachia from Muscidifurax uniraptor (Hymenoptera: Pteromalidae) to Drosophila simulans (Diptera: Drosophilidae). Heredity 82: 163Ð169.
Wade, M. J., and N. W. Chang. 1995. Increased male fertility
in Tribolium confusum beetles after infection with the in-tracellular parasite, Wolbachia. Nature (Lond.) 373: 72Ð74.
Werren, J. H. 1997. Biology of Wolbachia. Annu. Rev.
Entomol. 42: 587Ð609.
Werren, J. H., D. Windsor, and L. R. Guo. 1995a.
Distribu-tion of Wolbachia among neotropical arthropods. Proc. R. Soc. Lond. Ser. B Biol. Sci. 262: 197Ð204.
Werren, J. H., W. Zhang, and L. R. Guo. 1995b. Evolution
and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc. R. Soc. Lond. Ser. B Biol. Sci. 261: 55Ð63.
West, S. A., J. M. Cook, J. H. Werren, and H.C.J. Godfray. 1998. Wolbachia in two insect host-parasitoid
communi-ties. Mol. Ecol. 7: 1457Ð1465.
Wright, J. D., and A. R. Barr. 1980. The ultrastructure and
symbiotic relationships of Wolbachia of mosquitoes of the
Aedes scutellarisgroup. J. Ultrastruct. Res. 72: 52Ð64.
Yen, J. H., and A. R. Barr. 1973. The etiological agent of
cytoplasmic incompatibility in Culex pipiens. J. Invertebr. Pathol. 22: 242Ð250.
Zhou, W., F. Rousset, and S. L. O’Neill. 1998. Phylogeny and
PCR-based classiÞcation of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. Ser. B Biol. Sci. 265: 509Ð515.