Polyhedron Vol. 9, No. l2, pp. 1491-1495, 1990 Printed in Great Britain
0277-5387/90 s3.c0+.00 0 1990 Pergamon Pwss plc
SYNTHESIS AND CRYSTAL STRUCTURE OF A TETRANUCLEAR MIXEDdMETAL ALKYLIDENE
COMPLEX CpWw(CO),@-O)(@-I)(@HTol)
SHIEMING PENG*t and GENE-HSIANG LEE
Department of Chemistry, National Taiwan University, Taipei 10764, Taiwan, R.O.C.
Department of Chemistry, National Tsing Hua University, Hsinchu 30043, Taiwan, R.O.C.
(Received 21 November 1989 ; accepted 27 February 1990)
Abstract-The solid-state structure of a tetranuclear mixed-metal cluster CpWOs3(CO)&- O)(p-H)(p-CHTol) (2) which possesses a bridging hydride, a bridging 0x0 and a bridging tolylalkylidene ligand is presented. Complex 2 crystallizes in the triclinic space. group PI, with a = 9.763(5), b = 10.759(4), c = 13.336(3) A, tl = 88.00(3), /I = 108.56(3), y = 100&l(3)“, 2 = 2 ; 3463 observed reflections with I > 30(I) were used in the refinement. The R values converged to RF = 0.057, R, = 0.058.
The reactivity of mixed-metal clusters has been of interest for many years.’ Research in this area is stimulated by a belief that the combination of metals having very different chemical properties within one compound may induce unique chemical transformations. In seeking to develop a systematic way to prepare the mixed-metal clusters of higher nuclear&y, Stone and co-workers have used Cp(C0)2W~R as a building block to prepare many mixed-metal clusters containing a bridging alkylidyne fragment.2 As the number of the mixed- metal alkylidyne clusters has increased dramatically in the past few years, there is increasing interest in examining the chemistry of the alkylidyne clusters because of the possible roles of the alkylidynes as reaction intermediates in, and/or models of various catalytic processes.3 In the present study we have explored the hydrogenation of a tetrahedral mixed- metal alkylidyne complex CpWOs3(CO)g(p3- CTol),(fl-H) (1)4 which produces an alkylidene complex CpWOs3(C0)g@-O)@-H)@-CHTol) (2) in
* Authors to whom correspondence should be addressed. t To whom inquiries concerning the X-ray crys- tallographic work should be addressed.
low yield (Scheme 1). The single-crystal X-ray diffraction study on complex 2 suggests that its gross geometry and ligand configuration are comp- lementary to those of the related, structurally char- acterized WOs, clusters CpWOs3(CO)g@O)@ H)(p-CHCH,Tol) (3)’ and CpWOs3(CO)g~-O)~- Cl)(p-CHCH2Tol) (4)6 (Scheme 2).
RESULTS AND DISCUSSION
Hydrogenation of complex 1 in refluxing xylenes solution (1 atm, 6 h) produced an orange-brown solution. The solvent was removed in vacua and the product purified by thin-layer chromatography (TLC), giving an orange product in low yield. The mass spectrum indicates a parent ion at m/z 1198, corresponding to a composition C22H140,00~3W. The 13C NMR spectrum (CDC13, 100.6 MHz, 213 K) exhibits nine OS-CO signals at 6 194.5, 184.4, 181.4, 180.9, 176.4, 173.2, 169.9, 169.7, 169.1 and the ‘H NMR spectrum (CDC13, 400 MHz, 294 K) shows two singlets at 6 5.52 and - 18.05, in addition to signals corresponding to one Cp ligand and one tolyl fragment. The IR spectrum (C,H,,) in the carbonyl region shows absorption bands at 2090(s), 2965(vs), 2028(vs), 202O(vs), 2014(s), 2007(m), 1491
1492 SHIE-MING PENG et al. +H2 (1) Scheme 1. (2) 1991(w), 1958(w), 1940(m) cm-‘, indicating that the molecule is analogous to the recently reported dioxo cluster C~WOS~(CO)~(,U-O)~(~-H).’ Based on these spectroscopic data, a formulation CpWOs, (CO)&O)@-H)(p-CHTol) is proposed for this complex. The exact source of the 0x0 ligand in 2 and the fate of the second alkylidyne in 1 are unknown. However, based on reports in the literature, we speculate that the 0x0 ligand may be derived from a CO ligand by C-O bond scission or from other possible sources (0, or HzO)’ in the solution and that the alkylidyne may be eliminated as a para- xylene molecule. 9
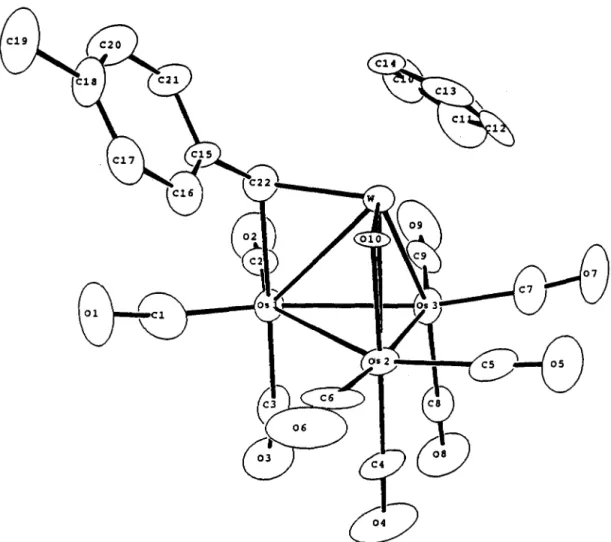
The molecular structure of 2 was determined by a single-crystal X-ray diffraction study. The ORTEP diagram and the scheme used for labelling the atoms are shown in Fig. 1. The bond distances and angles are listed in Tables 1 and 2, respectively. The mol- ecule consists of one tungsten atom and three osmium atoms which define a tetrahedral core associated with the expected 60 outer valence elec- trons. The tungsten atom is coordinated to a Cp, a bridging 0x0 and a bridging alkylidene ligand, whereas each of the three basal osmium atoms is associated with three terminal CO ligands arranged in a pseudo-octahedral geometry.
The 0x0 ligand 0( 10) bridges the W-OS(~) edge and the W-O(lO)-Os(2) interaction is char- acterized by the short tungsten-oxygen bond dis- tance (W-0( 1) = 1.77 A), the comparatively long Os(2)-0(10) bond length (2.13 A) and the small W--O(lO)-Os(2) angle (94.6”). These data are compatible with the parameters for the typical
W=O+Os bonding interaction as has been found previously in the related WOs, complexes. ‘O In addition, there is also a doubly bridging alkylidene ligand (p-CHTol) which is associated with the second W-OS edge of the triangular face defined by the atoms W, OS(~) and OS(~). The W-C(22) distance (2.08 A) is slightly shorter than the Os(l)-C(22) distance (2.24 A) and the tolyl sub- stituent is oriented toward the bridging 0x0 ligand. Finally, the structurally undetermined bridging hydride ligand is proposed to be associated with the third Os-Os edge of this WOs2 triangle. Consistent with our postulate, this metal-metal vector displays a relatively large Os(l)-Os(2) distance (2.997 A) and possesses two enlarged OS-OS-CO angles (113-l 14”) and two regular OS-OS-CO angles (89-94”). The other two OS-OS distances within the molecule are slightly smaller, with OS(~)- OS(~) = 2.844(2) and OS(~)-OS(~) = 2.846(2) A. After the position of the bridging hydride ligand is established, the formal electron counts for the W, OS(~), OS(~) and OS(~) atoms are calculated to be 17, 18.5, 19.5 and 17ee, respectively.
The structure of 2 is different from those of the analogous hydrido-alkylidene complex 3 and the chloro-alkylidene complex 4 in terms of the location of the bridging hydride and the configuration of the bridging alkylidene ligand (Scheme 2). In complex 3, the bridging hydride is located on the OS-OS edge eclipsed to the W-OS edge which is associated with the alkylidene fragment ; therefore, the metal- metal edges supporting the bridging hydride, 0x0 and alkylidene ligands are aligned in a zigzag
CP y w.+Tol
/“I\
0 \ (co)30s~~s/Hr’(co), wll; CP QH,Tol Q CH2Tol (2), anti (3), syn Scheme 2. (4L synCpWOs,(CO),(p-O)(p-H)(p-CHTol)
Fig. 1. The molecular structure of CpWOs3(CO)& l-O)(p-H)@-CHTol) (2) showing the atomic
numbering scheme.
Table 1. Selected interatomic distances (A) of complex 2 (ESADs in parentheses)
Os( l)-Os(2) 2.977(2) Os(l)-Os(3) 2.844(2)
OS(~)-OS(~) 2.846(2) W-Os( 1) 2.857(2) W-Os(2) 2.874(2) W-Os(3) 2.629(2) W-0(10) 1.77(2) 0s(2)--0(10) 2.13(.‘) w-C(22) 2.08(2) Os( ljC(22) 2.24(2) Os(l)-C(l) 1.92(3) Os(l)-C(2) 1.95(3) Os(l)--c(3) 1.96(3) Os(2>--c(4) 1.86(3) Os(2)-4v) 1.94(3) Os(2)-C(6) 1.68(4) Os(3)--c(7) 1.87(3) Os(3)--c(8) 1.95(3) Os(3)-C(9) 1.80(3) C(l)--o(l) 1.13(3) C(2)-O(2) 1.08(4) C(3)--0(3) 1.10(4) C(4)-O(4) 1.1 l(4) C(5)-0(5) 1.15(3) C(6)-0(6) 1.30(5) C(7)-O(7) 1.17(3) C(8)---O(8) 1.08(4) C(9)--O(9) 1.19(4) w-C(10) 2.34(4) W-qll) 2.39(4) W-C(l2) 2.35(2) W--X(13) 2.37(3) W-C(l4) 2.31(3) C(22)-C(15) 1.53(3)
arrangement. In contrast, the bridging chloro
ligand of complex 4 is located at a position similar to that of the bridging hydride in complex 2 ; there- fore, the respective metal-metal edges are arranged in a triangular pattern. In addition, because the bridging chloro ligand is a three-electron-donor, the bond distance between the osmium atoms linked by chloro ligand is not in the range of a typical OS-OS single bond.
On the other hand, the a-hydrogen of the alkyl- idene ligand in both complexes 3 and 4 is oriented toward the bridging 0x0 ligand ; therefore, the con- figuration of the bridging alkylidene (denoted as syn) is in contrast to that of complex 2 (denoted as anti). We propose that the formation of the anti configuration for complex 2 is due to its greater thermodynamic stability with respect to the unob- served syn configuration, and the latter is destabil- ized because of the unfavourable steric repulsion between the tolyl substituent and the Cp ligand.
1494 SHIE-MING PENG et al.
Table 2. Selected bond angles (“) of complex 2 (ESADs in parentheses) W-os( l)-os(2) Os( l)-W-as(2) w-os(3)--os( 1) W--Os(2)-Os(3) OS(~)-W--OS(~) OS(~)--Os(3)--Os(l) Gs(l)_-c(l>--o(l) Gs(l)_-c(3)--0(3) Gs(2)--c(5)_-0(5) Gs(3)-(?7)-G(7) Gs(3)--c(9)--0(9) w-Os( l)-c(22) Os(2)--0( 10)--w w-Os(2)--0( 10) Os( 1)--c(22)--c( 15) Os( l)--Os(2)-C(6) os(2)--os(lfl(3) 58.98(4) 62.58(4) 62.79(4) 54.72(5) 62.1 l(5) 63.08(5) 172(3) 172(3) 176(3) 174(3) 178(2) 46.3(5) 94.6(7) 37.9(5) 123(2) 113(l) 89(l) w-os(2jos( 1) W-os( l)-Os(3) Os( l)--W-as(3) W--Q(3)--OS(~) Gs( l)-os(2)--os(3) Gs(3)--os(l )--OS(~) Gs(l>--c(2~(2) Gs(2)--c(4>--0(4) Gs(2)-C(6)--0(6) Gs(3)_-c@)-G@) Os( l)-C(22)--w Os( l)-W-c(22) os(2)--w-o( 10) W-c(22)--C( 15) Gs( 1 )--os(2)--c(4) Gs(2)-@(l)--c(l) 58.43(4) 54.91(4) 62.29(4) 63.17(5) 58.42(4) 58.49(4) 178(2) 177(4) 169(3) 175(4) 82.7(8) 51.1(6) 47.5(5) 119(2) 94.4(9) 114(l) EXPERIMENTAL
IR spectra were recorded using a Perkin-Elmer 580 spectrometer calibrated using the absorption of cyclohexane at 2138.5 cm-’ and the absorption of polystyrene film at 1944.5 cn-‘. Both ‘H and 13C NMR spectra were recorded using a Bruker AM-
400 instrument. Mass spectra were obtained on a
JEOL-HXl 10 instrument operating in electron impact, field desorption or fast atom bombardment modes. All reactions were performed under a nitro- gen atmosphere using deoxygenated solvents dried with an appropriate reagent. The progress of reac- tions was monitored by analytical TLC (5735 Kie- selgel 60 FZs4, E. Merck) and the products were separated on the commercially available pre- parative TLC plates (Kieselgel60 FZs4, E. Merck). The mixed-metal complexes C~WOS,(CO)&~- CTol)&-H) (1) were prepared according to the literature method.4
Preparation of CPWOS ~(COMP-WP-WP-
CHTol) (2)
A xylene solution (45 cm’) of CpWOs3(CO)9@3- CTO~)~(,U-H) (1) (56 mg, 0.043 mmol) was heated at reflux under 1 atm of hydrogen. After refluxing for 6 h, the solvent was evaporated in uacuo and the residue was separated on a preparative TLC plate (hexanedichloromethane, 3 : 2), giving the alkyl- idene complex 2 (6 mg, 0.005 11111101, 12%) as red- orange crystals after recrystallization from dichlo- romethane-methanol. In addition, some starting materials (10 mg, 0.008 mmol, 18%) were also re- covered from the residue of the reaction mixture.
Spectroscopic data for complex 2. MS (EI, ‘920s, ‘84W), m/z 1198(M+); IR(C6Hr2): v(CO), 2090(s), 2065(vs), 2028(vs), 202O(vs), 2014(s), 2007(m), 1991(w), 1958(w), 1940(m) cm-‘; ‘H NMR (400 MHz, CDC13, 294 K) : 6 7.05-6.45 (m, 4H), 5.94 (s, 5H), 5.52 (s, lH), 2.39 (s, 3H), - 18.05 (s, 1H); 13C- (H} NMR (100.4 MHz, CDC13, 213 K) : OS-CO, 6 194.5, 184.4, 181.4, 180.9, 176.4, 173.2, 169.9, 169.7, 169.1.
Structural determination of complex 2
Crystals suitable for diffraction analysis were obtained from a dichloromethanemethanol solu- tion. A crystal was mounted on a glass fibre. Diffraction measurements were carried out on a Nonius CAD-4 fully automated four-circle diffrac- tometer. The unit cell was determined and refined using setting angles of 25 randomly selected reflec- tions, with 29 angles in the range of 2.5-50”, obtained by using the CAD-4 automatic search, centre, index and least-squares routines. All data reduction and structure refinement were performed using the NRCC-SDP-VAX packages. The struc- ture was solved by the Patterson method and refined by least-squares ; all non-hydrogen atoms were refined with anisotropic thermal parameters. The data collection parameters are summarized in Table 3. Tables of atomic coordinates, anisotropic ther- mal parameters, structure factor amplitudes, and non-essential bond distance and angle parameters have been deposited as supplementary data and are available from the authors (S.M.P.).
CpWOs,(CO),(p-O)(p-H)@CHTol)
Table 3. Experimental data for the X-ray diffraction study of complex 2
1495
(A) Crystal data a (A) b (A) c (A) a (“) B (“) Y (“) F(OO0) Temperature (K) Crystal system Space group Z Formula V(A3) Molecular weight Density (talc.) (g cm- ‘) 9.763(5) 10.759(4) 13.336(3) 88.00(3) 108.56(3) 100.44(3) 1056 297 Triclinic
Pi
2 C22H140100~3W, 1305.6(9) 1192.8 3.034(B) Data collection, reduction, solution and refinement Data collection instrument
Radiation (monochromated in incident beam) Scan method
Scan range Scan speed Crystal size (mm)
Linear absorption coefficient (mm- ‘) Transmission factors : max, min.
Number of unique data, total with Z > 30(Z) Number of atoms and parameters refined Largest and mean A/a
R; R,“ (%) G.O.F.’
Residual electron density (e A- ‘) : max./min.
Nonius CAD-4 MO-K, (,I = 0.71073 A) 20-8 scan mode 0.65+0.35 tan 0 Variable ; l . lO-8.24”/min 0.25 x 0.40 x 0.40 19.10 0.99,0.55 4574,3463 36,326 0.228, 0.001 5.7; 5.8 3.71 4.431-2.88 aw-I = o*(F).
‘S = [X w~F0-F,~2/(N,-Nv)]‘~2 (N, = number of observations; N, = number of variables).
REFERENCES
D. A. Roberts and G. L. Geoffroy, in Compre- hensive Organometallic Chemistry (Edited by G. Wilkinson, F. G. A. Stone and E. W. Abel), Vol. 6, Ch. 40. Pergamon Press, Oxford (1982).
(a) F. G. A. Stone, in Inorganic Chemistry: Toward the 21st Century (Edited by M. H. Chisholm), ACS Symp. Ser. No. 211, p. 383. American Chemical Society, Washington, D.C. (1983) ; (b) S. J. Davies, J. A. K. Howard, R. J. Musgrove and F. G. A. Stone, Angew. Chem. Znt. Edn Engl. 1989, 28, 624 and refs cited therein.
(a) E. L. Muetterties and J. Stein, Chem. Rev. 1979, 79, 479 ; (b) L. R. Beanan and J. B. Keister, Organ- ometallics 1985, 4, 1713.
(a) J. T. Park, J. R. Shapley, C. Bueno, J. W. Ziller and M. R. Churchill, Organometallics 1988,7,2307;
5. 6. 7. 8. 9. 10.
(b) J. T. Park, J. R. Shapley, M. R. Churchill and C. Bueno, J. Am. Chem. Sot. 1983,105,6182.
M. R. Churchill and Y. J. Li, J. Organomet. Chem. 1985,291, 61.
Y. Chi, J. R. Shapley, J. W. Ziller and M. R. Chur- chill, Organometallics 1987,6, 301.
Y. Chi, L.-S. Hwang, G.-H. Lee and S.-M. Peng, J. Chem. Sot., Chem. Commun. 1988, 1456.
M. R. Churchill, C. Bueno, J. T. Park and J. R. Shapley, Znorg. Chem. 1984,23, 1017.
(a) J. B. Keister, J. Chem. Sot., Chem. Commun. 1979, 214; (b) G. Fachinetti, R. Lazzaroni and S. Pucci, Angew. Chem. Znt. Edn Engl. 1981,20, 1063 ; (c)J. B. Keister, M. W. Payne and M. J. Muscatella, Organometallics 1983,2,219.
M. R. Churchill and Y. J. Li, J. Organomet. Chem. 1985,294,367.