The Analysis and Application of Biological
Hydrodynamics on the Freshwater Snails Sinotaia
quadrata and Thiara granifera
Song-Yue Yang
[1]Bing-Shi Lin
[2]Wen-Lian Chang
[3]ABSTRACT Freshwater snails are important biological indicators of water quality and the primary source of sustenance for firefly larvae in Taiwan. Currents may promote the transport of nutrients and the metabolism of aquatic organisms. However, they may simultaneously exert stresses on organisms. In this study, the morphology, drag, lifts, adhesive forces and dislodgment velocities of Sinotaia
quadrata and Thiara granifera were measured. The relationships among
hydrodynamics, morphology and mechanical characteristics were derived from the results of the measurements. The results indicated that the streamline shell could reduce drag and lift under high current velocity. The morphology of T. granifera was superior to that of S. quadrata for resisting hydrodynamic stresses because it is streamlined. Theoretically, the T. granifera should be better able to resist dislodgment than S. quadrata; however, the results of the dislodgment experiment did not confirm this expectation but showed that T. granifera was more easily dislodged than S. quadrata. The analysis of the dislodgement model showed that the mechanism of dislodgment of freshwater snails consists of two parts - the actual dislodgment mechanism, involving drag, lift, and buoyancy, and the dislodgment-resistance mechanism, involving adhesive force and weight. In conclusion, the hydrodynamic forces were determined primarily by the morphology of the shell, and the adhesive forces served as a buffer of the direct effects of hydrodynamic forces. The dislodgment velocity was determined by the interaction between hydrodynamic and biological mechanisms.
Key Words: freshwater snail, hydrodynamics, morphology, Sinotaia quadrata,
Thiara granifera
石田螺與瘤蜷的生物流體力學分析與應用
楊松岳
[1]林秉石
[2]張文亮
[3] 摘 要 淡水螺是台灣水質的重要生物指標,同時也是螢火蟲的主要食物來源。對於水中生 物而言,水流可以增加營養物質的傳輸與新陳代謝,但是同時也會施加應力在生物體上。本研 究中將針對石田螺與瘤蜷的型態、阻力、升力、吸附力與衝落流速進行量測。根據所量測的結 〔1〕經濟部水利署水利規劃試驗所工程員(通訊作者)Engineer, Water Resources Planning Institute, Water Resources Agency, Ministry of Economic Affairs, Taichung 413, Taiwan, R. O. C. (Corresponding Author)
E-mail:acton@ms14.url.com.tw 〔2〕國立台灣大學生物環境系統工程學系碩士
Master, Department of Bioenvironmental Systems Engineering, National Taiwan University. 〔3〕國立台灣大學生物環境系統工程學系教授
果,求得流體力學、形態與機械特性的關係。由本實驗的結果可以發現在高流速下流線型的螺 殼將可以減少阻力與昇力,流線型的螺殼型態使得瘤蜷在減少水中應力上表現較石田螺佳。理 論上,瘤蜷應較石田螺更能抵擋水流的沖落,但是衝落實驗的結果並非如此,瘤蜷反而較石田 螺更容易衝落。經過衝落模式的分析可以將淡水螺的沖落機制分成兩個部分:衝落機制,包括 阻力、升力與浮力;及抵抗衝落機制,包括吸附力與重力。總而言之,水流作用力主要是由螺 殼的型態所決定,吸附力則可減緩水流直接的作用力,而衝落流速則是由水流作用力與生物力 的交互作用下而決定。 關鍵詞:淡水螺、流體力學、型態、石田螺、瘤蜷。
1.INTRODUCTION
Various environmental variables that may influence the abundance of freshwater snails or the variety of species have been catalogued. They include depth, flow, species of substrate, type of vegetation, hardness, pH and drainage area and others (Appleton, 1978; Pip, 1978; Okland, 1983; Thomas and Tait, 1984; Watters, 1992). However, Dillon (2000) suggested that the current velocity might be the most important variable, although separating the effects of depth, substrate and current in the lotic environment is difficult. The current can carry dissolved gases and nutrients for aquatic organisms, removing their waste, and dispersing their gametes and spores, but hydrodynamic forces cause various stresses in their bodies (Koehl, 1982; Koehl, 1984; Denny et
al., 1985; Koehl, 1986).
Pace (1973) categorized aquatic habitat of Taiwan freshwater snails into five types - river and stream, irrigation canals, flood plain, ponds and lakes. Most of Taiwan’s cultivated lands are rice paddies, which require irrigation through an extensive system of reservoirs, irrigation canals and flood plains. The habitats of freshwater snails are being significantly decreased by increasing human population and activities, and the native snails are being replaced by alien snails, such as Pomacea
canaliculata, and their numbers are being
reduced by the overuse of insecticide and water pollution.
Chao (2000) suggested that Sinotaia
quadrata and Thiara granifera can be used as
biological indicators of the deterioration of
aquatic ecosystems in Taiwan. The firefly larvae feed on them primarily, so the recovery of fireflies must be based on the recovery of freshwater snails. The adult shell of S.
quadrata is solid and narrow and the length of
an adult shell is around 2.5cm. Its embryonic shell is small and has shorter, more closely spaced chaetae. It is frequently found in the mud or silt of lakes, ponds, rice paddies, irrigation canals and streams. The shell of T.
granifera is characterized by coarse, rectangular
nodules formed by reticulation of axial ribs and spiral cords. The length of adult shell is around 1.5 cm and the body whorlis longer than half of the total height of the shell. It is one of the most widespread freshwater snails in Taiwan (Pace, 1973).
The aim of this study is to measure the morphology, drag, lift, adhesive force and dislodgment velocity of each of S. quadrata and
T. granifera under various flow velocities. The
analysis of those parameters yielded the relationships among hydrodynamics, morphology and mechanical properties. Then, the magnitudes of forces and dislodgment velocities were predicted using the dislodgment model. This calculation offers a basis for hydraulic engineering designs that conserve the ecology and control the population of freshwater snails.
2.MATERIALS AND METHODS
Freshwater snails were collected from King-Long Lake and upstream from this lake in Taipei, Taiwan (120o 37' E, 25o 04' N). Before experimentation, the freshwater snails were kept in a tank for one week for acclimation. Figure 1 presents photographs of S. quadrata and T. granifera.
(A) (B)
Fig.1 The freshwater snails used in this study (A) Sinotaia quadrata and (B) Thiara granifera.
(1) Morphology
The volumes (V) of freshwater snails were determined by measuring the weight change of a suspended specimen in air and a water beaker. The anterior–posterior length, l, and the left–right width of the shell, w, were measured to the nearest 0.001 cm using electronic vernier calipers (Mitutoyo). Profile areas (Aap) and
planform areas (Apl) were photographed on the
anterior–posterior axis and the dorsal side of the shell using digital cameras. A comparison was made with a reference scale and Aap and Apl were
determined using AutoCAD. The weight was expressed as a function of V, the areas as a function of V2/3and the lengths as a function of
V1/3.
(2) Hydrodynamics
Drag in the direction of flow is caused primarily by an upstream–downstream difference in pressure (Vogel, 1994). As water moves past freshwater snails, the presence of the shell changes the pattern of flow, so the pressure of the upstream part of the shell exceeds that of the downstream part. Drag can be expressed as follows. 2
2
1
u
A
C
D
=
D apρ
w (1)where CD is the drag coefficient; ρw is the
fluid density, and u is the current velocity. Lift, acting perpendicular to the direction of flow, is caused by the pressure difference above and below the sides of an organism. Lift can be expressed as, 2
2
1
u
A
C
L
=
L plρ
w (2)where CL is the lift coefficient.
The drags and lifts of live freshwater snails in currents of different velocities were measured in a flume made of acrylic plates (Fig. 2). As the freshwater snail was placed on the sensor, the orientations of the shells were adjusted such that the anterior of the shell pointed in the direction of flow. After the foot of freshwater snails stretched out from its shell and re-attached to the sensor, the measurement was made. The drag and lift measurements were made at different current velocities from 0 to 110 cms-1 to simulate its actual habitat. The current velocities, controlled by the frequency converter of the pump, were measured before the experiment using an electromagnetic current meter (ACM-200P Electromagnetic velometer) placed 1 cm above the substratum.
The drags were measured by vertically orientating an aluminum beam in the flow. A couple of strain gauges (KFW-5-120-D16- 11-L1M2S) detected the displacement of the
beams and transferred the signal to a transducer (Omega DP41-S). The freshwater snails did not significantly influence the flow through the flow tank since they obstructed less than 2% of the flume’s cross section. The measurement was corrected for the relatively tiny shear force that acted on the exposed area of the beam (Denny, 1989). The lifts of the freshwater snails were measured in the same experimental channel using the same transducer. The only difference was that the aluminum beam was oriented parallel to the flow.
Each pair of drags, lifts and water velocities was used to calculate the drag coefficient CD and
the lift coefficient CL. CD and CL were estimated
as functions of the logarithm of the Reynolds number, Re, using standard least-square linear regression. The Reynolds number is defined as, w c w
uL
Re
μ
ρ
=
(3)where Lc is the characteristic length of the
object (in the direction of flow) and μw is the
dynamic viscosity of the fluid.
(3) Adhesive and shear forces
The measurements of the adhesive force (Ta)
and the shear force (Ts) of live snails on acrylic
plate were made by holding an electronic digital tensiometer (Digital force gauge FGE-0_5X) via a nylon loop attached to the center of the shell. Each snail was allowed to re-adhere to the acrylic plate for a few minutes, before it was pulled separately in the vertical and horizontal directions until it detached from the plate. The measurements of both adhesive and shear forces on each specimen were performed three times and averaged. Each pair of adhesive and shear forces was adopted to determine the frictional coefficient μs. The variation of the
adhesive force was estimated as a function of weight, W, using standard least-square linear regression.
Fig.2 Schematic diagram of a unidirectional flume (A. water flow tank; B. drag device; C. lift device; 1. frequency converter; 2. receiving chamber; 3.stainless steel screens; 4.flume channel; 5. electromagnetic current meter; 6, transducer for current meter; 7. transducer for strain gages; 8. storage tank; 9. delivery pipe; 10, 11. aluminum beams; 12, 13. strain gages.)
A C B 1 2 4 5 6 7 8 9 10 11 3 12 13 Unit: mm
(4) Dislodgment Experiment
The dislodgment experiments on freshwater snails were conducted in the same flume. The experimental dislodgment velocities (ud), which
were defined as those at which the freshwater snails dislodged, can be expressed as a normal cumulative probability function (Pd), to estimate
the likelihood of dislodgment by current velocities. (Alfaro and Carpenter, 1999)
du
s
u
u
s
P
ud d avg d∫
−∞⎥
⎥
⎦
⎤
⎢
⎢
⎣
⎡
−
−
=
2 22
)
(
exp
2
1
π
(4)where uavg is the mean of the experimental
dislodgment velocities, and s is the standard deviation of the experimental dislodgment velocities. The experimental data, following a normal cumulative probability function, were estimated. Then, the weight of the specimen was estimated as a function of dislodgment velocity, ud.
(5) Model Simulation
According to Fig. 3, the freshwater snail was assumed to be dislodged if the drag exceeded the friction force.
s
F
D
>
(5)The force balance perpendicular to the flow direction could be expressed as,
N
L
B
W
T
a+
=
+
+
(6)where Ta was the adhesive force; W was the weight; B was the buoyancy, and N was the normal force. W is given by
V
g
W
=
ρ
bio⋅
⋅
(7)where ρbio is the density of the snail, g is the
acceleration due to gravity, and V is the volume of the snail. B is given by,
V
g
B
=
ρ
w⋅
⋅
(8)Equation (6) can be rearranged as,
L
B
W
T
N
=
a+
−
−
(9)Equations (2), (7) and (8) are substituted into Eq. (9) to yield,
2
2
1
)
(
gV
C
A
u
T
N
=
a+
ρ
bio−
ρ
w−
L plρ
w (10)The normal force on the freshwater snail can be converted into the friction force by
applying the friction coefficient (μs). The friction
force (Fs) was expressed as follows;
N
F
s=
μ
s (11)Equation (10) is substituted into Eq. (11).
]
2
1
)
(
[
2u
A
C
gV
T
F
s=
μ
s a+
ρ
bio−
ρ
w−
L plρ
w (12)Equations (1) and (12) are substituted into (5) to yield, ] 2 1 ) ( [ 2 1C A u2 T gV C A u2 w pl L w bio a s w ap D ρ >μ + ρ −ρ − ρ (13)
The critical dislodgment velocity is given,
[
]
)
(
)
(
2
al L s ap D w w bio aA
C
A
C
gV
T
u
+
−
+
>
μ
ρ
ρ
ρ
(14)3.RESULTS AND DISCUSSIONS
(1) Effect of shell shape on hydrodynamic
forces
Table 1 presents the relationships between the body volume and various morphological parameters. The density (ρbio) of S. libertina
(1.59 g/cm3) exceeded that of T. granifera (1.45 g/cm3). For a given volume, S. quadrata had a greater projected area (Aap) than T. granifera, but a smaller planform area (Apl). The ratio of the length to the width (l/w) of S. quadrata (1.52) was smaller than that of T. granifera (2.24), revealing that the shell of T. granifera was more streamline than that of S. quadrata.
Figures 4 and 5 plot the relationships between drag and lift coefficient and Reynolds number Re ranging from 10,000 and 19,000. The regression coefficients are presented in Tab. 2 and 3. Drag and lift coefficients at Reynolds number of 15,000, are calculated compared at Tab. 4.
The drag coefficients for two species decreased slightly with Re. At Reynolds number of 15,000, the drag coefficient of S.
quadrata (0.7843) was greater than that of S. libertina (0.3265). The drag coefficient of S. quadrata was statistically distinguishable to
that of T. granifera due to no overlap in their 95% confidence intervals.
The lift coefficient of S. quadrata decreased slightly with increasing Re. Although the lift
coefficient of T. granifera appeared to increase slightly, the effect was not statistically significant. At Reynolds number of 15,000, the lift coefficient of S. quadrata (0.2451) was greater than that of T. granifera (0.0972). The lift coefficient of S. quadrata was statistically distinguishable to that of T. granifera due to no overlap in their 95% confidence intervals.
The length-width ratio of shell of S.
quadrata is lower than that of T. granifera, so
the former has a higher drag coefficient, since drag is caused primarily by an upstream–downstream pressure difference. A more streamline shell exhibits a smaller pressure difference between upstream and downstream. Additionally, lift is caused by a pressure difference between the top and bottom sides of an organism (Vogel, 1994), so the lift coefficients of S. quadrata exceeded those of T.
granifera.
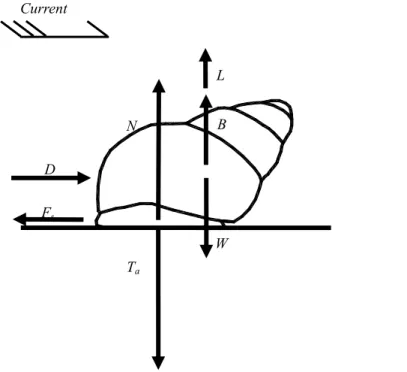
Fig.3 Schematic diagram of forces exerted on freshwater snail (L is the lift; B is the buoyancy; N is the normal force; W is the weight; Ta is the adhesive force; Fs is the friction force; D is the drag.)
Table 1 Morphological relationships for the freshwater snails
K1 K2 K3 K4 K5 l/w S. quadrata 1.5900 1.4416 1.6565 1.8213 1.1993 1.5186 R2 0.9679 0.6942 0.8706 0.8153 0.9231 n 16 11 10 18 18 T. granifera 1.4478 1.2069 1.8405 2.6514 1.1829 2.2414 R2 0.9218 0.7123 0.9797 0.9104 0.7593 n 21 10 6 21 21
W, weight; Aap, area projected along the anterior–posterior axis of the test; Apl, planform area projected along the oral–aboral axis; l, total length; w, total width.
W=K1(V);Aap=K2(V)2/3; Apl=K3(V)2/3; l=K4(V)1/3; w=K5(V)1/3.
T
aF
sD
W
L
B
N
Current
0.01 0.1 1 10 10000 12000 14000 16000 18000 Reynolds Number Re D rag Coef fi cient CD S. quadrata T. granifera
Fig.4 The relation between drag coefficient and Reynolds number
0.01 0.1 1 10000 12000 14000 16000 18000 Reynolds Number Re Lift Coe ffic ie nt CL S. quadrata T. granifera
Fig.5 The relation between lift coefficient and Reynolds number S. quadrata
T. granifera
S. quadrata
(2) Adhesive and shear forces
Table 5 shows that the adhesive forces of S.
quadrata and T. granifera both increased
significantly with weight; however, the variance of organisms leaded to that the effects were not statistically significant. The variances of the adhesive force of gastropods are presented in other research (Denny and Blanchette, 2000). The friction coefficients, the ratios of shear forces (Ts) to adhesive forces (Ta), of S. quadrata
and T. granifera, were 0.278 ± 0.159 and 0.323 ± 0.103 (mean ± standard deviation) from which values the normal force could be transformed into the friction force in the dislodgment model.
(3) Dislodgment experiment and
dislodgment model
Figure 6 and Table 6 present the cumulative probability functions of dislodgment. S.
quadrata and T. granifera were dislodged at
current velocities of 72 ± 24 and 67 ± 20 cm/s (mean ± standard deviation), respectively. Table 7 plots the relationship between the weights of the dislodged freshwater snails and the experimental dislodgment velocities and showed that the experimental dislodgment velocities were not significantly correlated (p>0.05) with the sizes of S. quadrata and T.
granifera.
Theoretically, the capacity of T. granifera to resist dislodgment should exceed that of S.
quadrata because it has a streamlined shell.
However, the dislodgment experiment did not confirm this hypothesis and showed that T.
granifera was more easily dislodged than S. quadrata in a strong current. The dislodgment
model was further calculated to elucidate the
mechanism of dislodgment.
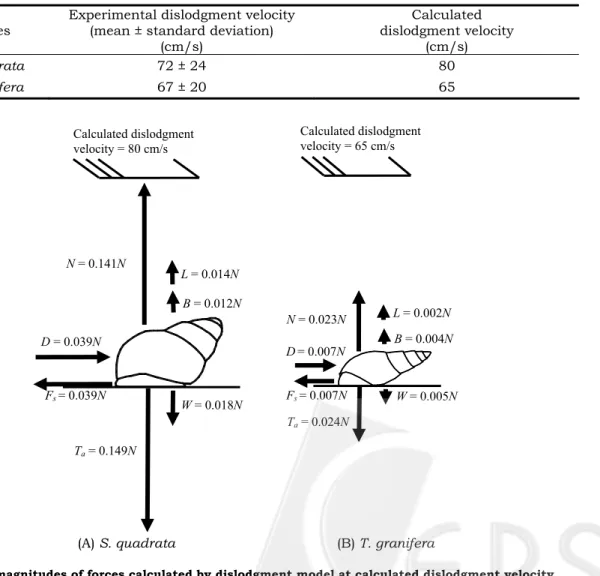
The mean volumes of S. quadrata (1.178 cm3) and T. granifera (0.375 cm3) of the dislodgment experiment herein were given as parameters that are substituted in the regression of morphology, hydrodynamic and adhesive forces. Then, the calculated dislodgment velocities of S. quadrata (80 cm/s) and T. granifera (65 cm/s) were obtained using the dislodgement model. According to Tab. 8, the calculated dislodgment velocities were close to and consistent with the experimental dislodgment velocities.
The calculated dislodgment velocities were substituted into the dislodgment model; then, the magnitudes of the drag, lift, buoyancy, weight and adhesive forces were obtained, and plotted in Fig. 7. The drag (0.039N) of S.
quadrata was almost three times the lift (0.014N)
and buoyancy (0.012N) at the calculated dislodgment velocity (80 cm/s). In contrast, the adhesive force (0.149N) was almost eight times the weight (0.018N). The normal force (0.141N) is the remainder of the vertical forces (adhesive force plus lift plus buoyancy minus weight) and can be converted into the frictional force (0.039N) by applying the friction coefficient (0.278).
The drag (0.007N) of S. quadrata was 3.5 times the lift (0.002N) and almost doubles the buoyancy (0.004N) at the calculated dislodgment velocity (65 cm/s). The adhesive force (0.024N) was almost five times the weight (0.005N). The normal force was 0.023N, which was transformed into the friction force (0.007N) by applying the friction coefficient (0.323).
Table 2 Drag coefficients as a function of Reynolds number
Species a b R2 d.f. p-value
S. quadrata 95.962 -0.4999 0.0596 14 >0.05
T. granifera 161.25 -0.6450 0.0848 9 >0.05 The function used was: CD = a×(Re)b, where CD is the drag coefficient, Re is Reynolds number and a
Table 3 Lift coefficients as a function of Reynolds number
Species a b R2 d.f. p-value
S. quadrata 62.049 -0.5755 0.3371 10 >0.05
T. granifera 0.0219 0.1633 0.0498 3 >0.05 The function used was: CL = a×(Re)b, where CL is the lift coefficient, Re is Reynolds number and a and
b are the constants used in the regression equation. Table 4 rag and lift coefficients calculated at Re = 1.5×104
Species CD 95%CI CL 95%CI
S. quadrata 0.7843 0.7099-0.9378 0.2451 0.2262-0.2696
T. granifera 0.3265 0.2851-0.3901 0.1053 0.0951-0.1165 95%CI: 95% confidence intervals
Table 5 Maximum adhesive force as a function of weight
a b R2 d.f. p-value
S. quadrata 0.0167 0.0887 0.4756 12 >0.05
T. granifera -0.0352 0.0985 0.4922 11 >0.05 The function used was: Ta = a + b × W, where Ta is freshwater snails’ maximum adhesive force (N), W is weight (g), a and b are the constants used in the regression equation.
0
10
20
30
40
50
60
70
80
90
100
0
20
40
60
80
100
Current velocity u (cm/s)
Probability of
dis
lodgment
P
d(%)
S. quadrata
T. granifera
Experimental dislodgment
velocity =72 cm/s
Experimental dislodgment
velocity = 67 cm/s
Fig.6 The relation between cumulative probabilities of dislodgment and current velocity
S. quadrata
Table 6 Cumulative probability functions of dislodgment as a function of experimental dislodgment velocity
Species uavg ud R2 d.f. p-value
S. quadrata 72 24 0.8658 21 <0.05
T. granifera 67 20 0.9661 17 <0.05 The function used was:
du s u u s P ud d avg d
∫
−∞ ⎥ ⎥ ⎦ ⎤ ⎢ ⎢ ⎣ ⎡ − − = 2 2 2 ) ( exp 2 1π , where ud is the experimental dislodgment velocities
(cm/s); uavg is the mean of the experimental dislodgment velocities (cm/s), and s is the standard
deviation of the experimental dislodgment velocities (cm/s).
Table 7 Experimental dislodgment velocities as a function of weight
Species a b R2 d.f. p-value
S. quadrata 1.6521 0.0039 0.0234 21 >0.05
T. granifera 0.3730 0.0025 0.0627 17 >0.05 The function used was: u = a + b× W, where W is the weight of snail, a and b are the constants used in the regression equation.
Table 8 The experimental and calculated dislodgment velocity of the freshwater snails
Species Experimental dislodgment velocity(mean ± standard deviation) (cm/s) Calculated dislodgment velocity (cm/s) S. quadrata 72 ± 24 80 T. granifera 67 ± 20 65
(A) S. quadrata (B) T. granifera
Fig.7 The magnitudes of forces calculated by dislodgment model at calculated dislodgment velocity
W = 0.018N D = 0.039N Fs = 0.039N Ta = 0.149N N = 0.141N B = 0.012N L = 0.014N W = 0.005N Ta = 0.024N Fs = 0.007N D= 0.007N N = 0.023N B = 0.004N L = 0.002N Calculated dislodgment velocity = 80 cm/s Calculated dislodgment velocity = 65 cm/s
The calculation using the dislodgment model indicated that the dislodgment velocity was governed by the interaction of hydrodynamic and biological mechanisms. The mechanisms of dislodgment were hydrodynamic, including drag, life and buoyancy; the mechanisms of resistance against dislodgment were biological, including adhesive forces and weight. Figure 7 shows that drag was the primary factor that governed hydrodynamic forces and significantly influenced dislodgment. In the mechanisms of resistance against dislodgment, the adhesive force was the primary factor - much stronger than the weight. In conclusion, the hydrodynamic forces were determined by the morphology of the shell, and the adhesive force served to buffer the direct effects of hydrodynamic forces.
S. quadrata has a stronger adhesive force, but suffers from a higher hydrodynamic force because it is not streamlined; T. granifera is more streamline, but has a weaker adhesive force. When hydrodynamic and biological mechanisms interact with each other, S.
quadrata can endure a higher current
velocity than T. granifera.
Table 7 revealed that differently sized snails were not expected to be dislodged differentially by the given current velocities. Even the adhesive force appears to depend on size; however, the drag and lift also dependent on sizes of the projections of the shell area perpendicular and parallel to the flow. The larger freshwater snails suffer greater hydrodynamic forces; even thought they have stronger adhesive forces.
(4) The application in engineering design
The probability of dislodgment of freshwater snails in currents of various velocities can be applied for hydraulic engineering design. The determination of the design velocity depends on the purpose of the hydrodynamic construction. For instance, the current velocity at which 10%of freshwater snails are dislodged can be applied as the ceiling design velocity in conserving the ecology of native freshwater snails. In contrast, if the hydrodynamic construction is designed to control the population of harmful freshwater snails, then the current velocity at which 90% of freshwater snails are dislodged can be applied as the floor design velocity.
ACKNOWLEDGEMENTS
We thank C. H. Lin, G. S. Gan, and X. I. Zao for assistance with fieldwork. We wish to thank the unknown reviewers for helpful suggestions and comments.
LIST OF SYMBOLS
Aap the area projected along the
anterior–posterior axis
Apl the planform area projected along the
oral–aboral axis
B the buoyancy CD the drag coefficient
CL the lift coefficient
D the drag
Fs the friction force
g the acceleration due to gravity L the lift
Lc the characteristic length of the object
l the total length of shell N the normal force n the number of samples
Pd the cumulative probability function
Re Reynolds number
s the standard deviation of experimental
dislodgment velocity
Ta the adhesive force
u the current velocity
uavgthe mean of experimental dislodgment velocity
ud the experimental dislodgment velocity
V the volume of freshwater snail w the width of shell
W the weight
μs the friction coefficient
μw the dynamic viscosity of the fluid
ρbio the density of organism
REFERENCES
1. Alfaro, A .C. and R. C. Carpenter (1999), “Physical and biological influencing zonation patterns of subtidal population of marine snail, Astraea (Lithopoma) undosa Wood 1828,” J. Exp. Mar. Biol. Ecol., 240:259~283.
2. Appleton, C. (1978), “Review of literature on abiotic factors influencing the distribution and life cycles of bilharziasis intermediate host snails,” Malacol. Rev., 11:1~25.
3. Chao, D. (2000), “Apply mollusc as biological indicator in environmental change and pollution assessment,” Environmental
Education Quarterly, 42:67~76 (in Chinese).
4. Denny, M. W. (1989), “A limpet shell that reduces drag: laboratory demonstration of a hydrodynamic mechanism and an exploration of its effectiveness in nature,”
Can. J. Zool., 67:2098~2106.
5. Denny, M. W. and C. L. Blanchette (2000), “Hydrodymanics, shell shape, behavior and survivorship in the owl limpet Lottia
Gigantea,” J. Exp. Biol., 203: 2623~2639.
6. Denny, M. W., T. L. Daniel and M. A. R. Koehl (1985), “Mechanical limits to size in wave-swept organisms,” Ecol. Monogr., 55:69~102.
7. Dillon, R. T., Jr. (2000), The Ecology of
Freshwater Molluscs, Cambridge University
Press, Cambridge, U.K.
8. Koehl, M. A. R. (1982), “The interaction of moving water and sessile organisms,” Sci.
Am., 247:124~134.
9. Koehl, M. A. R. (1984), “How do benthic organisms withstand moving water?” Am.
Zool., 24:57~70.
10. Koehl, M. A. R. (1986), “Seaweeds in moving water: Form and mechanical function,” pp. 603-634, In T. J. Givnish [ed.], On the
Economy of Plant Form and Function,
Cambridge University Press.
11. Okland, J. (1983), “Factors regulating the distribution of freshwater snails (Gastropoda) in Norway,” Malacologia, 24:277~288.
12. Pace, G. L. (1973), “The freshwater snail of Taiwan (Formosa),” Malacological Review, Supplement 1:1~118.
13. Pip, E. (1978), “A survey of the ecology and composition of submerged aquatic snail-plant communities,” Can. J. Zool., 56:2263~2279.
14. Thomas, J. and A. Tait (1984), “Control of the snail hosts of schistosomiasis by environmental manipulation: a field and laboratory appraisal in the Ibadan area, Nigeria,” Philos. Trans. R. Soc. Lond. Ser. B, 305:201~253.
15. Vogel, S. (1994), Life in Moving Fluids: The
Physical Biology of Flow, Princeton
University Press, Princeton, U.S.A.
16. Watters, G. (1992), “Unionids, fishes, and the species-area curve,” J. Biogeogr., 19:481~90.